Genetic Mosaics and the Germ Line Lineage
Abstract
:1. Embryonic Origin of the Mammalian Germ Line
2. Germ Line and Zygotic de novo Point Mutation Rates
3. Mosaicism Patterns Depending on Germ Line Lineage
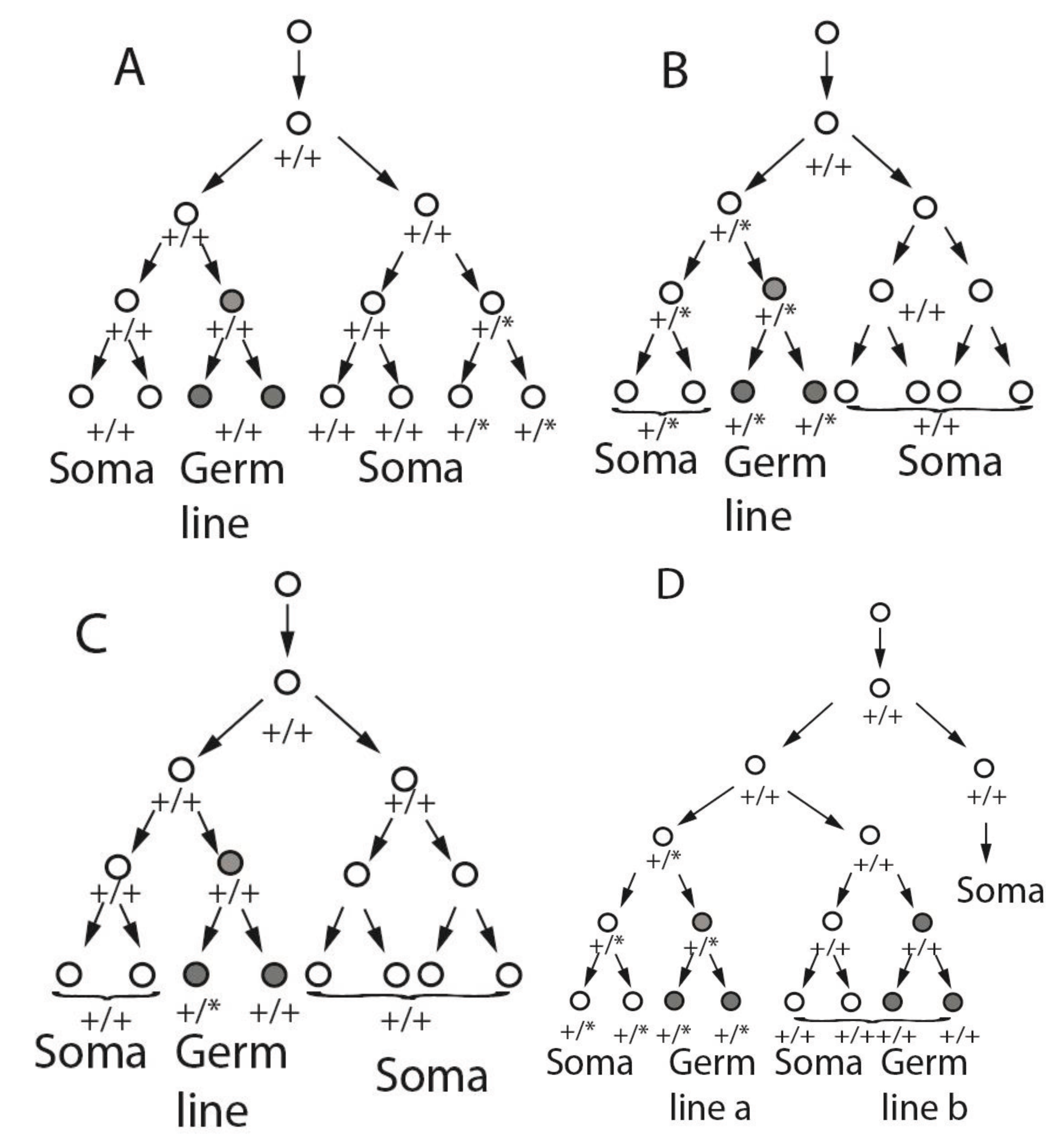
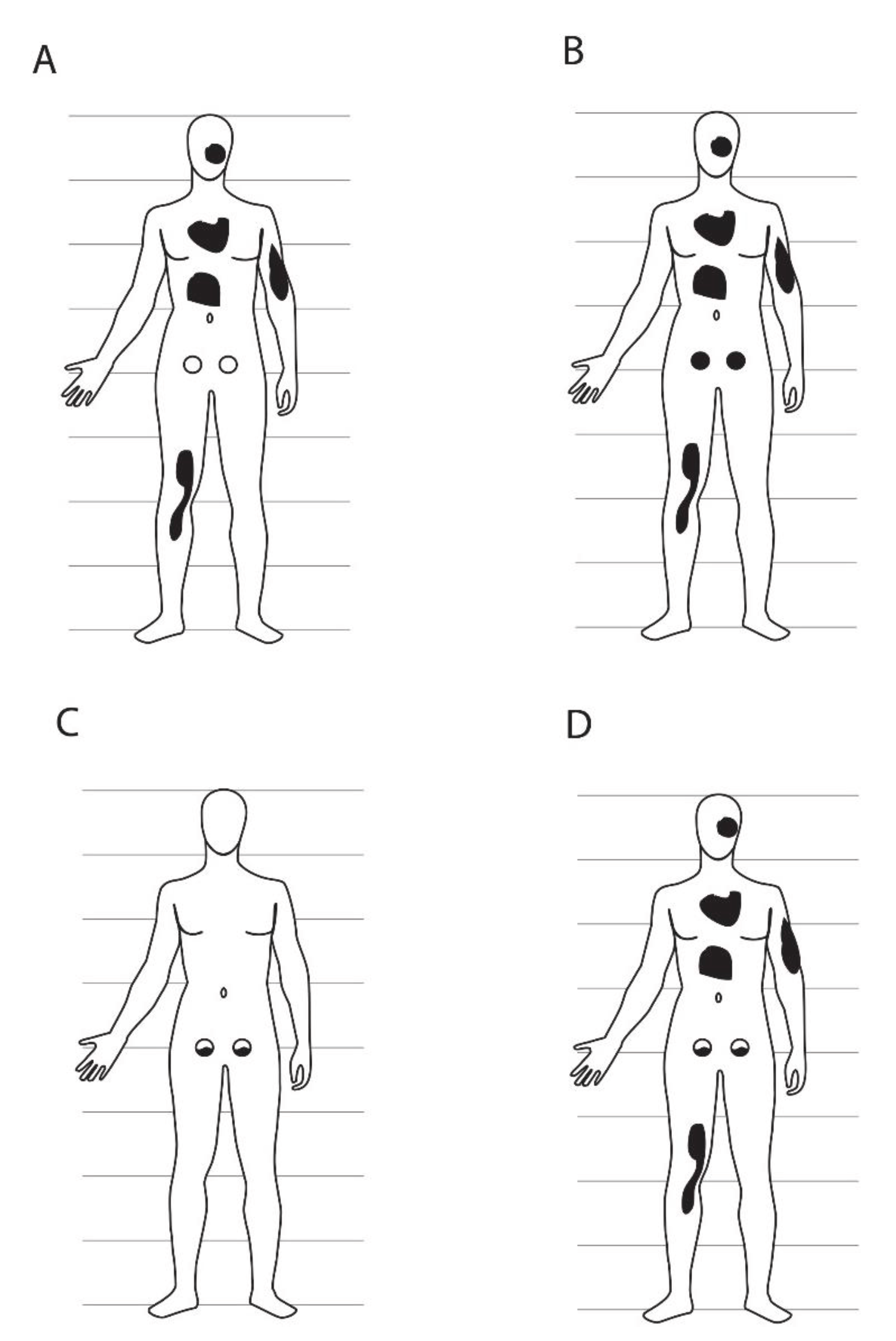
4. Observational Tests of Combined Germ Line and Somatic Mosaicism
| Disorder and Clinical Status of Affected Offspring | Gene and Chromosome | Somatic Mosaicism in Parent * | Germ Line Mosaicism in Parent * | Mosaic Parent and Clinical Status |
|---|---|---|---|---|
| X-linked dominant protoporphyria [55], One severely affected | ALAS2 ChrX | Yes Sequencing, 13% mutant allele in peripheral blood and buccal mucosa | Yes Aff/unaff half-sibs share same maternal X haplotype with/without mutation | Mother Mildly affected |
| Androgen insensitivity [56] Two siblings differentially affected, one raised as female one as male | AR ChrX | Yes Allele specific oligo hybridization <10% mutant allele in peripheral blood | Yes Aff/unaff sibs share same maternal X haplotype with/without mutation | Mother Unaffected |
| Osteogenesis imperfecta [57] Two half-siblings, one deceased pre-term, one deceased neonatally | COL1A1 Chr17 | Yes RFLP, 20% mutant allele in peripheral blood, hair bulbs, absence of mutant allele in fibroblasts | Yes RFLP, 14% mutant allele in sperm | Father Unaffected |
| Osteogenesis imperfecta [58] One deceased perinatally | COL1A1 Chr17 | Probably Allele-specific hybridization, variable proportion mutant allele 26% in peripheral blood 45%–50% in fibroblasts | Probably Allele-specific hybridization, 36%–40% mutant allele in sperm | Father Mildly affected |
| Osteogenesis imperfecta [59] Two siblings, one more severe deceased at 3 years | COL1A1 Chr17 | Yes Allele specific hybridization, library colony count, variable proportion mutant allele in peripheral blood (~10%), fibroblasts (~25%) | Possibly Allele specific hybridization, library colony count, 40%–45% mutant allele in sperm | Father Unaffected |
| Osteogenesis imperfecta [60] Two half-siblings, severely affected deceased neonatally | COL1A2 Chr7 | Probably Southern blot, variable stoichiometry of mutant allele in peripheral blood (40%), fibroblasts (almost 50%) | Probably Southern blot, 40% mutant allele in sperm | Father Moderately affected |
| Osteogenesis imperfecta [61] One affected proband, two deceased prenatally | COL1A2 Chr7 | Yes 25% mutant allele in peripheral blood, fibroblasts | Possibly 40% mutant allele in sperm | Father Unaffected |
| Rubinstein-Taybi syndrome [62] One significantly affected (FII) | CREBBP Chr16 | Possibly Sanger sequencing, small secondary peak of mutant allele in saliva, blood (not quantified) | Possibly Sanger sequencing, small secondary peak of mutant allele in sperm (not quantified) | Father Very mildly affected if at all |
| Dyskeratosis congenital [63] Two affected males, two unaffected mutation carrier het females with skewed X-inactivation | DKC1 ChrX | Yes Allele-specific PCR, mutant allele observed but <5% in peripheral blood, saliva | Yes Aff/unaff brothers share same maternal X haplotype with/without mutation | Mother Unaffected |
| Duchenne muscular dystrophy [64] Family DL114, one affected proband | DMD ChrX | Possibly Southern blot, mutant allele band less than 50% in peripheral blood | Yes Aff/unaff sibs share same maternal X haplotype with/without mutation | Mother |
| Duchenne muscular dystrophy [65] Proband hemizygous, severely affected from age 3 years | DMD ChrX | Yes Microsatellite genotyping and PCR deletion detection Three alleles detected in maternal lymphocytes | Yes Aff/unaff brothers share same maternal X haplotype with/without mutation | Mother Unaffected |
| Haemophilia B [66] One severely affected hemizygous male of heterozygous mother, mosaicism analyzed in her father (“grandfather”) | FIX ChrX | Yes DHPLC, 35% mutant allele in peripheral blood | Yes Aff/unaff half-sisters share same grandpaternal X haplotype with/without mutation | Grandfather Mildly affected in clotting assay |
| Facioscapulohumeral muscular dystrophy [67] One affected in each of two families (F4, F13) | FSHD1 Chr4 | Probably Southern blot Signal of mutant versus normal RFLP band in peripheral blood lower than in non-mosaic affected progeny (semi-quantitative) | Yes Southern blot Aff/unaff sibs share same maternal haplotype with/without mutation in both families | Mothers (2 families) Possibly affected |
| Haemophilia A [68] One affected male proband, mutation from mosaic mother | FVIII ChrX | Possibly Southern blot, causal mutant allele much less than 50% in peripheral blood | Possibly Southern blot, three gene alleles among progeny | Mother Unaffected |
| Haemophilia A [69] One affected male proband, mutation from heterozygous unaffected mother, mosaic was maternal grandfather | FVIII ChrX | Yes Sequencing and PAGE, normal and mutant allele of X-linked gene present in peripheral blood, buccal cells | Yes Sequencing and PAGE, 2 sisters of proband’s mother, normally obligate mutation carriers, lacked mutant allele | Grandfather Unaffected |
| Lesch-Nyhan syndrome [70] Proband hemizygous for mutation, undiagnosed brother deceased age 1 month | HPRT1 ChrX | Yes Cultured cell clones with or without mutation | Yes Aff/unaff sisters share same maternal X haplotype with/without mutation (heterozygous) | Mother Unaffected |
| Hunter disease [71] Proband hemizygous for mutation | IDS ChrX | Yes Allele-specific hybridization, quantitative PCR, variable mutant allele frequencies 7% in lymphocytes, leukocytes, 22% in fibroblasts, 1/35 hair bulbs | Yes Aff/unaff brother/sister share same maternal X haplotype with/without mutation | Mother Unaffected |
| CRASH syndrome [72] Proband hemizygous for mutation, mother heterozygous carrier, one affected hemizygous uncle | L1CAM ChrX | Yes RFLP, SSCP, mutant allele signal less than in true heterozygotes in family. | Yes Aff/unaff siblings share same grandmaternal X haplotype with/without mutation | Grandmother Unaffected |
| Neurofibromatosis [73] Mosaic mother is proband, affected daughter simple heterozygote for mutation | NF2 Chr22 | Yes Quantitative Sanger sequencing, 18% mutant allele in peripheral blood | Yes Aff sister/unaff brother share same maternal haplotype with/without mutation | Mother Affected diagnosed age 23 years |
| Lowe syndrome [74] One affected hemizygous mutation carrier in family LS04FR, heterozygous unaffected mother | OCRL ChrX | Possibly Single strand conformation analysis, small proportion of mutant allele detected in urine, none in blood, buccal or hair bulb | Yes 1 carrier, 2 normal sisters share same grandmaternal X haplotype with/without mutation | Grandmother Unaffected |
| Hypophosphatemic rickets [75] One affected diagnosed age 19 months, 56% mutant allele as per simple heterozygote | PHEX ChrX | Yes Single-base extension and DHPLC, 60% mutant allele in lymphocytes, 6%–94% mutant allele in multiple independent hair bulbs | Yes Aff/unaff sisters share same paternal X haplotype with/without mutation (heterozygous) | Father Affected, treatment initiated age 2 years, grandparents unaffected |
| Polycystic kidney disease [76] One affected diagnosed age 17 years | PKD1 Chr16 | Yes Next-generation sequencing, 3% mutant allele in peripheral blood, 4% in buccal cells (below detection limit by Sanger sequencing) | Yes Sanger, next-generation sequencing, 10% mutant allele in sperm | Father Affected, diagnosed age 50 years |
| Alzheimer disease [77] Mosaic mother is proband, onset age 42 years, deceased age 58 years. One daughter inherited mutation fully heterozygous, more severe, onset age 27 years, deceased age 39 years | PSEN1 Chr14 | Yes Allele-specific hybridization, mutant allele in peripheral blood, autopsy cerebral cortex much lower signal than in heterozygous daughter (qualitative), mutant detected by sequencing cerebral cortex but not peripheral blood DNA | Yes 1 aff/2 unaff sibs share same maternal haplotype with/without mutation | Mother Affected |
| Retinoblastoma [78] Three families (139, 345, 385) each with one bilaterally affected proband | RB1 Chr13 | Yes PCR SSCP, mutant allele less than 50% in peripheral blood in all three mosaic parents | Yes Aff/unaff sibs share same parental haplotype with/without mutation (all 3 families). In one family, mutation observed in 20%–30% of father’s sperm | Father (two families) Mother (one family) All unaffec ted |
| Retinoblastoma [79] Families D, E one bilaterally affected proband in each | RB1 Chr13 | Yes RFLP, sequencing individual PCR clones from peripheral blood, 10% clones mutation positive in fam D, 12% in fam E. Single-sperm PCR RFLP, 7% mutation-carrying sperm in fam E | Yes Aff/unaff sibs or half-sibs share same paternal haplotype with/without mutation | Fathers (two families, one bilaterally, one unilaterally affected) |
| Spinal muscular atrophy [80] One affected inheriting mutation independently from both parents, father het carrier, paternal grandmother is candidate mosaic | SMN1 Chr5 | Possibly Microsatellite genotyping showed 3 chr5 haplotypes, qPCR showed intermediate gene dosage in peripheral blood | Possibly Affected/unaffected progeny share same grandmaternal haplotype with/without mutation | Grandmother Unaffected |
| Anophthalmia syndrome [81] One severely affected, second deceased pre-term not studied | SOX2 Chr3 | Yes RFLP by DHPLC, mutant allele present with lower signal in blood, mouthwash of parent than in non-mosaic affected heterozygous offspring (qualitative) | Yes Aff/unaff sibs share same maternal haplotype with/without mutation | Mother Unaffected |
| 46,XY disorder of sexual development [82] Two fully sex-reversed XY siblings | SRY ChrY | Yes Normal and mutant SRY alleles seen for Y chromosome in peripheral blood (qualitative) | Yes Normal and mutant SRY alleles seen for Y chromosome in sperm (qualitative) | Father Unaffected |
5. Examples of Combined Somatic and Germ Line Mosaicism
6. Number and Time of Specification of FGCs
7. Use of High Throughput Sequencing for Developmental Lineage Studies in Humans
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Felici, M. Origin, Migration, and Proliferation of Human Primordial Germ Cells. In Oogenesis; Coticchio, C., Albertini, D.F., de Santis, L., Eds.; Springer-Verlag: London, UK, 2013; pp. 19–37. [Google Scholar]
- McKay, D.G.; Hertig, A.T.; Adams, E.C.; Danziger, S. Histochemical observations on the germ cells of human embryos. Anat. Rec. 1953, 117, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Chiquoine, A.D. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat. Rec. 1954, 118, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M.; Clark, J.M.; Gong, D.; Fenderson, B.A. Origin and migration of primordial germ cells in mammals. Gamete Res. 1981, 4, 333–362. [Google Scholar] [CrossRef]
- Hardisty, M.W. The numbers of vertebrate primordial germ cells. Biol. Rev. Camb. Philos. Soc. 1967, 42, 265–287. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Muller, F. Developmental Stages in Human Embryos; Washington, C.I.O., Ed.; Carnegie Institution of Washington: Washington, DC, USA, 1987. [Google Scholar]
- Ginsburg, M.; Snow, M.H.; McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar] [PubMed]
- Fujimoto, T.; Miyayama, Y.; Fuyuta, M. The origin, migration and fine morphology of human primordial germ cells. Anat. Rec. 1977, 188, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Pereda, J.; Zorn, T.; Soto-Suazo, M. Migration of human and mouse primordial germ cells and colonization of the developing ovary: An ultrastructural and cytochemical study. Microsc. Res. Tech. 2006, 69, 386–395. [Google Scholar] [CrossRef] [PubMed]
- De Felici, M. Experimental approaches to the study of primordial germ cell lineage and proliferation. Hum. Reprod. Update 2004, 10, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Fulton, N.; Cowan, G.; Coutts, S.; Saunders, P.T. Conserved and divergent patterns of expression of DAZl, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC dev. Biol. 2007, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Diedrichs, F.; Mlody, B.; Matz, P.; Fuchs, H.; Chavez, L.; Drews, K.; Adjaye, J. Comparative molecular portraits of human unfertilized oocytes and primordial germ cells at 10 weeks of gestation. Int. J. Dev. Biol. 2012, 56, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, D.H.; Quade, B.J.; Wang, T.Y.; Quigley, C.; Crum, C.P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 9585–9590. [Google Scholar] [CrossRef] [PubMed]
- Perrett, R.M.; Turnpenny, L.; Eckert, J.J.; O’Shea, M.; Sonne, S.B.; Cameron, I.T.; Wilson, D.I.; Rajpert-de Meyts, E.; Hanley, N.A. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol. Reprod. 2008, 78, 852–858. [Google Scholar] [CrossRef] [PubMed]
- De Felici, M. Primordial germ cell biology at the beginning of the xxi century. Int. J. Dev. Biol. 2009, 53, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Luebeck, E.G.; Byers, B.; Moolgavkar, S.H. On the number of founding germ cells in humans. Theor. Biol. Med. Model. 2005, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, M.N.; Gartler, S.M. The applications of genetic mosaicism to developmental problems. Annu. Rev. genet. 1971, 5, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, L.G.; Spinner, N.B. A genomic view of mosaicism and human disease. Nat. Rev. Genet. 2013, 14, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.P. Recent advances in the study of somatic mosaicism and diseases other than cancer. Curr. Opin. Genet. Dev. 2014, 26C, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lupski, J.R. Genome mosaicism—One human, multiple genomes. Science 2013, 341, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.P. Somatic gene mutation and human disease other than cancer: An update. Mutat. Res. 2010, 705, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.B.; Lee, W.R. The developmental basis for germline mosaicism in mouse and drosophila melanogaster. Genetica 1998, 102–103, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Kruskall, M.S.; Yunis, J.J.; Knoll, J.H.; Uhl, L.; Alosco, S.; Ohashi, M.; Clavijo, O.; Husain, Z.; Yunis, E.J. Disputed maternity leading to identification of tetragametic chimerism. N. Engl. J. Med. 2002, 346, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, Q.; Gao, S.; Su, Y.; Deng, Z. Congenital tetragametic blood chimerism explains a case of questionable paternity. J. Forensic Sci. 2011, 56, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Dal, G.M.; Erguner, B.; Sagiroglu, M.S.; Yuksel, B.; Onat, O.E.; Alkan, C.; Ozcelik, T. Early postzygotic mutations contribute to de novo variation in a healthy monozygotic twin pair. J. Med. Genet. 2014, 51, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.C.; Glusman, G.; Smit, A.F.; Huff, C.D.; Hubley, R.; Shannon, P.T.; Rowen, L.; Pant, K.P.; Goodman, N.; Bamshad, M.; et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 2010, 328, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, P.; Gauthier, J.; Myers, R.A.; Casals, F.; Hamdan, F.F.; Griffing, A.R.; Cote, M.; Henrion, E.; Spiegelman, D.; Tarabeux, J.; et al. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am. J. Hum. Genet. 2010, 87, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.F.; Keebler, J.E.; DePristo, M.A.; Lindsay, S.J.; Zhang, Y.; Casals, F.; Idaghdour, Y.; Hartl, C.L.; Torroja, C.; Garimella, K.V.; et al. Variation in genome-wide mutation rates within and between human families. Nat. Genet. 2011, 43, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, J.J.; Shi, Y.; Gujral, M.; Zheng, H.; Malhotra, D.; Jin, X.; Jian, M.; Liu, G.; Greer, D.; Bhandari, A.; et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 2012, 151, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, X. De novo mutations discovered in 8 mexican american families through whole genome sequencing. BMC Proc. 2014, 8, S24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Eichler, E.E. Properties and rates of germline mutations in humans. Trends genet. TIG 2013, 29, 575–584. [Google Scholar] [CrossRef]
- Silva, S.; Martins, Y.; Matias, A.; Blickstein, I. Why are monozygotic twins different? J. Perinat. Med. 2011, 39, 195–202. [Google Scholar] [PubMed]
- Baranzini, S.E.; Mudge, J.; van Velkinburgh, J.C.; Khankhanian, P.; Khrebtukova, I.; Miller, N.A.; Zhang, L.; Farmer, A.D.; Bell, C.J.; Kim, R.W.; et al. Genome, epigenome and rna sequences of monozygotic twins discordant for multiple sclerosis. Nature 2010, 464, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasap, P.; Kulawonganunchai, S.; Srichomthong, C.; Tongsima, S.; Suphapeetiporn, K.; Shotelersuk, V. Whole genome and exome sequencing of monozygotic twins with trisomy 21, discordant for a congenital heart defect and epilepsy. PLOS ONE 2014, 9, e100191. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Oka, S.; Matsui, T.; Hashimoto, A.; Arinuma, Y.; Komiya, A.; Fukui, N.; Tsuchiya, N.; Tohma, S. Genome, epigenome and transcriptome analyses of a pair of monozygotic twins discordant for systemic lupus erythematosus. Hum. Immunol. 2013, 74, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhu, S.; Hu, P.; Liu, D.; Li, Q.; Li, Z.; Zhang, X.; Xie, Y.; Chen, X. Genomic and epigenomic analyses of monozygotic twins discordant for congenital renal agenesis. Am. J. Kidney Dis. 2014, 64, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Schutte, B.C.; Richardson, R.J.; Bjork, B.C.; Knight, A.S.; Watanabe, Y.; Howard, E.; de Lima, R.L.; Daack-Hirsch, S.; Sander, A.; et al. Mutations in IRF6 cause van der woude and popliteal pterygium syndromes. Nat. Genet. 2002, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Serpa, R.; van Vliet, G.; Samuels, M.E.; Deladoey, J. Somatic mutations are not observed by exome sequencing of lymphocyte DNA from monozygotic twins discordant for congenital hypothyroidism due to thyroid dysgenesis. Horm. Res. Paediatr. 2015, 83, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.S.; Spehlmann, M.E.; Raedler, A.; Stade, B.; Thomsen, I.; Rabionet, R.; Rosenstiel, P.; Schreiber, S.; Franke, A. Whole genome and exome sequencing of monozygotic twins discordant for Crohn’s disease. BMC Genom. 2014, 15, 564. [Google Scholar] [CrossRef]
- Reumers, J.; de Rijk, P.; Zhao, H.; Liekens, A.; Smeets, D.; Cleary, J.; van Loo, P.; van Den Bossche, M.; Catthoor, K.; Sabbe, B.; et al. Optimized filtering reduces the error rate in detecting genomic variants by short-read sequencing. Nat. Biotechnol. 2012, 30, 61–68. [Google Scholar] [CrossRef]
- Weber-Lehmann, J.; Schilling, E.; Gradl, G.; Richter, D.C.; Wiehler, J.; Rolf, B. Finding the needle in the haystack: Differentiating “identical” twins in paternity testing and forensics by ultra-deep next generation sequencing. Forensic Sci. Int. Genet. 2014, 9, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Montpetit, A.; Rousseau, M.; Wu, S.Y.; Greenwood, C.M.; Spector, T.D.; Pollak, M.; Polychronakos, C.; Richards, J.B. Somatic point mutations occurring early in development: A monozygotic twin study. J. Med. Genet. 2014, 51, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, A.S. Genetics: The rate of human mutation. Nature 2012, 488, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Kondrashov, A.S. Direct estimates of human per nucleotide mutation rates at 20 loci causing mendelian diseases. Hum. Mutat. 2003, 21, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.E. Saturation of the human phenome. Curr. Genom. 2010, 11, 482–499. [Google Scholar] [CrossRef]
- Huang, A.Y.; Xu, X.; Ye, A.Y.; Wu, Q.; Yan, L.; Zhao, B.; Yang, X.; He, Y.; Wang, S.; Zhang, Z.; et al. Postzygotic single-nucleotide mosaicisms in whole-genome sequences of clinically unremarkable individuals. Cell Res. 2014, 24, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Lindhurst, M.J.; Sapp, J.C.; Teer, J.K.; Johnston, J.J.; Finn, E.M.; Peters, K.; Turner, J.; Cannons, J.L.; Bick, D.; Blakemore, L.; et al. A mosaic activating mutation in AKT1 associated with the proteus syndrome. N. Engl. J. med. 2011, 365, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.J.; Trahair, T.N.; Kirk, E.P. Mutant AKT1 in proteus syndrome. N. Engl. J. Med. 2011, 365, 2141–2142. [Google Scholar] [CrossRef] [PubMed]
- Schwindinger, W.F.; Francomano, C.A.; Levine, M.A. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory g protein of adenylyl cyclase in mccune-albright syndrome. Proc. Natl. Acad. Sci. USA 1992, 89, 5152–5156. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, L.S.; Shenker, A.; Gejman, P.V.; Merino, M.J.; Friedman, E.; Spiegel, A.M. Activating mutations of the stimulatory g protein in the mccune-albright syndrome. N. Engl. J. Med. 1991, 325, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Yoon, S.R.; Calabrese, P.; Arnheim, N. A germ-line-selective advantage rather than an increased mutation rate can explain some unexpectedly common human disease mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 10143–10148. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Yoon, S.R.; Calabrese, P.; Arnheim, N. Positive selection for new disease mutations in the human germline: Evidence from the heritable cancer syndrome multiple endocrine neoplasia type 2B. PLOS Genet. 2012, 8, e1002420. [Google Scholar] [CrossRef] [PubMed]
- Goriely, A.; McVean, G.A.; Rojmyr, M.; Ingemarsson, B.; Wilkie, A.O. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science 2003, 301, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.R.; Choi, S.K.; Eboreime, J.; Gelb, B.D.; Calabrese, P.; Arnheim, N. Age-dependent germline mosaicism of the most common noonan syndrome mutation shows the signature of germline selection. Am. J. Hum. Genet. 2013, 92, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Ducamp, S.; Schneider-Yin, X.; de Rooij, F.; Clayton, J.; Fratz, E.J.; Rudd, A.; Ostapowicz, G.; Varigos, G.; Lefebvre, T.; Deybach, J.C.; et al. Molecular and functional analysis of the C-terminal region of human erythroid-specific 5-aminolevulinic synthase associated with X-linked dominant protoporphyria (XLDPP). Hum. Mol. Genet. 2013, 22, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, A.L.; Brinkmann, A.O.; Niermeijer, M.F.; Bakker, L.; Halley, D.J.; Drop, S.L. Germ-line and somatic mosaicism in the androgen insensitivity syndrome: Implications for genetic counseling. Am. J. Hum. Genet. 1997, 60, 1003–1006. [Google Scholar] [PubMed]
- Cohn, D.H.; Starman, B.J.; Blumberg, B.; Byers, P.H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am. J. Hum. Genet. 1990, 46, 591–601. [Google Scholar] [PubMed]
- Wallis, G.A.; Starman, B.J.; Zinn, A.B.; Byers, P.H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am. J. Hum. Genet. 1990, 46, 1034–1040. [Google Scholar] [PubMed]
- Namikawa, C.; Suzumori, K.; Fukushima, Y.; Sasaki, M.; Hata, A. Recurrence of osteogenesis imperfecta because of paternal mosaicism: Gly862-->ser substitution in a type I collagen gene (COL1A1). Hum. Genet. 1995, 95, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.J.; Wenstrup, R.J.; Byers, P.H.; Cohn, D.H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a mutation in the COL1A2 gene of type I collagen. The mosaic parent exhibits phenotypic features of a mild form of the disease. Hum. Mutat. 1992, 1, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.M.; Schwartz, M.; Raghunath, M.; Steinmann, B.; Skovby, F. Gly802asp substitution in the pro alpha 2(I) collagen chain in a family with recurrent osteogenesis imperfecta due to paternal mosaicism. Eur. J. Hum. Genet. 1996, 4, 39–45. [Google Scholar] [PubMed]
- Chiang, P.W.; Lee, N.C.; Chien, N.; Hwu, W.L.; Spector, E.; Tsai, A.C. Somatic and germ-line mosaicism in rubinstein-taybi syndrome. Am. J. Med. Genet. A 2009, 149A, 1463–1467. [Google Scholar] [CrossRef]
- Vulliamy, T.J.; Knight, S.W.; Heiss, N.S.; Smith, O.P.; Poustka, A.; Dokal, I.; Mason, P.J. Dyskeratosis congenita caused by a 3' deletion: Germline and somatic mosaicism in a female carrier. Blood 1999, 94, 1254–1260. [Google Scholar] [PubMed]
- Bakker, E.; Veenema, H.; den Dunnen, J.T.; van Broeckhoven, C.; Grootscholten, P.M.; Bonten, E.J.; van Ommen, G.J.; Pearson, P.L. Germinal mosaicism increases the recurrence risk for ‘new’ duchenne muscular dystrophy mutations. J. Med. Genet. 1989, 26, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Bunyan, D.J.; Robinson, D.O.; Collins, A.L.; Cockwell, A.E.; Bullman, H.M.; Whittaker, P.A. Germline and somatic mosaicism in a female carrier of duchenne muscular dystrophy. Hum. Genet. 1994, 93, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Mitchell, M.J.; Smith, M.P.; Savidge, G.F. Germline mosaicism resulting in the transmission of severe hemophilia b from a grandfather with a mild deficiency. Am. J. Med. genet. A 2004, 129A, 13–15. [Google Scholar] [CrossRef]
- Kohler, J.; Rupilius, B.; Otto, M.; Bathke, K.; Koch, M.C. Germline mosaicism in 4q35 facioscapulohumeral muscular dystrophy (FSHD1A) occurring predominantly in oogenesis. Hum. Genet. 1996, 98, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Gitschier, J. Maternal duplication associated with gene deletion in sporadic hemophilia. Am. J. Hum. Genet. 1988, 43, 274–279. [Google Scholar] [PubMed]
- Casey, G.J.; Rodgers, S.E.; Hall, J.R.; Rudzki, Z.; Lloyd, J.V. Grandpaternal mosaicism in a family with isolated haemophilia A. Br. J. Haematol. 1999, 107, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Willers, I. Germline mosaicism complicates molecular diagnosis of lesch-nyhan syndrome. Prenat. Diagn. 2004, 24, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Froissart, R.; Maire, I.; Bonnet, V.; Levade, T.; Bozon, D. Germline and somatic mosaicism in a female carrier of hunter disease. J. Med. Genet. 1997, 34, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Vits, L.; Chitayat, D.; van Camp, G.; Holden, J.J.; Fransen, E.; Willems, P.J. Evidence for somatic and germline mosaicism in crash syndrome. Hum. Mutat. 1998, S11, S284–S287. [Google Scholar] [CrossRef]
- Bijlsma, E.K.; Wallace, A.J.; Evans, D.G. Misleading linkage results in an NF2 presymptomatic test owing to mosaicism. J. Med. Genet. 1997, 34, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Satre, V.; Monnier, N.; Berthoin, F.; Ayuso, C.; Joannard, A.; Jouk, P.S.; Lopez-Pajares, I.; Megabarne, A.; Philippe, H.J.; Plauchu, H.; et al. Characterization of a germline mosaicism in families with lowe syndrome, and identification of seven novel mutations in the OCRL1 gene. Am. J. Hum. Genet. 1999, 65, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Goji, K.; Ozaki, K.; Sadewa, A.H.; Nishio, H.; Matsuo, M. Somatic and germline mosaicism for a mutation of the phex gene can lead to genetic transmission of x-linked hypophosphatemic rickets that mimics an autosomal dominant trait. J. Clin. Endocrinol. Metab. 2006, 91, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Y.; Blumenfeld, J.; Michaeel, A.; Donahue, S.; Bobb, W.; Parker, T.; Levine, D.; Rennert, H. Autosomal dominant polycystic kidney disease caused by somatic and germline mosaicism. Clin. Genet. 2015, 87, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.A.; Poulter, M.; Campbell, T.A.; Uphill, J.B.; Adamson, G.; Geddes, J.F.; Revesz, T.; Davis, M.B.; Wood, N.W.; Collinge, J.; et al. Somatic and germline mosaicism in sporadic early-onset alzheimer’s disease. Hum. Mol. Genet. 2004, 13, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Sippel, K.C.; Fraioli, R.E.; Smith, G.D.; Schalkoff, M.E.; Sutherland, J.; Gallie, B.L.; Dryja, T.P. Frequency of somatic and germ-line mosaicism in retinoblastoma: Implications for genetic counseling. Am. J. Hum. Genet. 1998, 62, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Munier, F.L.; Thonney, F.; Girardet, A.; Balmer, A.; Claustre, M.; Pellestor, F.; Senn, A.; Pescia, G.; Schorderet, D.F. Evidence of somatic and germinal mosaicism in pseudo-low-penetrant hereditary retinoblastoma, by constitutional and single-sperm mutation analysis. Am. J. Hum. Genet. 1998, 63, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Eggermann, T.; Zerres, K.; Anhuf, D.; Kotzot, D.; Fauth, C.; Rudnik-Schoneborn, S. Somatic mosaicism for a heterozygous deletion of the survival motor neuron (SMN1) gene. Eur. J. Hum. Genet. 2005, 13, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Faivre, L.; Williamson, K.A.; Faber, V.; Laurent, N.; Grimaldi, M.; Thauvin-Robinet, C.; Durand, C.; Mugneret, F.; Gouyon, J.B.; Bron, A.; et al. Recurrence of SOX2 anophthalmia syndrome with gonosomal mosaicism in a phenotypically normal mother. Am. J. Med. Genet. A 2006, 140, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.S.; Tho, S.P.; Zhang, Y.Y.; Plouffe, L., Jr.; Hansen, K.A.; Khan, I.; McDonough, P.G. Paternal somatic and germ-line mosaicism for a sex-determining region on Y (SRY) missense mutation leading to recurrent 46,XY sex reversal. Fertil. Steril. 1997, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Dakouane Giudicelli, M.; Serazin, V.; le Sciellour, C.R.; Albert, M.; Selva, J.; Giudicelli, Y. Increased achondroplasia mutation frequency with advanced age and evidence for G1138A mosaicism in human testis biopsies. Fertil. Steril. 2008, 89, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Rey, R.A.; Venara, M.; Coutant, R.; Trabut, J.B.; Rouleau, S.; Lahlou, N.; Sultan, C.; Limal, J.M.; Picard, J.Y.; Lumbroso, S. Unexpected mosaicism of R201H-GNAS1 mutant-bearing cells in the testes underlie macro-orchidism without sexual precocity in mccune-albright syndrome. Hum. Mol. Genet. 2006, 15, 3538–3543. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Calabrese, P.; Tiemann-Boege, I.; Shinde, D.N.; Yoon, S.R.; Gelfand, D.; Bauer, K.; Arnheim, N. The molecular anatomy of spontaneous germline mutations in human testes. PLOS Biol. 2007, 5, e224. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.J.; Burgoyne, P.S. In situ analysis of fetal, prepuberal and adult xx----xy chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 1991, 112, 265–268. [Google Scholar] [PubMed]
- Warr, N.; Greenfield, A. The molecular and cellular basis of gonadal sex reversal in mice and humans. Wiley Interdiscipl. Rev. Dev. Biol. 2012, 1, 559–577. [Google Scholar] [CrossRef]
- Hulten, M.A.; Patel, S.D.; Tankimanova, M.; Westgren, M.; Papadogiannakis, N.; Jonsson, A.M.; Iwarsson, E. On the origin of trisomy 21 down syndrome. Mol. Cytogenet. 2008, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Zlotogora, J. Germ line mosaicism. Hum. Genet. 1998, 102, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.; Wolstenholme, J.; Murdoch, A.P.; Butler, T.J. Mitotic activity during preimplantation development of human embryos. J. Reprod. Fertil. 1995, 103, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, V.; Daly, A.F.; Thiry, A.; Petrossians, P.; Fina, F.; Rostomyan, L.; Silvy, M.; Enjalbert, A.; Barlier, A.; Beckers, A. Mccune-albright syndrome: A detailed pathological and genetic analysis of disease effects in an adult patient. J. Clin. Endocrinol. Metab. 2014, 99, E2029–E2038. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Huch, M.; van Boxtel, R.; Karthaus, W.; Wedge, D.C.; Tamuri, A.U.; Martincorena, I.; Petljak, M.; Alexandrov, L.B.; Gundem, G.; et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 2014, 513, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Oetting, W.S.; Greenblatt, M.S.; Brookes, A.J.; Karchin, R.; Mooney, S.D. Germline & somatic mosaicism: The 2014 annual scientific meeting of the human genome variation society. Hum. Mutat. 2015, 36, 390–393. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuels, M.E.; Friedman, J.M. Genetic Mosaics and the Germ Line Lineage. Genes 2015, 6, 216-237. https://doi.org/10.3390/genes6020216
Samuels ME, Friedman JM. Genetic Mosaics and the Germ Line Lineage. Genes. 2015; 6(2):216-237. https://doi.org/10.3390/genes6020216
Chicago/Turabian StyleSamuels, Mark E., and Jan M. Friedman. 2015. "Genetic Mosaics and the Germ Line Lineage" Genes 6, no. 2: 216-237. https://doi.org/10.3390/genes6020216
APA StyleSamuels, M. E., & Friedman, J. M. (2015). Genetic Mosaics and the Germ Line Lineage. Genes, 6(2), 216-237. https://doi.org/10.3390/genes6020216





