Abstract
Proximity ligation assays such as circularized chromosome conformation capture and high-throughput chromosome capture assays have shed light on the structural organization of the interphase genome. Functional topologically associating domains (TADs) that constitute the building blocks of genomic organization are disrupted and reconstructed during the cell cycle. Epigenetic memory, as well as the sequence of chromosomes, regulate TAD reconstitution. Sub-TAD domains that are invariant across cell types have been identified, and contacts between these domains, rather than looping, are speculated to drive chromatin folding. Replication domains are established simultaneously with TADs during the cell cycle and the two correlate well in terms of characteristic features, such as lamin association and histone modifications. CCCTC-binding factor (CTCF) and cohesin cooperate across different cell types to regulate genes and genome organization. CTCF elements that demarcate TAD boundaries are commonly disrupted in cancer and promote oncogene activation. Chromatin looping facilitates interactions between distant promoters and enhancers, and the resulting enhanceosome complex promotes gene expression. Deciphering the chromatin tangle requires comprehensive integrative analyses of DNA- and protein-dependent factors that regulate genomic organization.
1. Introduction
Genomic DNA in each human diploid cell, which is 2 m in total length, is folded around histone proteins to form the compact nucleosome, which in addition to DNA and the histone octamer core, contains the tail regions of histone proteins. The predominant histone octamer core contains two of each of the four histones, H2A, H2B, H3, and H4, around which ≈147 bp of DNA is wound. The nucleosome also contains the histone H1, which binds outside the nucleosome “bead” to the linker DNA (20–80 bp) that connects adjacent nucleosomes. In the last decade, the emergence of new techniques, such as proximity ligation assays, have provided novel and fundamental insights into chromatin organization at different stages of the cell cycle. The mechanisms by which regulatory elements such as enhancers and CCCTC-binding factor (CTCF) sites control genome organization have also been elucidated. This review focuses on recent insights into genome organization, regulatory mechanisms of genome organization, and the significance of genome organization to gene regulation, development, and disease.
2. Chromosome Compartmentalization in the Nucleus
The tangled mass of chromatin is spatially organized in the nucleus within very distinct topological domains. Larger chromosomes and chromosomes with more heterochromatin regions are seen to be present on the nuclear periphery while smaller chromosomes and those with more euchromatin regions are localized at the center of the nucleus [1,2]. The existence of such topological domains has been brought to light by recently developed molecular and genomic techniques such as the circularized chromosome conformation capture assays [3,4]. Based on Hi-C data, two major chromatin compartments have been defined: the A compartment that is made up of the more active and open chromatin, and the B compartment that is made up of inactive and closed chromatin (Figure 1). These compartments can be as large as several Mb; their existence was first suggested by electron microscopy analysis [5], which showed densely-stained chromatin near the nuclear periphery and nucleoli. Active chromatin is concentrated near the center of the nucleus because this is also where transcription factors (TF), RNA polymerases, and other transcriptional regulatory factors are concentrated [6]. In contrast, peripheral nuclear lamina is predominantly composed of intermediate filaments and proteins that provide mechanical support. Laminin polypeptides of the nuclear lamina have a high affinity for inactive chromatin regions with high A/T content, which are referred to as matrix attachment regions (MARs).
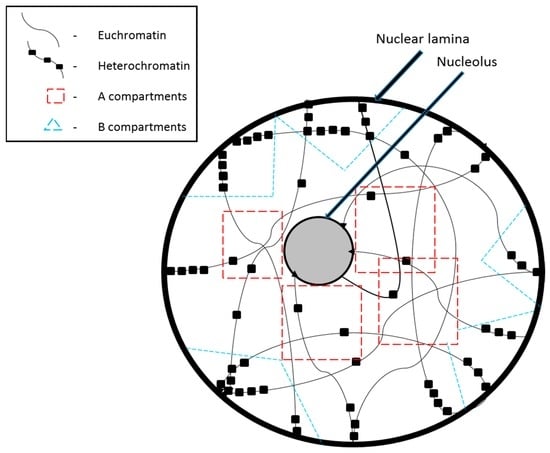
Figure 1.
Chromatin organization in the nucleus during interphase.
Within each of the A and B compartments, there are sub-domains called topologically associating domains (TADs) where interactions are at their highest levels [7]. TADs are highly conserved across various cell lines indicating that they must be important in laying the foundation for the building of the bigger compartments. The A and B compartments appear to be cell-type specific [8]. They can be reorganized to lie closer to the nuclear periphery or the center based on chromatin modifications, which in turn depend on what genes are necessary at eachpoint during development [9]. However, the extent of conservation at TADs is only valid during the interphase. During mitosis, specifically at the metaphase stage, the chromatin takes up an altogether different conformation and to achieve this, even the TADs need to be broken down. Several models have been proposed to explain chromosomal reorganization during mitosis. Electron microscopy and immunofluorescence assays suggest that chromosomes may be organized as hierarchically folded chromatin fibers or chromatin loops, held together along a central axis made of only condensin [10] or of condensin and topoisomerase II [11,12]. Through polymer simulations and genomic analysis, it was discovered that TADs are formed by progressive loop extrusion mediated by loop extruding factors [13].
Genome organization is partly driven by the high affinity of active chromatin regions for other active regions, which also explains why interactions between euchromatin and heterochromatin are infrequently observed. The interactions listed by their order of strength from strongest to weakest are: within A compartments > within B compartments > between A and B compartments [8]. Approximately 70% of chromatin looping events, which facilitate gene regulation by distant regulatory elements [14,15,16], occur within TADs [17]. The typical length scale of chromatin looping is 10–200 kb, whereas that of TADs can be as large as 2000 kb. Recent insights have suggested that TADs can be regarded as looped domains that are held together and demarcated by convergent CTCF sites. In addition, distinct domains within TADs, or sub-TADs, have been reported, which vary between cell types, unlike TADs. Gene regulation at a distance can occur through contacts between sub-TADs without requiring direct chromatin looping of gene promoters with regulatory elements; thus, chromatin folding may be based on sub-TAD interactions rather than chromatin looping mechanisms [18]. However, further studies are required to better understand thesub-TADs and their potential importance indefining genome architecture and the genomic regulatory landscape.
3. Plasticity of TADs
Within a TAD, the activity of individual DNA sequence elements is correlated [19], which indicates non-local TAD-specific regulatory mechanisms. Gene expression from promoter sequences in the same TAD was better correlated than gene expression from the same promoter sequences, but in different TADs [20]. TAD boundaries are typically defined by CTCF sites, promoters, and short interspersed nuclear elements (SINE), and sub-TAD regions are demarcated by CTCF and cohesin-binding sites [21]. CTCF, cohesin, and mediator proteins are critical for chromatin folding and for facilitating long-range regulatory interactions. Knockout of CTCF results in disruption of the associated chromatin loops and the dysregulation of relevant genes [22]. In addition, the deletion of CTCF sites alters the frequency of interactions between loci in adjacent TADs and hence the expression of neighboring genes [23,24,25,26]. The binding of CTCF and other specific factors may by itself intrinsically demarcate boundaries, which is likely the case at most boundaries. Boundaries may also be established indirectly by the aggregation of genetic loci present within TADs, driven by factors such as sequence homology [27].
Several inversions and deletions have been described that disrupt or modify TAD boundaries. For instance, a 0.3 Mb inversion at the Tfap2c (Transcription factor AP-2 gamma) and Bmp7 (Bone morphogenetic protein 7) locus in mouse embryos results in the shifting of a TAD boundary, activation of Tfap2c, and silencing of Bmp7 [28]. The loss or modification of TAD boundaries also results in the activation of oncogenes in cancer cells. TAD boundaries and specifically CTCF binding domains are frequent targets of alteration in cancer cells. In acute myeloid leukemia, as a result of chromosome inversion, two TAD boundaries are disrupted, and an enhancer is dislocated to the TADcontaining the Evi1oncogene [29]. However, this is not the case in the majority of other diseases, wherein single nucleotide polymorphisms are frequently found in regulatory elements rather than in TAD boundaries [30].
4. Overlap of Topologically Associating Domains and Replication Domains
Interphase TADs are disrupted during mitosis, requiring reconstitution of the genome architecture after each round of mitosis. Replication domains, defined as 400–800-kb DNA regions that replicate synchronously, are established during the G1 phase of the cell cycle [31]. At the same time, TADs and inter-TAD boundaries are also established. Regions that replicate synchronously are spatially proximal to each other. Whereas TADs are retained during the G2 phase, the determinants of replication are lost, which suggests that other factors in addition to the chromatin organization determine the establishment of replication domains. However, during the G0 phase, both TADs and replication domains are maintained.
The intimate relationship between TADs, replication domains, and transcriptional regulation is also evident in the spatial organization of the replication domains. Approximately 50% of the genome replicates synchronously (i.e., ‘constitutive replication domains’) [32]. Constitutive replication domains, which are generally transcriptionally active, are positioned near the center of the nucleus where transcriptional regulatory elements are also concentrated. The remainder of the genome replicates more asynchronously, exhibits unique compartmentalization, and is reorganized when required during development (i.e., ‘developmental replication domains’) [33]. Developmental replication domains are also translocated to the nuclear center when required. Compared to constitutive replication domains, developmental replication domains exhibit only a moderate enrichment in chromatin marks, which facilitates the switch from early to late replication, and vice versa [31,34]. A and B compartments of the genome can be subdivided into more specific compartments based on these different features (Table 1).

Table 1.
Classification of the genomic domains based on various chromatin features.
The concept that early acting replication domains correspond to the regions with high euchromatin content has been recently dispelled. In addition to constitutively acting genes, specific developmental genes also replicate early in specific cell types. Moreover, gene expression can be altered without any change in the replication timing [32,34]. Genes can be classified into three categories based on the correlation of expression with replication timing [35]: those that are expressed only during early replication, those that are expressed during early and late replication, and those that are expressed only during late replication. These results suggest that multiple distinct mechanisms regulate gene expression and replication timing.
5. Replication Origins and Nuclear Organization
Pre-replication complexes (pre-RCs) are recruited to specific chromatin sites where they further recruit additional components to form pre-initiation complexes [36,37]. Although the eukaryotic genome contains numerous origins of replication, only ≈20% are initiated during a somatic cell cycle [38]. The subset of the replication origins utilized by each cell differs [39], which could be important for the simultaneous coordination of DNA replication, transcription, and other nuclear events. A significant number of replication origins are located near transcription start sites, CpG islands, and regions exhibiting DNase hypersensitivity. Moreover, high GC content has been associated with replication origin activity [40]. The establishment of a specific chromatin structure and organization likely determines global DNA replication timing and transcription. However, the chromatin state is reciprocally influenced by DNA sequences that initiate replication, referred to as replicators [41,42]. Interactions with structural nuclear components, such as those between MARs and intermediate filaments, can also regulate replication initiation [43], and specific histone modifications are associated specifically with early or late replication [44] (Table 1). Differences in nucleotide base composition may drive the complicated yet intricate relationships between replication timing, histone modification, and genome organization. In-depth analysis of functional elements, transcriptional units, replication and topological domains have revealed variations in sequence GC content. The high amounts of GC observed at early replicating domains result from small-scale increases near functional elements. At late replicating domains a gradual accumulation of A/T nucleotides is facilitated by changes in the deoxyribose nucleotide triphosphate(dNTP) pool available during the late S phase [45].
6. Reconstitution of Topologically Associating Domains Following Mitosis
Although chromosomes are reorganized into chromatids during mitosis, the chromatin status of specific regions is retained [46], which could contribute to the high fidelity reconstitution of the A and B compartments. DNA sequence homology could also drive domain reconstitution. During interphase, centromeric and sub-centromeric regions can fuse to form chromocenters [47,48]. In addition, differences in the distribution of repetitive sequence elements between A and B compartments could contribute to their selective compartmentalization. Long interspersed nuclear elements (LINE) are enriched in heterochromatic regions (i.e., B compartment), whereas SINE are enriched in euchromatic regions (i.e., A compartment) [8].
The distribution of lamina-associated domains (LADs) could also regulate the chromosome position and shape in the nucleus. LADs tether chromatin to the nuclear lamina through important tethering proteins; lamin B receptor (LBR) and lamin A/C. The LBR and lamin A/C are both constituent proteins of the lamina that are sequentially expressed during cell differentiation. Deletion of both proteins causes dislocation of LADs from the nuclear lamina to the nuclear center [49]. Chromosomes that are attached to the lamina appear flattened compared to their unattached counterparts. Repressive chromatin modifications, which include histone H3 lysine 27 tri-methylation (H3K27me3) and histone H3 lysine 9 di-/ tri-methylation (H3K9me2/me3), of developmentally regulated LADs, also called facultative LADs, during differentiation is associated with the silencing of genes and the translocation of corresponding domains to the nuclear and nucleolar periphery. In vitro experiments have revealed that whereas chromatin modifications occur before mitosis, domain dislocation and changes in gene expression manifest only after the completion of mitosis [50,51,52], suggesting that active signaling mechanisms during mitosis suppress domain reconstitution.
7. Regulatory Elements in a Divided Genome
Enhancers are important cis-acting DNA regulatory elements that promote TF. Enhancers do not always affect the promoters that are closest to them in the linear DNA sequence, and may only be active in specific tissue types and at specific development stages. The first enhancer sequence, which was identified in an intron of the mouse immunoglobulin heavy chain gene, increased the transcription of various nuclear factors. Enhancers may potentially function by turning on transcription of a specific locus in more cells or by increasing the number of RNA molecules transcribed from a specific locus [53].
Enhancers are typically located several kb or Mb away from the gene promoters that they act on. The mechanisms of enhancer-mediated gene transcription at a distance have recently been elucidated [54,55,56]. Chromatin looping brings the transcription initiation factors of the pre-initiation complexes that are bound at the distant enhancer site close to the gene promoter site [57]. According to the enhanceosome model, TF are recruited to clustered DNA binding enhancer sites in an orderly and cooperative manner, and transcription occurs only when all factors are present. An alternative model is the billboard model, in which the binding and function of each TF are independently regulated. The enhanceosome model proposes that gene repression of nucleosome DNA can be overcome by the binding of factors with increasing avidity. Although short and highly degenerate TF recognition sequences frequently occur in the genome, less than half of these sites are actually occupied.
Members of a TF family may share a common DNA binding domain but may be recruited by other unique elements for distinct purposes. For example, the FOXM1 (forkhead box M1) transcription factor is recruited via protein–protein interactions with CHR (cell cycle homology region) elements rather than by interaction with the consensus RYAAAYA binding motif, to specifically regulate late cell cycle processes [58]. Analysis of the protein interactome of FOX (forkhead box) TF family members also revealed differences in protein–protein interactions depending on whether FOX TF members were chromatin-bound at the time point of the analysis [59]. Therefore, the roles of TFs in gene regulation can depend on their interactions with other proteins, including transcriptional co-regulators. TFs involved in maintaining the pluripotency of stem cells or specifying cell fates, such as OCT4 (octamer-binding transcription factor 4), can recognize binding sites in silent chromatin, which triggers chromatin remodeling and the further recruitment of other factors [60].
A genome-wide analysis of enhancer–promoter interactions in human primary resting CD4+ Tcells identified 2373 such interactions involving 2067 enhancers and 1619 promoters., with 91% of these being single promoter–enhancer interactions [53]. The majority of these interactions were within 50kb regions and additionally, a number of promoter–promoter and enhancer–enhancer interactions were identified. While enhancer–promoter interactions did increase gene transcription, about 23% of the enhancers were found to be bound by the insulator protein, CTCF. As CTCF is the key player in demarcating TADs, there is a good chance that the resultant increase in gene transcription is brought about by boundary disruption that brings an enhancer and a promoter in adjacent TADs closer together (Figure 2). Co-expression was seen at promoters that interact and of genes driven by a common enhancer; however, these interactions may be tissue-specific.
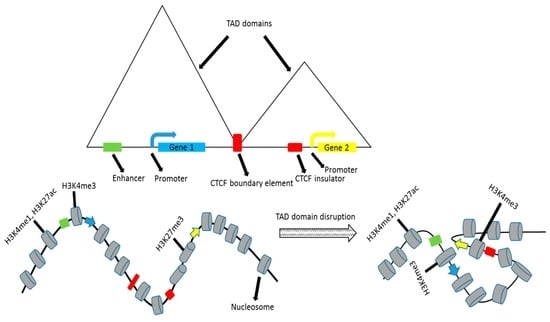
Figure 2.
Effects of disrupting topologically associating domains (TAD) on gene regulation. CTCF: CCCTC-binding factor.
CTCF and cohesin act cooperatively in genome organization [61,62,63,64,65,66]. CTCF binding sites can function as insulators and block enhancer activity when inserted between a promoter and its enhancer; however, this phenomenon may be an artefact of the in vitro system it was characterized in. CTCF binding sites are concentrated in gene-dense regions and are largely tissue-invariant [67,68]. The CTCF-cohesin complex also regulates the transcription of genes in the viral genome, including those involved in pathogenicity [69,70]. CTCF sites in mammalian genomes, such as in rat, mouse, dog, and short-tailed opossum, have evolved over time through the expansion of SINE elements that carry the CTCF binding motif [71]. Epigenetic mechanisms and transposable elements also regulate genome organization and function in higher eukaryotes. In addition to its structural role in organizing sister chromatids during mitosis and meiosis, cohesin has important post-mitotic functions, which is evident from its significance in post-mitotic cells. High-affinity cohesin binding sites are associated with CTCF binding sites [64,65]. At weaker cohesin binding sites, mediator components, loading factors such as Nipbl (Nipped-B-like protein), and TF are required for efficient cohesin binding [72,73]. Thus, cohesin has CTCF-dependent and -independent gene regulatory functions [74].
Numerous databases, such as the Encyclopedia of DNA Elements (ENCODE) database, are available for predicting regulatory sequences through in silico analysis. Whereas CTCF sites are defined by a consensus sequence, enhancer regions are not. Additionally, regulatory sequences may function differently based on the tissue type and/or the developmental stage of the organism. Therefore, it will be necessary to experimentally validate computationally predicted regulatory sequence elements. Cross-disciplinary perspectives that combine structural and functional genomics will be required for a genome-wide understanding of the mechanisms of gene regulation [75].
8. Future Perspectives
Although proximity ligation assays have contributed to our understanding of genome organization and chromatin folding, much more remains to be discovered. Further research is required to understand how TADs reconstitute with high fidelity following every cell division. Additional studies are also required to understand the spatial and temporal relationships among replication origins, gene regulatory elements, and epigenetic modifications that regulate if and when two genomic regions are brought into close proximity. Long non-coding RNAs, which are associated with the genome architecture, may be critical to answering some of these questions. Finally, conceptual and experimental models that go beyond single-factor binding models will be required to define both the underlying molecules and mechanisms that govern genome organization.
Acknowledgments
This paper was supported by the KU-Research Professor Program of Konkuk University, Seoul, South Korea.
Conflicts of Interest
The authors declare that there are no conflict of interests regarding the publication of this article.
References
- Tanabe, H.; Müller, S.; Neusser, M.; von Hase, J.; Calcagno, E.; Cremer, M.; Solovei, I.; Cremer, C.; Cremer, T. Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates. Proc. Natl. Acad. Sci. USA 2002, 99, 4424–4429. [Google Scholar] [CrossRef] [PubMed]
- Bolzer, A.; Kreth, G.; Solovei, I.; Koehler, D.; Saracoglu, K.; Fauth, C.; Müller, S.; Eils, R.; Cremer, C.; Speicher, M.R.; et al. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 2005, 3, e157. [Google Scholar] [CrossRef] [PubMed]
- Simonis, M.; Klous, P.; Splinter, E.; Moshkin, Y.; Willemsen, R.; de Wit, E.; van Steensel, B.; de Laat, W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 2006, 38, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McCord, R.P.; Ho, Y.J.; Lajoie, B.R.; Hildebrand, D.G.; Simon, A.C.; Becker, M.S.; Alt, F.W.; Dekker, J. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 2012, 148, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.G. Fine structure of heterochromatin in certain cell nuclei. Nature 1967, 214, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Green, J.; Das Neves, R.P.; Wallace, H.A.; Smith, A.J.; Hughes, J.; Gray, N.; Taylor, S.; Wood, W.G.; Higgs, D.R.; et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J.Cell. Biol. 2008, 182, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Peric-Hupkes, D.; Meuleman, W.; Pagie, L.; Bruggeman, S.W.; Solovei, I.; Brugman, W.; Gräf, S.; Flicek, P.; Kerkhoven, R.M.; van Lohuizen, M.; et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 2010, 38, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kireeva, N.; Lakonishok, M.; Kireev, I.; Hirano, T.; Belmont, A.S. Visualization of early chromosome condensation: A hierarchical folding, axial glue model of chromosome structure. J. Cell Biol. 2004, 166, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Strukov, Y.G.; Belmont, A.S. Mitotic chromosome structure: Reproducibility of folding and symmetry between sister chromatids. Biophys. J. 2009, 96, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Samejima, K.; Samejima, I.; Vagnarelli, P.; Ogawa, H.; Vargiu, G.; Kelly, D.A.; de Lima Alves, F.; Kerr, A.; Green, L.C.; Hudson, D.F.; et al. Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIα. J. Cell Biol. 2012, 199, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Fudenberg, G.; Imakaev, M.; Lu, C.; Goloborodko, A.; Abdennur, N.; Mirny, L.A. Formation of Chromosomal Domains by Loop Extrusion. CellRep. 2016, 5, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- García-González, E.; Escamilla-Del-Arenal, M.; Arzate-Mejía, R.; Recillas-Targa, F. Chromatin remodeling effects on enhancer activity. Cell. Mol. Life Sci. 2016, 73, 2897–2910. [Google Scholar] [CrossRef] [PubMed]
- Kadauke, S.; Blobel, G.A. Chromatin loops in gene regulation. Biochim. Biophys. Acta 2009, 1789, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kolovos, P.; van de Werken, H.J.G.; Kepper, N.; Zuin, J.; Brouwer, R.W.W.; Kockx, C.E.M.; Wendt, K.S.; van Ijcken, W.F.J.; Grosveld, F.; Knoch, T.A. Targeted Chromatin Capture (T2C): A novel high resolution high throughput method to detect genomic interactions and regulatory elements. Epigenetics Chromatin 2014, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Lajoie, B.R.; Jain, G.; Dekker, J. The long-range interaction landscape of gene promoters. Nature 2012, 489, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Berlivet, S.; Paquette, D.; Dumouchel, A.; Langlais, D.; Dostie, J.; Kmita, M. Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of HoxA Genes in Developing Limbs. PLoS Genet. 2013, 9, e1004018. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Cremins, J.E.; Sauria, M.E.G.; Sanyal, A.; Gerasimova, T.I.; Lajoie, B.R.; Bell, J.S.K.; Ong, C.-T.; Hookway, T.A.; Guo, C.; Sun, Y.; et al. Architectural Protein Subclasses Shape 3D Organization of Genomes during Lineage Commitment. Cell 2013, 153, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Valton, A.L.; Dekker, J. TAD disruption as oncogenic driver. Curr. Opin. Genet. Dev. 2016, 36, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Symmons, O.; Uslu, V.V.; Tsujimura, T.; Ruf, S.; Nassari, S.; Schwarzer, W.; Ettwiller, L.; Spitz, F. Functional and topological characteristics of mammalian regulatory domains. Genome Res. 2014, 24, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T.; Klein, F.A.; Langenfeld, K.; Glaser, J.; Huber, W.; Spitz, F. A discrete transition zone organizes the topological and regulatory autonomy of the adjacent TFAP2C and BMP7 genes. PLoS Genet. 2015, 11, e1004897. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, D.G.; Spielmann, M.; Mundlos, S. Breaking TADs: how alterations of chromatin domains result in disease. Trends. Genet. 2016, 32, 225–237. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Vos, E.S.M.; Holwerda, S.J.B.; Valdes-Quezada, C.; Verstegen, M.J.A.M.; Teunissen, H.; Splinter, E.; Wijchers, P.J.; Krijger, P.H.L.; de Laat, W. CTCF Binding Polarity Determines Chromatin Looping. Mol. Cell 2015, 60, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, Q.; Canzio, D.; Shou, J.; Li, J.; Gorkin, D.U.; Jung, I.; Wu, H.; Zhai, Y.; Tang, Y.; et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell 2015, 162, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Dowen, J.M.; Fan, Z.P.; Hnisz, D.; Ren, G.; Abraham, B.J.; Zhang, L.N.; Weintraub, A.S.; Schuijers, J.; Lee, T.I.; Ko, K.; et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 2014, 159, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Narendra, V.; Rocha, P.P.; An, D.; Raviram, R.; Skok, J.A.; Mazzoni, E.O.; Reinberg, D. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 2015, 347, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Groschel, S.; Sanders, M.A.; Hoogenboezem, R.; de Wit, E.; Bouwman, B.A.M.; Erpelinck, C.; van der Velden, V.H.; Havermans, M.; Avellino, R.; van Lom, K.; et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell 2014, 157, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Dadon, D.B.; Powell, B.E.; Fan, Z.P.; Borges-Rivera, D.; Shachar, S.; Weintraub, A.S.; Hnisz, D.; Pegoraro, G.; Lee, T.I.; et al. 3D Chromosome Regulatory Landscape of Human Pluripotent Cells. Cell Stem Cell 2016, 18, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Dileep, V.; Ay, F.; Sima, J.; Vera, D.L.; Noble, W.S.; Gilbert, D.M. Topologically associating domains and their long-range contact are established during early G1 coincident with the establishment of the replication-timing program. Genome Res. 2015, 25, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, I.; Ryba, T.; Itoh, M.; Yokochi, T.; Schwaiger, M.; Chang, C.W.; Lyou, Y.; Townes, T.M.; Schübeler, D.; Gilbert, D.M. Global reorganization of replication domains during embryonic stem cell differentiation. PLoS Biol. 2008, 6, e245. [Google Scholar] [CrossRef] [PubMed]
- Ryba, T.; Hiratani, I.; Lu, J.; Itoh, M.; Kulik, M.; Zhang, J.; Dalton, S.; Gilbert, D.M. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res. 2010, 20, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.P.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, I.; Ryba, T.; Itoh, M.; Rathjen, J.; Kulik, M.; Papp, B.; Fussner, E.; Bazett-Jones, D.P.; Plath, K.; Dalton, S.; et al. Genomewide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res. 2010, 20, 155–169. [Google Scholar] [CrossRef] [PubMed]
- DePamphilis, M.L.; Blow, J.J.; Ghosh, S.; Saha, T.; Noguchi, K.; Vassilev, A. Regulating the licensing of DNA replication origins in metazoa. Curr. Opin.Cell Biol. 2006, 18, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Depamphilis, M.L.; de Renty, C.M.; Ullah, Z.; Lee, C.Y. ‘‘The Octet’’: Eightprotein kinases that control mammalian DNA replication. Front. Physiol. 2012, 3, 368. [Google Scholar] [CrossRef] [PubMed]
- Cayrou, C.; Coulombe, P.; Vigneron, A.; Stanojcic, S.; Ganier, O.; Peiffer, I.; Rivals, E.; Puy, A.; Laurent-Chabalier, S.; Desprat, R.; et al. Genome-scale analysis of metazoan replication origins reveals their organization in specific but flexible sites defined by conserved features. Genome Res. 2011, 21, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Fragkos, M.; Ganier, O.; Coulombe, P.; Mechali, M. DNA replication origin activation in space and time. Nat. Rev. Mol. Cell Biol. 2015, 16, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Majocchi, S.; Aritonovska, E.; Mermod, N. Epigenetic regulatory elements associate with specific histone modifications to prevent silencing of telomeric genes. Nucleic Acids Res. 2014, 42, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.L.; Aladjem, M.I. The chromatin backdrop of DNA replication: Lessons from genetics and genome-scale analyses. Biochim. Biophys.Acta 2012, 1819, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, L.; Lin, C.M.; Singhania, S.; Bouhassira, E.E.; Aladjem, M.I. Preventing gene silencing with human replicators. Nat. Biotechnol. 2006, 24, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.K.; Aladjem, M.I. Chromatin structure and replication origins: Determinants of chromosome replication and nuclear organization. J. Mol. Biol. 2014, 426, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Mechali, M.; Yoshida, K.; Coulombe, P.; Pasero, P. Genetic and epigenetic determinants of DNA replication origins, position and activation. Curr. Opin. Genet. Dev. 2013, 23, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, E.; Yehuda, Y.; Marjavaara, L.; Keszthelyi, A.; Chabes, A.; Tanay, A.; Simon, I. The mutation spectrum in genomic late replication domains shapes mammalian GC content. Nucleic Acids Res. 2016, 44, 4222–4232. [Google Scholar] [CrossRef] [PubMed]
- Terrenoire, E.; McRonald, F.; Halsall, J.A.; Page, P.; Illingworth, R.S.; Taylor, A.M.; Davison, V.; O’Neill, L.P.; Turner, B.M. Immunostaining of modified histones defines high-level features of the human metaphase epigenome. Genome Biol. 2010, 11, R110. [Google Scholar] [CrossRef] [PubMed]
- Wijchers, P.J.; Geeven, G.; Eyres, M.; Bergsma, A.J.; Janssen, M.; Verstegen, M.; Zhu, Y.; Schell, Y.; Vermeulen, C.; de Wit, E.; et al. Characterization and dynamics of pericentromere-associated domains in mice. Genome Res. 2015, 25, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.; Schermelleh, L.; During, K.; Engelhardt, A.; Stein, S.; Cremer, C.; Cremer, T. Differences in centromere positioning of cycling and postmitotic human cell types. Chromosoma 2004, 112, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Finlan, L.E.; Sproul, D.; Thomson, I.; Boyle, S.; Kerr, E.; Perry, P.; Ylstra, B.; Chubb, J.R.; Bickmore, W.A. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008, 4, e1000039. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.I.; Spector, D.L. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J.Cell Biol. 2008, 180, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.L.; Zullo, J.M.; Bertolino, E.; Singh, H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 2008, 452, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, I.; Wei, G.; Wangsa, D.; Tang, Q.; Zhao, K. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 2012, 22, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tavoosidana, G.; Sjölinder, M.; Göndör, A.; Mariano, P.; Wang, S.; Kanduri, C.; Lezcano, M.; Sandhu, K.S.; Singh, U.; et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat. Genet. 2006, 38, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Dostie, J.; Richmond, T.A.; Arnaout, R.A.; Selzer, R.R.; Lee, W.L.; Honan, T.A.; Rubio, E.D.; Krumm, A.; Lamb, J.; Nusbaum, C.; et al. Chromosome conformation capture carbon copy (5C): A massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006, 16, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Kolovos, P.; Knoch, T.A.; Grosveld, F.G.; Cook, P.R.; Papantonis, A. Enhancers and silencers: An integrated and simple model for their function. Epigenetics Chromatin 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Erokhin, M.; Vassetzky, Y.; Georgiev, P.; Chetverina, D. Eukaryotic enhancers: common features, regulation, and participation in diseases. Cell. Mol. Life Sci. 2015, 72, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Muller, G.A.; Quaas, M.; Fischer, M.; Han, N.; Stutchbury, B.; Sharrocks, A.D.; Engeland, K. The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol. Cell. Biol. 2013, 33, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, W.; Wang, J.; Malovannaya, A.; Xi, Y.; Li, W.; Guerra, R.; Hawke, D.H.; Qin, J.; Chen, J. Proteomic analyses reveal distinct chromatin-associated and soluble transcription factor complexes. Mol. Syst. Biol. 2015, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Taberlay, P.C.; Kelly, T.K.; Liu, C.C.; You, J.S.; De Carvalho, D.D.; Miranda, T.B.; Zhou, X.J.; Liang, G.; Jones, P.A. Polycomb-repressed genes have permissive enhancers that initiate reprogramming. Cell 2011, 147, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Wendt, K.S.; Yoshida, K.; Itoh, T.; Bando, M.; Koch, B.; Schirghuber, E.; Tsutsumi, S.; Nagae, G.; Ishihara, K.; Mishiro, T.; et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008, 451, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Parelho, V.; Hadjur, S.; Spivakov, M.; Leleu, M.; Sauer, S.; Gregson, H.C.; Jarmuz, A.; Canzonetta, C.; Webster, Z.; Nesterova, T.; et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 2008, 132, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Seitan, V.C.; Hao, B.; Tachibana-Konwalski, K.; Lavagnolli, T.; Mira-Bontenbal, H.; Brown, K.E.; Teng, G.; Carroll, T.; Terry, A.; Horan, K.; et al. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature 2011, 476, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Rubio, E.D.; Reiss, D.J.; Welcsh, P.L.; Disteche, C.M.; Filippova, G.N.; Baliga, N.S.; Aebersold, R.; Ranish, J.A.; Krumm, A. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. USA 2008, 105, 8309–8314. [Google Scholar] [CrossRef] [PubMed]
- Stedman, W.; Kang, H.; Lin, S.; Kissil, J.L.; Bartolomei, M.S.; Lieberman, P.M. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/IGF2 insulators. EMBO J. 2008, 27, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Tark-Dame, M.; Jerabek, H.; Manders, E.M.M.; Heermann, D.W.; van Driel, R. Depletion of the chromatin looping proteins ctcf and cohesin causes chromatin compaction: Insight into chromatin folding by polymer modelling. PLoS Comput. Biol. 2014, 10, e1003877. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Abdullaev, Z.K.; Smith, A.D.; Ching, K.A.; Loukinov, D.I.; Green, R.D.; Zhang, M.Q.; Lobanenkov, V.V.; Ren, B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 2007, 128, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Lindblad-Toh, K.; Garber, M.; Zuk, O.; Lin, M.F.; Parker, B.J.; Washietl, S.; Kheradpour, P.; Broad Institute Sequencing Platform and Whole Genome Assembly Team; Baylor College of Medicine Human Genome Sequencing Center Sequencing Team; Genome Institute at Washington University; et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature 2011, 478, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Wikramasinghe, P.; Showe, L.; Lieberman, P.M. Cohesins repress Kaposi’s sarcoma-associated herpesvirus immediate early gene transcription during latency. J. Virol. 2012, 86, 9454–9464. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wiedmer, A.; Yuan, Y.; Robertson, E.; Lieberman, P.M. Coordination of KSHV latent and lytic gene control by CTCF-cohesin mediated chromosome conformation. PLoS Pathog. 2011, 7, e1002140. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Wilson, M.D.; Ballester, B.; Schwalie, P.C.; Brown, G.D.; Marshall, A.; Kutter, C.; Watt, S.; Martinez-Jimenez, C.P.; Mackay, S.; et al. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 2010, 328, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Schwalie, P.C.; Ross-Innes, C.S.; Hurtado, A.; Brown, G.D.; Carroll, J.S.; Flicek, P.; Odom, D.T. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 2010, 20, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kagey, M.H.; Newman, J.J.; Bilodeau, S.; Zhan, Y.; Orlando, D.A.; van Berkum, N.L.; Ebmeier, C.C.; Goossens, J.; Rahl, P.B.; Levine, S.S.; et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010, 467, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Beygo, J.; Citro, V.; Sparago, A.; De Crescenzo, A.; Cerrato, F.; Heitmann, M.; Rademacher, K.; Guala, A.; Enklaar, T.; Anichini, C.; et al. The molecular function and clinical phenotype of partial deletions of the IGF2/H19 imprinting control region depends on the spatial arrangement of the remaining CTCF-binding sites. Hum. Mol. Genet. 2013, 22, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, C.; Raphael, B.J. Identification of hierarchical chromatin domains. Bioinformatics 2016, 32, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).