Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy
Abstract
1. Introduction
2. Clinical Manifestations
2.1. cALD
2.2. AMN
2.3. Adrenal Insufficiency
3. X-ALD Patients’ CNS Lesions in Postmortem Studies and MR Images
3.1. Brain Lesions in Patients with cALD or AMN
3.2. Spinal Cord Lesions
3.3. Peripheral Neuropathy
4. Imaging of CNS Lesions
5. The ABCD1 Gene and Its Expression
5.1. The X-Linked ABCD1 Gene
5.2. Individual Variability of Disease Phenotypes
5.3. ALDP, the ABCD1-Encoded Protein
5.4. ALDP Expression in Human Cells
5.5. VLCFA Metabolism and Myelin
5.6. Mutant Abcd1 Animal Models
5.6.1. Abcd1-Knockout (KO) Mouse
5.6.2. Other ABCD1-Mutant Animals Have Also Been Studied
5.6.3. Abcd2 KO and Abcd1: Abcd2 Double KO Mice
6. Main Characteristics of Neural Cell Populations in Health and VLCFA-Related Diseases
6.1. OLs
6.2. Neurons and Axons
6.3. Microglia, Macrophages, Immune Cells
6.3.1. Microglia
6.3.2. Resident Macrophages
6.3.3. Circulating Monocytes
6.4. Astrocytes
6.5. Endothelial Cells
6.6. Schwann Cells
6.7. Adrenal Cells
7. Lessons from X-ALD Patients’ Fibroblasts
8. Previous Views of X-ALD Pathogenesis
9. Revisiting X-ALD Pathophysiology
9.1. Adrenomyeloneuropathy
9.1.1. The Human Disease
9.1.2. The Mouse Disease
9.2. cALD
10. Future of Research on X-ALD Pathogenesis
10.1. Multi-Omics
10.2. Human Induced Pluripotent Stem Cells (iPSCs)
10.3. Single-Cell and Spatial Transcriptomics
10.4. Environmental Research in Mice
10.5. Non-Human Primates (NHPs)
10.6. CNS Imaging
11. Temporary Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAVs | adeno-associated vectors |
| ABC | ATP synthase (ATP)-binding cassette |
| ABCD1 | ATP-binding cassette subfamily B member-1 gene |
| ACOX1 | acyl-CoA (coenzyme A) oxidase gene |
| ACTH | adrenocorticotropic hormone |
| ALDP | ALD protein |
| AMN | adrenomyeloneuropathy |
| AAP | amyloid precursor protein |
| BBB | blood–brain barrier |
| cALD | cerebral ALD |
| C1q | complement component 1q |
| CAMs | CNS border-associated macrophages |
| CAT | catalase |
| CBA | chicken β-actin |
| CE | cholesterol esters |
| CerS2 | ceramide synthase 2 |
| CNP | 2′,3′-cyclic nucleotide 3′-phosphodiesterase |
| CNS | central nervous system |
| COVID-19 | coronavirus disease-2019 |
| CSF | cerebral spinal fluid |
| CYP4F2 | cytochrome P450 family 4 subfamily F member 2 |
| dACOX1 | Drosophila mutant acyl-CoA oxidase gene |
| DAMPs | damage-associated molecular patterns |
| Dbp | D site-binding protein |
| DRG | dorsal root ganglia |
| ELOVL1 | very-long-chain-fatty acid elongase-1 |
| ER | endoplasmic reticulum |
| FLAIR | fluid-attenuated inversion recovery MRI sequences |
| GFAP | glial fibrillary acidic protein |
| GLUT1 | glucose transporter protein type-1 |
| GPX1 | glutathione peroxidase 1 |
| HSC | hematopoietic stem cells |
| HSD17B4 | hydroxysteroid-17-β-dehydrogenase-4 |
| I2Rs | type-2 imidazoline receptors |
| IFN-γ | interferon-γ |
| IL | interleukin |
| iPSCs | induced pluripotent stem cells |
| KO | knockout |
| LD | lipid droplets |
| MAO-B | monoamine oxidase-B |
| MCT1 | monocarboxylate transporter-1 |
| MFGE8 | milk fat globule-epidermal growth factor 8 |
| MFP2 | multifunctional protein-2 |
| MRI | magnetic resonance imaging |
| NAMPs | neurodegeneration-associated molecular patterns |
| NEX | neuronal helix–loop–helix protein |
| OL | oligodendrocyte |
| OPC | oligodendrocyte progenitor cell |
| PAMPs | pathogen-associated molecular patterns |
| PET | positron emission tomography |
| Pex5 | peroxin-5 |
| Pex19p | peroxisomal biogenesis factor-19 |
| pmp-4 | peroxisomal membrane protein-4 gene |
| PMP70 | 70 kDa peroxisomal membrane protein |
| PNS | peripheral nervous system |
| R/GCP | red/green color vision gene |
| S1P | sphingosine-1-P |
| SOD2 | superoxide Dismutase 2 |
| TGF-β | transforming growth factor-β |
| TNF | tumor necrosis factor |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| TSPO | translocator protein |
| VLCFA | very-long-chain fatty acids |
| WAM | white matter-restricted microglia |
| X-ALD | X-linked adrenoleukodystrophy |
References
- Gaviglio, A.; McKasson, S.; Singh, S.; Ojodu, J. Infants with Congenital Diseases Identified through Newborn Screening-United States, 2018–2020. Int. J. Neonatal Screen. 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.B.; Jones, R.O.; Hubbard, W.C.; Tortorelli, S.; Orsini, J.J.; Caggana, M.; Vogel, B.H.; Raymond, G.V. Newborn screening for X-linked adrenoleukodystrophy. Int. J. Neonatal Screen. 2016, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, N.; Takashima, S.; Kawai, H.; Kubota, K.; Sasai, H.; Orii, K.; Ogawa, M.; Ohnishi, H. Advanced diagnostic system and introduction of newborn screening of adrenoleukodystrophy and peroxisomal disorders in Japan. Int. J. Neonatal Screen. 2021, 7, 58. [Google Scholar] [CrossRef]
- Moser, A.B.; Seeger, E.; Raymond, G.V. Newborn Screening for X-Linked Adrenoleukodystrophy: Past, Present, and Future. Int. J. Neonatal Screen. 2022, 8, 16. [Google Scholar] [CrossRef]
- Matteson, J.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Olney, R.S.; Tang, H. Adrenoleukodystrophy newborn screening in California since 2016: Programmatic outcomes and follow-up. Int. J. Neonatal Screen. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.L.; Li, H.; Hagar, A.F.; Jerris, S.C.; Wittenauer, A.; Wilcox, W. Newborn screening for X-linked Adrenoleukodystrophy in Georgia: Experiences from a pilot study screening of 51,081 newborns. Int. J. Neonatal Screen. 2020, 6, 81. [Google Scholar] [CrossRef]
- Priestley, J.R.C.; Adang, L.A.; Drewes Williams, S.; Lichter-Konecki, U.; Menello, C.; Engelhardt, N.M.; DiPerna, J.C.; DiBoscio, B.; Ahrens-Nicklas, R.C.; Edmondson, A.C.; et al. Newborn Screening for X-Linked Adrenoleukodystrophy: Review of Data and Outcomes in Pennsylvania. Int. J. Neonatal Screen. 2022, 8, 24. [Google Scholar] [CrossRef]
- Baker, C.V.; Cady Keller, A.; Lutz, R.; Eveans, K.; Baumert, K.; DiPerna, J.C.; Rizzo, W.B. Newborn Screening for X-Linked Adrenoleukodystrophy in Nebraska: Initial Experiences and Challenges. Int. J. Neonatal Screen. 2022, 8, 29. [Google Scholar] [CrossRef]
- Schwan, K.; Youngblom, J.; Weisiger, K.; Kianmahd, J.; Waggoner, R.; Fanos, J. Family Perspectives on Newborn Screening for X-Linked Adrenoleukodystrophy in California. Int. J. Neonatal Screen. 2019, 5, 42. [Google Scholar] [CrossRef]
- Burton, B.K.; Hickey, R.; Hitchins, L.; Shively, V.; Ehrhardt, J.; Ashbaugh, L.; Peng, Y.; Basheeruddin, K. Newborn screening for X-linked adrenoleukodystrophy: The initial Illinois experience. Int. J. Neonatal Screen. 2022, 8, 6. [Google Scholar] [CrossRef]
- Blom, M.; Bredius, R.G.M.; Weijman, G.; Dekkers, E.; Kemper, E.A.; van den Akker-van Marle, M.E.; van der Ploeg, C.P.B.; van der Burg, M.; Schielen, P. Introducing Newborn Screening for Severe Combined Immunodeficiency (SCID) in the Dutch Neonatal Screening Program. Int. J. Neonatal Screen. 2018, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Albersen, M.; van der Beek, S.L.; Dijkstra, I.M.E.; Alders, M.; Barendsen, R.W.; Bliek, J.; Boelen, A.; Ebberink, M.S.; Ferdinandusse, S.; Goorden, S.M.I.; et al. Sex-specific newborn screening for X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 2023, 46, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Barendsen, R.W.; Dijkstra, I.M.; Visser, W.F.; Alders, M.; Bliek, J.; Boelen, A.; Bouva, M.J.; van der Crabben, S.N.; Elsinghorst, E.; van Gorp, A.G. Adrenoleukodystrophy newborn screening in the Netherlands (SCAN Study): The X-factor. Front. Cell Dev. Biol. 2020, 8, 499. [Google Scholar] [CrossRef]
- Bonaventura, E.; Alberti, L.; Lucchi, S.; Cappelletti, L.; Fazzone, S.; Cattaneo, E.; Bellini, M.; Izzo, G.; Parazzini, C.; Bosetti, A. Newborn screening for X-linked adrenoleukodystrophy in Italy: Diagnostic algorithm and disease monitoring. Front. Neurol. 2023, 13, 1072256. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, C.; Eichler, F.S.; Berger, J. The genetic landscape of X-linked adrenoleukodystrophy: Inheritance, mutations, modifier genes, and diagnosis. Appl. Clin. Genet. 2015, 8, 109–121. [Google Scholar]
- Schaumburg, H.H.; Powers, J.M.; Raine, C.S.; Spencer, P.S.; Griffin, J.W.; Prineas, J.W.; Boehme, D.M. Adrenomyeloneuropathy: A probable variant of adrenoleukodystrophy. II. General pathologic, neuropathologic, and biochemical aspects. Neurology 1977, 27, 1114–1119. [Google Scholar] [CrossRef]
- Schaumburg, H.H.; Powers, J.M.; Raine, C.S.; Suzuki, K.; Richardson, E.P. Adrenoleukodystrophy: A clinical and pathological study of 17 cases. Arch. Neurol. 1975, 32, 577–591. [Google Scholar] [CrossRef]
- Schaumburg, H.H.; Powers, J.M.; Spencer, P.S.; Raine, C.S.; Prineas, J.W.; Boehme, D.M. The myeloneuropathy variant of adrenoleukodystrophy. J. Neuropathol. Exp. Neurol. 1976, 35, 312. [Google Scholar] [CrossRef]
- Powell, H.; Tindall, R.; Schultz, P.; Paa, D.; O’Brien, J.; Lampert, P. Adrenoleukodystrophy: Electron microscopic findings. Arch. Neurol. 1975, 32, 250–260. [Google Scholar] [CrossRef]
- Budka, H.; Sluga, E.; Heiss, W.D. Spastic paraplegia associated with Addison’s disease: Adult variant of adreno-leukodystrophy. J. Neurol. 1976, 213, 237–250. [Google Scholar] [CrossRef]
- Gumbinas, M.; Liu, H.M.; Dawson, G.; Larsen, M.; Green, O. Progressive spastic paraparesis and adrenal insufficiency. Arch. Neurol. 1976, 33, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Schaumburg, H.; Powers, J.; Kishimoto, Y.; Koilodny, E.; Suzuki, K. Fatty acid abnormality in adrenoleukodystrophy. J. Neurochem. 1976, 26, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Mosser, J.; Douar, A.M.; Sarde, C.O.; Kioschis, P.; Feil, R.; Moser, H.; Poustka, A.M.; Mandel, J.L.; Aubourg, P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993, 361, 726–730. [Google Scholar] [CrossRef]
- Aubourg, P.; Blanche, S.; Jambaqué, I.; Rocchiccioli, F.; Kalifa, G.; Naud-Saudreau, C.; Rolland, M.-O.; Debré, M.; Chaussain, J.-L.; Griscelli, C.; et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N. Engl. J. Med. 1990, 322, 1860–1866. [Google Scholar] [CrossRef]
- Raymond, G.V.; Aubourg, P.; Paker, A.; Escolar, M.; Fischer, A.; Blanche, S.; Baruchel, A.; Dalle, J.-H.; Michel, G.; Prasad, V. Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 538–548. [Google Scholar] [CrossRef]
- Forss-Petter, S.; Werner, H.; Berger, J.; Lassmann, H.; Molzer, B.; Schwab, M.H.; Bernheimer, H.; Zimmermann, F.; Nave, K.A. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 1997, 50, 829–843. [Google Scholar] [CrossRef]
- Lu, J.-F.; Lawler, A.M.; Watkins, P.A.; Powers, J.M.; Moser, A.B.; Moser, H.W.; Smith, K.D. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 1997, 94, 9366–9371. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shinnoh, N.; Kondo, A.; Yamada, T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 1997, 232, 631–636. [Google Scholar] [CrossRef]
- Pujol, A.; Hindelang, C.; Callizot, N.; Bartsch, U.; Schachner, M.; Mandel, J.L. Late onset neurological phenotype of the X-ALD gene inactivation in mice: A mouse model for adrenomyeloneuropathy. Hum. Mol. Genet. 2002, 11, 499–505. [Google Scholar] [CrossRef]
- Powers, J.M. Adreno-leukodystrophy (adreno-testiculo-leukomyelo-neuropathic-complex). Clin. Neuropathol. 1985, 4, 181–199. [Google Scholar]
- Engelen, M.; Kemp, S.; De Visser, M.; van Geel, B.M.; Wanders, R.J.; Aubourg, P.; Poll-The, B.T. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J. Rare Dis. 2012, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Bezman, L.; Moser, A.B.; Raymond, G.V.; Rinaldo, P.; Watkins, P.A.; Smith, K.D.; Kass, N.E.; Moser, H.W. Adrenoleukodystrophy: Incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 2001, 49, 512–517. [Google Scholar] [CrossRef]
- Koto, Y.; Sakai, N.; Lee, Y.; Kakee, N.; Matsuda, J.; Tsuboi, K.; Shimozawa, N.; Okuyama, T.; Nakamura, K.; Narita, A. Prevalence of patients with lysosomal storage disorders and peroxisomal disorders: A nationwide survey in Japan. Mol. Genet. Metab. 2021, 133, 277–288. [Google Scholar] [CrossRef]
- van Geel, B.r.M.; Assies, J.; Weverling, G.J.; Barth, P.G. Predominance of the adrenomyeloneuropathy phenotype of X-linked adrenoleukody strophy in the Netherlands: A survey of 30 kindreds. Neurology 1994, 44, 2343. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, W.; Cohen-Cole, S.A.; Mickel, S.F. Adrenoleukodystrophy: Frequency of presentation as a psychiatric disorder. Biol. Psychiatry 1987, 22, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Güler, A.S.; Fis, N.P.; Berkem, M. X-Linked adrenoleukodystrophy in a 7-year-old boy presenting with psychiatric symptoms. Eur. Child. Adolesc. Psychiatry 2011, 20, 275–276. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B.; Frayer, K.K.; Chen, W.; Schulman, J.D.; O’Neill, B.P.; Kishimoto, Y. Adrenoleukodystrophy: Increased plasma content of saturated very long chain fatty acids. Neurology 1998, 51, 334–334-a. [Google Scholar] [CrossRef]
- Aubourg, P.; Bougnères, P.F.; Rocchiccioli, F. Capillary gas-liquid chromatographic-mass spectrometric measurement of very long chain (C22 to C26) fatty acids in microliter samples of plasma. J. Lipid Res. 1985, 26, 263–267. [Google Scholar] [CrossRef]
- Edwin, D.; Speedie, L.; Naidu, S.; Moser, H. Cognitive impairment in adult-onset adrenoleukodystrophy. Mol. Chem. Neuropathol. 1990, 12, 167–176. [Google Scholar] [CrossRef]
- Rosebush, P.I.; Garside, S.; Levinson, A.J.; Mazurek, M.F. The neuropsychiatry of adult-onset adrenoleukodystrophy. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 315–327. [Google Scholar] [CrossRef]
- Mehta, A.M.; Prabhu, M.; Krishnan, G. Adult-onset adrenoleukodystrophy presenting with status epilepticus and psychosis. BMJ Case Rep. 2021, 14, e244757. [Google Scholar] [CrossRef]
- Levinson, A.J.; Mazurek, M.F. Late-onset adrenoleukodystrophy associated with long-standing psychiatric symptoms. J. Clin. Psychiatry 1999, 60, 460–468. [Google Scholar]
- Bouquet, F.; Dehais, C.; Sanson, M.; Lubetzki, C.; Louapre, C. Dramatic worsening of adult-onset X-linked adrenoleukodystrophy after head trauma. Neurology 2015, 85, 1991–1993. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Liedtke, W.; Petersen, D.; Opitz, H.; Poremba, M. Very-late-onset adrenoleukodystrophy: Possible precipitation of demyelination by cerebral contusion. Neurology 1992, 42, 367. [Google Scholar] [CrossRef]
- Raymond, G.V.; Seidman, R.; Monteith, T.S.; Kolodny, E.; Sathe, S.; Mahmood, A.; Powers, J.M. Head trauma can initiate the onset of adreno-leukodystrophy. J. Neurol. Sci. 2010, 290, 70–74. [Google Scholar] [CrossRef]
- Budhram, A.; Pandey, S.K. Activation of cerebral X-linked adrenoleukodystrophy after head trauma. Can. J. Neurol. Sci. 2017, 44, 597–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moser, H.; Smith, K.; Watkins, P.; Powers, J.; Moser, A. X-linked adrenoleukodystrophy. In The Metabolic and Molecular Bases of Inherited Disease; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Griffin, J.W.; Goren, E.; Schaumburg, H.; Engel, W.K.; Loriaux, L. Adrenomyeloneuropathy: A probable variant of adrenoleukodystrophy. I. Clinical and endocrinologic aspects. Neurology 1977, 27, 1107–1113. [Google Scholar] [CrossRef]
- Powers, J.M.; DeCiero, D.P.; Ito, M.; Moser, A.B.; Moser, H.W. Adrenomyeloneuropathy: A neuropathologic review featuring its noninflammatory myelopathy. J. Neuropathol. Exp. Neurol. 2000, 59, 89–102. [Google Scholar] [CrossRef]
- Corre, C.S.; Grant, N.; Sadjadi, R.; Hayden, D.; Becker, C.; Gomery, P.; Eichler, F.S. Beyond gait and balance: Urinary and bowel dysfunction in X-linked adrenoleukodystrophy. Orphanet J. Rare Dis. 2021, 16, 14. [Google Scholar] [CrossRef]
- Hofereiter, J.; Smith, M.D.; Seth, J.; Tudor, K.I.; Fox, Z.; Emmanuel, A.; Murphy, E.; Lachmann, R.H.; Panicker, J. Bladder and bowel dysfunction is common in both men and women with mutation of the ABCD1 gene for X-linked adrenoleukodystrophy. JIMD Rep. 2015, 22, 77–83. [Google Scholar]
- Silveri, M.; De Gennaro, M.; Gatti, C.; Bizzarri, C.; Mosiello, G.; Cappa, M. Voiding dysfunction in X-linked adrenoleukodystrophy: Symptom score and urodynamic findings. J. Urol. 2004, 171, 2651–2653. [Google Scholar] [CrossRef]
- Kararizou, E.; Karandreas, N.; Davaki, P.; Davou, R.; Vassilopoulos, D. Polyneuropathies in teenagers: A clinicopathological study of 45 cases. Neuromuscul. Disord. 2006, 16, 304–307. [Google Scholar] [CrossRef]
- Engelen, M.; van der Kooi, A.J.; Kemp, S.; Wanders, R.J.; Sistermans, E.A.; Waterham, H.R.; Koelman, J.T.; van Geel, B.M.; de Visser, M. X-linked adrenomyeloneuropathy due to a novel missense mutation in the ABCD1 start codon presenting as demyelinating neuropathy. J. Peripher. Nerv. Syst. 2011, 16, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, B.M.; Bezman, L.; Loes, D.J.; Moser, H.W.; Raymond, G.V. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2001, 49, 186–194. [Google Scholar] [CrossRef]
- Aubourg, P.; MandelL, J.-L. X-linked adrenoleukodystrophy. Ann. N. Y. Acad. Sci. 1996, 804, 461–476. [Google Scholar] [CrossRef] [PubMed]
- van Geel, B.M.; Koelman, J.H.; Barth, P.G.; Ongerboer de Visser, B.W. Peripheral nerve abnormalities in adrenomyeloneuropathy: A clinical and electrodiagnostic study. Neurology 1996, 46, 112–118. [Google Scholar] [CrossRef]
- Curiel, J.; Steinberg, S.J.; Bright, S.; Snowden, A.; Moser, A.B.; Eichler, F.; Dubbs, H.A.; Hacia, J.G.; Ely, J.J.; Bezner, J. X-linked adrenoleukodystrophy in a chimpanzee due to an ABCD1 mutation reported in multiple unrelated humans. Mol. Genet. Metab. 2017, 122, 130–133. [Google Scholar] [CrossRef]
- O’Neill, B.P.; Moser, H.W.; Saxena, K.M.; Marmion, L.C. Adrenoleukodystrophy: Clinical and biochemical manifestations in carriers. Neurology 1984, 34, 798–801. [Google Scholar] [CrossRef]
- Huffnagel, I.C.; Dijkgraaf, M.G.; Janssens, G.E.; van Weeghel, M.; van Geel, B.M.; Poll-The, B.T.; Kemp, S.; Engelen, M. Disease progression in women with X-linked adrenoleukodystrophy is slow. Orphanet J. Rare Dis. 2019, 14, 30. [Google Scholar] [CrossRef]
- Naidu, S.; Washington, C.; Thirumalai, S.; Smith, K.; Moser, H.; Watkins, P. X-Chromosome Inactivation in Symptomatic Heterozygotes of X-Linked Adrenoleukodystrophy; Lippincott-Raven Publ: Philadelphia, PA, USA, 1997; Volume 42, p. 33. [Google Scholar]
- Maier, E.M.; Kammerer, S.; Muntau, A.C.; Wichers, M.; Braun, A.; Roscher, A.A. Symptoms in carriers of adrenoleukodystrophy relate to skewed X inactivation. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2002, 52, 683–688. [Google Scholar] [CrossRef]
- Salsano, E.; Tabano, S.; Sirchia, S.M.; Colapietro, P.; Castellotti, B.; Gellera, C.; Rimoldi, M.; Pensato, V.; Mariotti, C.; Pareyson, D. Preferential expression of mutant ABCD1 allele is common in adrenoleukodystrophy female carriers but unrelated to clinical symptoms. Orphanet J. Rare Dis. 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, S.S.; Naidu, S.; Blevins, L.S.; Ladenson, P.W. Assessment of adrenal function in women heterozygous for adrenoleukodystrophy. J. Clin. Endocrinol. Metab. 1997, 82, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Fatemi, A.; Huang, H.; Nagae-Poetscher, L.; Wakana, S.; Barker, P.B.; Van Zijl, P.; Moser, H.W.; Mori, S.; Raymond, G.V. Diffusion tensor–based imaging reveals occult abnormalities in adrenomyeloneuropathy. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 58, 758–766. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B.; Naidu, S.; Bergin, A. Clinical aspects of adrenoleukodystrophy and adrenomyeloneuropathy. Dev. Neurosci. 1991, 13, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Huffnagel, I.C.; Linthorst, G.E.; Wanders, R.J.; Engelen, M. Adrenoleukodystrophy–neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 2016, 12, 606–615. [Google Scholar] [CrossRef]
- Laureti, S.; Casucci, G.; Santeusanio, F.; Angeletti, G.; Aubourg, P.; Brunetti, P. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison’s disease in young adult male patients. J. Clin. Endocrinol. Metab. 1996, 81, 470–474. [Google Scholar]
- Hsieh, S.; White, P.C. Presentation of primary adrenal insufficiency in childhood. J. Clin. Endocrinol. Metab. 2011, 96, E925–E928. [Google Scholar] [CrossRef]
- Danner, B.; Gonzalez, A.D.; Corbett, W.C.; Alhneif, M.; Etemadmoghadam, S.; Parker-Garza, J.; Flanagan, M.E. Brain banking in the United States and Europe: Importance, challenges, and future trends. J. Neuropathol. Exp. Neurol. 2024, 83, 219–229. [Google Scholar] [CrossRef]
- Vanderdonckt, P.; Aloisi, F.; Comi, G.; de Bruyn, A.; Hartung, H.P.; Huitinga, I.; Kuhlmann, T.; Lucchinetti, C.F.; Metz, I.; Reynolds, R.; et al. Tissue donations for multiple sclerosis research: Current state and suggestions for improvement. Brain Commun. 2022, 4, fcac094. [Google Scholar] [CrossRef]
- Powers, J.M.; Liu, Y.; Moser, A.B.; Moser, H.W. The inflammatory myelinopathy of adreno-leukodystrophy: Cells, effector molecules, and pathogenetic implications. J. Neuropathol. Exp. Neurol. 1992, 51, 630–643. [Google Scholar] [CrossRef]
- Reinecke, C.; Knoll, D.; Pretorius, P.; Steyn, H.; Simpson, R. The correlation between biochemical and histopathological findings in adrenoleukodystrophy. J. Neurol. Sci. 1985, 70, 21–38. [Google Scholar] [CrossRef]
- Schaumburg, H.H.; Powers, J.M.; Suzuki, K.; Raine, C.S. Adreno-leukodystrophy (sex-linked Schilder disease). Ultrastructural demonstration of specific cytoplasmic inclusions in the central nervous system. Arch. Neurol. 1974, 31, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.B.; Schaumburg, H.H.; Powers, J.M. Histochemical characteristics of the striated inclusions of adrenoleukodystrophy. J. Histochem. Cytochem. 1976, 24, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Schaumburg, H. The adrenal cortex in adreno leukodystrophy. Arch. Pathol. Lab. Med. 1973, 96, 305–310. [Google Scholar]
- Powers, J.M. Adreno-leukodystrophy: A personal historical note. Acta Neuropathol. 2005, 109, 124–127. [Google Scholar] [CrossRef]
- Menkes, J.H.; Corbo, L.M. Adrenoleukodystrophy: Accumulation of cholesterol esters with very long chain fatty acids. Neurology 1977, 27, 928. [Google Scholar] [CrossRef]
- Gong, Y.; Laheji, F.; Berenson, A.; Li, Y.; Moser, A.; Qian, A.; Frosch, M.; Sadjadi, R.; Hahn, R.; Maguire, C.A.; et al. Role of Basal Forebrain Neurons in Adrenomyeloneuropathy in Mice and Humans. Ann. Neurol. 2024, 95, 442–458. [Google Scholar] [CrossRef]
- Schlüter, A.; Espinosa, L.; Fourcade, S.; Galino, J.; López, E.; Ilieva, E.; Morató, L.; Asheuer, M.; Cook, T.; McLaren, A.; et al. Functional genomic analysis unravels a metabolic-inflammatory interplay in adrenoleukodystrophy. Hum. Mol. Genet. 2012, 21, 1062–1077. [Google Scholar] [CrossRef]
- Budka, H. A historical look using virtual microscopy: The first case report of adrenomyeloneuropathy (AMN). Free Neuropathol. 2023, 4, 18. [Google Scholar]
- Probst, A.; Ulrich, J.; Heitz, P.U.; Herschkowitz, N. Adrenomyeloneuropathy. A protracted, pseudosystematic variant of adrenoleukodystrophy. Acta Neuropathol. 1980, 49, 105–115. [Google Scholar] [CrossRef]
- Satoh, S.; Monma, N.; Satoh, T.; Satodate, R.; Saiki, K. Adrenoleukodystrophy: Report of an Autopsy Case with Adrenoleukomyeloneuropathy. Acta Patholigica Jpn. 1986, 36, 1055–1066. [Google Scholar] [CrossRef]
- Martin, J.J.; Dompas, B.; Ceuterick, C.; Jacobs, K. Adrenomyeloneuropathy and adrenoleukodystrophy in two brothers. Eur. Neurol. 1980, 19, 281–287. [Google Scholar] [CrossRef]
- Martin, J.J.; Lowenthal, A.; Ceuterick, C.; Gacoms, H. Adrenomyeloneuropathy. A report on two families. J. Neurol. 1982, 226, 221–232. [Google Scholar] [CrossRef]
- Julien, J.J.; Vallat, J.M.; Vital, C.; Lagueny, A.; Ferrer, X.; Darriet, D. Adrenomyeloneuropathy: Demonstration of inclusions at the level of the peripheral nerve. Eur. Neurol. 1981, 20, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Koyama, A.; Koike, R.; Ohno, T.; Atsumi, T.; Miyatake, T. Adrenomyeloneuropathy: Report of a family and electron microscopical findings in peripheral nerve. J. Neurol. 1985, 232, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P. Pathologic alterations of nerves. Peripher. Neuropathy 1993, 1, 514–595. [Google Scholar]
- Powers, J.M.; DeCiero, D.P.; Cox, C.; Richfield, E.K.; Ito, M.; Moser, A.B.; Moser, H.W. The dorsal root ganglia in adrenomyeloneuropathy: Neuronal atrophy and abnormal mitochondria. J. Neuropathol. Exp. Neurol. 2001, 60, 493–501. [Google Scholar] [CrossRef]
- Mallack, E.J.; Turk, B.R.; Yan, H.; Price, C.; Demetres, M.; Moser, A.B.; Becker, C.; Hollandsworth, K.; Adang, L.; Vanderver, A. MRI surveillance of boys with X-linked adrenoleukodystrophy identified by newborn screening: Meta-analysis and consensus guidelines. J. Inherit. Metab. Dis. 2021, 44, 728–739. [Google Scholar] [CrossRef]
- Liberato, A.P.; Mallack, E.J.; Aziz-Bose, R.; Hayden, D.; Lauer, A.; Caruso, P.A.; Musolino, P.L.; Eichler, F.S. MRI brain lesions in asymptomatic boys with X-linked adrenoleukodystrophy. Neurology 2019, 92, e1698–e1708. [Google Scholar] [CrossRef]
- Kemp, S.; Pujol, A.; Waterham, H.R.; van Geel, B.M.; Boehm, C.D.; Raymond, G.V.; Cutting, G.R.; Wanders, R.J.; Moser, H.W. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: Role in diagnosis and clinical correlations. Hum. Mutat. 2001, 18, 499–515. [Google Scholar] [CrossRef]
- Pasco, A.; Kalifa, G.; Sarrazin, J.; Adamsbaum, C.; Aubourg, P. Contribution of MRI to the diagnosis of cerebral lesions of adrenoleukodystrophy. Pediatr. Radiol. 1991, 21, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Loes, D.; Fatemi, A.; Melhem, E.; Gupte, N.; Bezman, L.; Moser, H.; Raymond, G. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology 2003, 61, 369–374. [Google Scholar] [CrossRef]
- Van der Knaap, M.S.; Valk, J. Magnetic Resonance of Myelination and Myelin Disorders, 3rd ed.; Springer, Ed.; Springer Science & Business Media: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Korenke, G.C.; Pouwels, P.J.; Frahm, J.; Hunneman, D.H.; Stoeckler, S.; Krasemann, E.; Jost, W.; Hanefeld, F. Arrested cerebral adrenoleukodystrophy: A clinical and proton magnetic resonance spectroscopy study in three patients. Pediatr. Neurol. 1996, 15, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Golay, X.; Fatemi, A.; Mahmood, A.; Raymond, G.V.; Moser, H.W.; Van Zijl, P.C.; Stanisz, G.J. Quantitative magnetization transfer characteristics of the human cervical spinal cord in vivo: Application to adrenomyeloneuropathy. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 61, 22–27. [Google Scholar] [CrossRef]
- Fatemi, A.; Smith, S.A.; Dubey, P.; Zackowski, K.M.; Bastian, A.J.; van Zijl, P.C.; Moser, H.W.; Raymond, G.V.; Golay, X. Magnetization transfer MRI demonstrates spinal cord abnormalities in adrenomyeloneuropathy. Neurology 2005, 64, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Sarde, C.-O.; Douar, A.-M.; Mosser, J.; Mandel, J.-L.; Aubourg, P. Abnormal messenger RNA expression and a missense mutation in patients with X-linked adrenoleukodystrophy. Hum. Mol. Genet. 1993, 2, 1949–1951. [Google Scholar] [CrossRef]
- Mallack, E.J.; Gao, K.; Engelen, M.; Kemp, S. Structure and function of the ABCD1 variant database: 20 years, 940 pathogenic variants, and 3400 cases of adrenoleukodystrophy. Cells 2022, 11, 283. [Google Scholar] [CrossRef]
- Wang, Y.; Busin, R.; Reeves, C.; Bezman, L.; Raymond, G.; Toomer, C.J.; Watkins, P.A.; Snowden, A.; Moser, A.; Naidu, S. X-linked adrenoleukodystrophy: ABCD1 de novo mutations and mosaicism. Mol. Genet. Metab. 2011, 104, 160–166. [Google Scholar] [CrossRef]
- Smith, K.D.; Kemp, S.; Braiterman, L.T.; Lu, J.-F.; Wei, H.-M.; Geraghty, M.; Stetten, G.; Bergin, J.S.; Pevsner, J.; Watkins, P.A. X-linked adrenoleukodystrophy: Genes, mutations, and phenotypes. Neurochem. Res. 1999, 24, 521–535. [Google Scholar] [CrossRef]
- Berger, J.; Molzer, B.; Fae, I.; Bernheimer, H. X-linked adrenoleukodystrophy (ALD): A novel mutation of the ALD gene in 6 members of a family presenting with 5 different phenotypes. Biochem. Biophys. Res. Commun. 1994, 205, 1638–1643. [Google Scholar] [CrossRef]
- Kemp, S.; Ligtenberg, M.J.; Vangeel, B.M.; Barth, P.G.; Wolterman, R.A.; Schoute, F.; Sarde, C.-O.; Mandel, J.-L.; Vanoost, B.A.; Bolhuis, P.A. Identification of a two base pair deletion in five unrelated families with adrenoleukodystrophy: A possible hot spot for mutations. Biochem. Biophys. Res. Commun. 1994, 202, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.P.; Lemos, M.; Sá-Miranda, C.; Azevedo, J.E. Molecular characterization of 21 X-ALD Portuguese families: Identification of eight novel mutations in the ABCD1 gene. Mol. Genet. Metab. 2002, 76, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Jardim, L.B.; da Silva, A.C.F.; Blank, D.; Villanueva, M.M.; Renck, L.; Costa, M.L.B.; Vargas, C.R.; Deon, M.; Coelho, D.l.M.; Vedolin, L. X-linked adrenoleukodystrophy: Clinical course and minimal incidence in South Brazil. Brain Dev. 2010, 32, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Korenke, G.C.; Fuchs, S.; Krasemann, E.; Doerr, H.G.; Wilichowski, E.; Hunneman, D.H.; Hanefeld, F. Cerebral adrenoleukodystrophy (ALD) in only one of monozygotic twins with an identical ALD genotype. Ann. Neurol. 1996, 40, 254–257. [Google Scholar] [CrossRef]
- Sobue, G.; Ueno-Natsukari, I.; Okamoto, H.; Connell, T.A.; Aizawa, I.; Mizoguchi, K.; Honma, M.; Ishikawa, G.; Mitsuma, T.; Natsukari, N. Phenotypic heterogeneity of an adult form of adrenoleukodystrophy in monozygotic twins. Ann. Neurol. 1994, 36, 912–915. [Google Scholar] [CrossRef]
- Di Rocco, M.; Doria-Lamba, L.; Caruso, U. Monozygotic twins with X-linked adrenoleukodystrophy and different phenotypes. Ann. Neurol. 2001, 50, 424. [Google Scholar] [CrossRef]
- Su, Q.; Chen, Y.; Fu, C.; Zhang, Y.; Zhang, Y.; Cao, Y.; Wang, X.; Zeng, Z.; Liu, C.; Yang, Z. Transcriptomic Analysis of Identical Twins with Different Onset Ages of Adrenoleukodystrophy. BMC Med. Genom. 2024. [Google Scholar] [CrossRef]
- Moser, H.; Moser, A.; Smith, K.; Bergin, A.; Borel, J.; Shankroff, J.; Stine, O.; Merette, C.; Ott, J.; Krivit, W. Adrenoleukodystrophy: Phenotypic variability and implications for therapy. J. Iherited Metab. Dis. 1992, 15, 645–664. [Google Scholar] [CrossRef]
- Richmond, P.A.; van der Kloet, F.; Vaz, F.M.; Lin, D.; Uzozie, A.; Graham, E.; Kobor, M.; Mostafavi, S.; Moerland, P.D.; Lange, P.F. Multi-omic approach to identify phenotypic modifiers underlying cerebral demyelination in X-linked adrenoleukodystrophy. Front. Cell Dev. Biol. 2020, 8, 520. [Google Scholar] [CrossRef]
- van Engen, C.E.; Ofman, R.; Dijkstra, I.M.; van Goethem, T.J.; Verheij, E.; Varin, J.; Vidaud, M.; Wanders, R.J.; Aubourg, P.; Kemp, S. CYP4F2 affects phenotypic outcome in adrenoleukodystrophy by modulating the clearance of very long-chain fatty acids. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1861–1870. [Google Scholar] [CrossRef]
- Maier, E.M.; Mayerhofer, P.U.; Asheuer, M.; Köhler, W.; Rothe, M.; Muntau, A.C.; Roscher, A.A.; Holzinger, A.; Aubourg, P.; Berger, J. X-linked adrenoleukodystrophy phenotype is independent of ABCD2 genotype. Biochem. Biophys. Res. Commun. 2008, 377, 176–180. [Google Scholar] [CrossRef]
- Matsukawa, T.; Asheuer, M.; Takahashi, Y.; Goto, J.; Suzuki, Y.; Shimozawa, N.; Takano, H.; Onodera, O.; Nishizawa, M.; Aubourg, P. Identification of novel SNPs of ABCD1, ABCD2, ABCD3, and ABCD4 genes in patients with X-linked adrenoleukodystrophy (ALD) based on comprehensive resequencing and association studies with ALD phenotypes. Neurogenetics 2011, 12, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Palakuzhiyil, S.V.; Christopher, R.; Chandra, S.R. Deciphering the modifiers for phenotypic variability of X-linked adrenoleukodystrophy. World J. Bological Chem. 2020, 11, 99. [Google Scholar] [CrossRef]
- Semmler, A.; Bao, X.; Cao, G.; Köhler, W.; Weller, M.; Aubourg, P.; Linnebank, M. Genetic variants of methionine metabolism and X-ALD phenotype generation: Results of a new study sample. J. Neurol. 2009, 256, 1277–1280. [Google Scholar] [CrossRef]
- Barbier, M.; Sabbagh, A.; Kasper, E.; Asheuer, M.; Ahouansou, O.; Pribill, I.; Forss-Petter, S.; Vidaud, M.; Berger, J.; Aubourg, P. CD1 gene polymorphisms and phenotypic variability in X-linked adrenoleukodystrophy. PLoS ONE 2012, 7, e29872. [Google Scholar] [CrossRef]
- Zierfuss, B.; Weinhofer, I.; Buda, A.; Popitsch, N.; Hess, L.; Moos, V.; Hametner, S.; Kemp, S.; Köhler, W.; Forss-Petter, S. Targeting foam cell formation in inflammatory brain diseases by the histone modifier MS-275. Ann. Clin. Transl. Neurol. 2020, 7, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Marques, M.; Ramos, B.; Kagan, J.C.; Ribeiro, D. Emerging roles of peroxisomes in viral infections. Trends Cell Biol. 2022, 32, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Surana, P.; Tang, S.; McDougall, M.; Tong, C.Y.W.; Menson, E.; Lim, M. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur. J. Pediatr. 2011, 170, 1007–1015. [Google Scholar] [CrossRef]
- Kimura-Ohba, S.; Kitamura, M.; Tsukamoto, Y.; Kogaki, S.; Sakai, S.; Fushimi, H.; Matsuoka, K.; Takeuchi, M.; Itoh, K.; Ueda, K. Viral entry and translation in brain endothelia provoke influenza-associated encephalopathy. Acta Neuropathol. 2024, 147, 77. [Google Scholar] [CrossRef]
- Constant, O.; Maarifi, G.; Blanchet, F.P.; Van de Perre, P.; Simonin, Y.; Salinas, S. Role of Dendritic Cells in Viral Brain Infections. Front. Immunol. 2022, 13, 862053. [Google Scholar] [CrossRef]
- Bin, N.-R.; Prescott, S.L.; Horio, N.; Wang, Y.; Chiu, I.M.; Liberles, S.D. An airway-to-brain sensory pathway mediates influenza-induced sickness. Nature 2023, 615, 660–667. [Google Scholar] [CrossRef]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-term neuroinflammation induced by influenza A virus infection and the impact on hippocampal neuron morphology and function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.K.; Sargent, B.F.; Ahmad, Z.-U.-A.; Tharmaratnam, K.; Dunai, C.; Egbe, F.N.; Martin, N.H.; Facer, B.; Pendered, S.L.; Rogers, H.C. Posthospitalization COVID-19 cognitive deficits at 1 year are global and associated with elevated brain injury markers and gray matter volume reduction. Nat. Med. 2025, 31, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Streit, S.; Dittmayer, C.; Manitius, R.V.; Radbruch, H.; Heppner, F.L. The neurobiology of SARS-CoV-2 infection. Nat. Rev. Neurosci. 2024, 25, 30–42. [Google Scholar] [CrossRef]
- Dunai, C.; Collie, C.; Michael, B.D. Immune-mediated mechanisms of COVID-19 neuropathology. Front. Neurol. 2022, 13, 882905. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Ren, A.L.; Digby, R.J.; Norton, E.J.; Ebrahimi, S.; Outtrim, J.G.; Chatfield, D.A.; Manktelow, A.E.; Leibowitz, M.M.; Newcombe, V.F. Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain 2022, 145, 4097–4107. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Pan, L.; Sha, J.; Bailey, M.; Faure-Kumar, E.; Williams, C.K.; Wohlschlegel, J.; Magaki, S.; Niu, C. Brain-wide alterations revealed by spatial transcriptomics and proteomics in COVID-19 infection. Nat. Aging 2024, 4, 1598–1618. [Google Scholar] [CrossRef]
- Jenkins, D. How do stochastic processes and genetic threshold effects explain incomplete penetrance and inform causal disease mechanisms? Philos. Trans. R. Soc. Biol. Sci. 2024, 379, 20230045. [Google Scholar] [CrossRef]
- Panzeri, I.; Pospisilik, J.A. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 2018, 14, 26–38. [Google Scholar] [CrossRef]
- Hartman IV, J.L.; Garvik, B.; Hartwell, L. Principles for the buffering of genetic variation. Science 2001, 291, 1001–1004. [Google Scholar] [CrossRef]
- Levine, E.; Hwa, T. Stochastic fluctuations in metabolic pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 9224–9229. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. USA 2012, 109, 10661–10668. [Google Scholar] [CrossRef]
- Singh, I.; Pujol, A. Pathomechanisms underlying X-adrenoleukodystrophy: A three-hit hypothesis. Brain Pathol. 2010, 20, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Mosser, J.; Lutz, Y.; Stoeckel, M.E.; Sarde, C.O.; Kretz, C.; Douar, A.M.; Lopez, J.; Aubourg, P.; Mandel, J.L. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum. Mol. Genet. 1994, 3, 265–271. [Google Scholar] [CrossRef]
- Kemp, S.; Mooyer, P.A.; Bolhuis, P.A.; van Geel, B.M.; Mandel, J.-L.; Barth, P.G.; Aubourg, P.; Wanders, R. ALDP expression in fibroblasts of patients with X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 1996, 19, 667–674. [Google Scholar] [CrossRef]
- Watkins, P.A.; Gould, S.J.; Smith, M.A.; Braiterman, L.T.; Wei, H.-M.; Kok, F.; Moser, A.B.; Moser, H.W.; Smith, K.D. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 1995, 57, 292. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Y.; Wang, W.; Lei, J.; Ying, Z.; Yang, G. Structural and functional insights of the human peroxisomal ABC transporter ALDP. Elife 2022, 11, e75039. [Google Scholar] [CrossRef]
- Gloeckner, C.J.; Mayerhofer, P.U.; Landgraf, P.; Muntau, A.C.; Holzinger, A.; Gerber, J.-K.; Kammerer, S.; Adamski, J.; Roscher, A.A. Human adrenoleukodystrophy protein and related peroxisomal ABC transporters interact with the peroxisomal assembly protein PEX19p. Biochem. Biophys. Res. Commun. 2000, 271, 144–150. [Google Scholar] [CrossRef]
- Contreras, M.; Sengupta, T.; Sheikh, F.; Aubourg, P.; Singh, I. Topology of ATP-binding domain of adrenoleukodystrophy gene product in peroxisomes. Arch. Biochem. Biophys. 1996, 334, 369–379. [Google Scholar] [CrossRef]
- van Roermund, C.W.; Visser, W.F.; IJlst, L.; van Cruchten, A.; Boek, M.; Kulik, W.; Waterham, H.R.; Wanders, R.J. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl–CoA esters. FASEB J. 2008, 22, 4201–4208. [Google Scholar] [CrossRef]
- van Roermund, C.W.; Visser, W.F.; IJlst, L.; Waterham, H.R.; Wanders, R.J. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2011, 1811, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Hama, K.; Fujiwara, Y.; Takashima, S.; Hayashi, Y.; Yamashita, A.; Shimozawa, N.; Yokoyama, K. Hexacosenoyl-CoA is the most abundant very long-chain acyl-CoA in ATP binding cassette transporter D1-deficient cells [S]. J. Lipid Res. 2020, 61, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, C.; Kunze, M.; Forss-Petter, S.; Berger, J. Impaired very long-chain acyl-CoA β-oxidation in human X-linked adrenoleukodystrophy fibroblasts is a direct consequence of ABCD1 transporter dysfunction. J. Biol. Chem. 2013, 288, 19269–19279. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Theodoulou, F.L.; Wanders, R.J. Mammalian peroxisomal ABC transporters: From endogenous substrates to pathology and clinical significance. Br. J. Pharmacol. 2011, 164, 1753–1766. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Xu, D.; Wang, L.; Mao, Y.-X.; Li, Y.; Cheng, M.-T.; Zhou, C.-Z.; Hou, W.-T.; Chen, Y. Structural basis of substrate recognition and translocation by human very long-chain fatty acid transporter ABCD1. Nat. Commun. 2022, 13, 3299. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Baes, M.; Ribeiro, D.; Ferdinandusse, S.; Waterham, H.R. The physiological functions of human peroxisomes. Physiol. Rev. 2023, 103, 957–1024. [Google Scholar] [CrossRef]
- McGuinness, M.; Zhang, H.-P.; Smith, K. Evaluation of pharmacological induction of fatty acid β-oxidation in X-linked Adrenoleukodystrophy. Mol. Genet. Metab. 2001, 74, 256–263. [Google Scholar] [CrossRef]
- Kemp, S.; Valianpour, F.; Mooyer, P.A.; Kulik, W.; Wanders, R.J. Method for measurement of peroxisomal very-long-chain fatty acid β-oxidation in human skin fibroblasts using stable-isotope-labeled tetracosanoic acid. Clin. Chem. 2004, 50, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Netik, A.; Forss-Petter, S.; Holzinger, A.; Molzer, B.; Unterrainer, G.; Berger, J. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): Implications for therapy. Hum. Mol. Genet. 1999, 8, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Wei, H.-M.; Lu, J.-F.; Braiterman, L.T.; McGuinness, M.C.; Moser, A.B.; Watkins, P.A.; Smith, K.D. Gene redundancy and pharmacological gene therapy: Implications for X-linked adrenoleukodystrophy. Nat. Med. 1998, 4, 1261–1268. [Google Scholar] [CrossRef]
- O’Neill, G.N.; Aoki, M.; Brown, R.H., Jr. ABCD1 translation–initiator mutation demonstrates genotype–phenotype correlation for AMN. Neurology 2001, 57, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Asheuer, M.; Bieche, I.; Laurendeau, I.; Moser, A.; Hainque, B.; Vidaud, M.; Aubourg, P. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2005, 14, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, F.; Zhou, J.M.; Ralston, E.; Murray, K.; Troalen, F.; Magal, E.; Robain, O.; Dubois-Dalcq, M.; Aubourg, P. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 1997, 3, 271–285. [Google Scholar] [CrossRef]
- Höftberger, R.; Kunze, M.; Weinhofer, I.; Aboul-Enein, F.; Voigtländer, T.; Oezen, I.; Amann, G.; Bernheimer, H.; Budka, H.; Berger, J. Distribution and cellular localization of adrenoleukodystrophy protein in human tissues: Implications for X-linked adrenoleukodystrophy. Neurobiol. Dis. 2007, 28, 165–174. [Google Scholar] [CrossRef]
- Weber, F.D.; Wiesinger, C.; Forss-Petter, S.; Regelsberger, G.; Einwich, A.; Weber, W.H.; Köhler, W.; Stockinger, H.; Berger, J. X-linked adrenoleukodystrophy: Very long-chain fatty acid metabolism is severely impaired in monocytes but not in lymphocytes. Hum. Mol. Genet. 2014, 23, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- De Marcos Lousa, C.; van Roermund, C.W.; Postis, V.L.; Dietrich, D.; Kerr, I.D.; Wanders, R.J.; Baldwin, S.A.; Baker, A.; Theodoulou, F.L. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. USA 2013, 110, 1279–1284. [Google Scholar] [CrossRef]
- Theodoulou, F.L.; Carrier, D.J.; Schaedler, T.A.; Baldwin, S.A.; Baker, A. How to move an amphipathic molecule across a lipid bilayer: Different mechanisms for different ABC transporters? Biochem. Soc. Trans. 2016, 44, 774–782. [Google Scholar] [CrossRef]
- Wanders, R.; Vreken, P.; Ferdinandusse, S.; Jansen, G.; Waterham, H.; Van Roermund, C.; Van Grunsven, E. Peroxisomal fatty acid α-and β-oxidation in humans: Enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem. Soc. Trans. 2001, 29, 250–267. [Google Scholar] [CrossRef]
- Wanders, R.J.; Waterham, H.R.; Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. 2016, 3, 83. [Google Scholar] [CrossRef]
- Theda, C.; Moser, A.B.; Powers, J.M.; Moser, H.W. Phospholipids in X-linked adrenoleukodystrophy white matter: Fatty acid abnormalities before the onset of demyelination. J. Neurol. Sci. 1992, 110, 195–204. [Google Scholar] [CrossRef]
- Wilson, R.; Sargent, J.R. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J. Neurochem. 1993, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Schaumburg, H.H.; Suzuki, K.; Kishimoto, Y.; Moser, A.E. Metabolic studies of adrenoleukodystrophy. In Myelination and Demyelination; Springer: Berlin/Heidelberg, Germany, 1978; pp. 601–619. [Google Scholar]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Frahm, J.; et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Powers, J. A pathogenetic hypothesis based on ultrastructural lesions in adrenal cortex, peripheral nerve and testis. Am. J. Pathol. 1974, 76, 481–500. [Google Scholar]
- Chrast, R.; Saher, G.; Nave, K.A.; Verheijen, M.H. Lipid metabolism in myelinating glial cells: Lessons from human inherited disorders and mouse models. J. Lipid Res. 2011, 52, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Lipid–protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1612, 1–40. [Google Scholar] [CrossRef]
- Powers, J.M.; Moser, H.W. Peroxisomal disorders: Genotype, phenotype, major neuropathologic lesions, and pathogenesis. Brain Pathol. 1998, 8, 101–120. [Google Scholar] [CrossRef]
- Raas, Q.; van de Beek, M.C.; Forss-Petter, S.; Dijkstra, I.M.; Deschiffart, A.; Freshner, B.C.; Stevenson, T.J.; Jaspers, Y.R.; Nagtzaam, L.; Wanders, R.J.; et al. Metabolic rerouting via SCD1 induction impacts X-linked adrenoleukodystrophy. J. Clin. Invest. 2021, 131, e142500. [Google Scholar] [CrossRef]
- Özgür-Günes, Y.; Chedik, M.; Le Stunff, C.; Fovet, C.M.; Bougnères, P. Long-Term Disease Prevention with a Gene Therapy Targeting Oligodendrocytes in a Mouse Model of Adrenomyeloneuropathy. Hum. Gene Ther. 2022, 33, 936–949. [Google Scholar] [CrossRef]
- Montoro, R.; Heine, V.M.; Kemp, S.; Engelen, M. Evolution of adrenoleukodystrophy model systems. J. Inherit. Metab. Dis. 2021, 44, 544–553. [Google Scholar] [CrossRef]
- Manor, J.; Chung, H.; Bhagwat, P.K.; Wangler, M.F. ABCD1 and X-linked adrenoleukodystrophy: A disease with a markedly variable phenotype showing conserved neurobiology in animal models. J. Neurosci. Res. 2021, 99, 3170–3181. [Google Scholar] [CrossRef]
- Özgür Günes, Y.; Le Stunff, C.; Vallat, J.; Bougnères, P. Peripheral Neuropathy in the Adreno-myelo-neuropathy Mouse Model. bioRxiv 2024. [Google Scholar] [CrossRef]
- Özgür Günes, Y.; Le Stunff, C.; Bougnères, P. Intracisternal AAV9-MAG-hABCD1 Vector Reverses Motor Deficits in Adult Adrenomyeloneuropathy Mice. Hum. Gene Ther. 2025, 36, 88–100. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Sasidharan, N.; Laheji, F.; Frosch, M.; Musolino, P.; Tanzi, R.; Kim, D.Y.; Biffi, A.; El Khoury, J.; Eichler, F. Microglial dysfunction as a key pathological change in adrenomyeloneuropathy. Ann. Neurol. 2017, 82, 813–827. [Google Scholar] [CrossRef]
- Launay, N.; Lopez-Erauskin, J.; Bianchi, P.; Guha, S.; Parameswaran, J.; Coppa, A.; Torreni, L.; Schlüter, A.; Fourcade, S.; Paredes-Fuentes, A.J.; et al. Imbalanced mitochondrial dynamics contributes to the pathogenesis of X-linked adrenoleukodystrophy. Brain 2024, 147, 2069–2084. [Google Scholar] [CrossRef]
- Fourcade, S.; Lopez-Erauskin, J.; Galino, J.; Duval, C.; Naudi, A.; Jove, M.; Kemp, S.; Villarroya, F.; Ferrer, I.; Pamplona, R. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 2008, 17, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, S.; López-Erauskin, J.; Ruiz, M.; Ferrer, I.; Pujol, A. Mitochondrial dysfunction and oxidative damage cooperatively fuel axonal degeneration in X-linked adrenoleukodystrophy. Biochimie 2014, 98, 143–149. [Google Scholar] [CrossRef] [PubMed]
- López-Erauskin, J.; Galino, J.; Ruiz, M.; Cuezva, J.M.; Fabregat, I.; Cacabelos, D.; Boada, J.; Martínez, J.; Ferrer, I.; Pamplona, R.; et al. Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2013, 22, 3296–3305. [Google Scholar] [CrossRef]
- Ruiz, M.; Jové, M.; Schlüter, A.; Casasnovas, C.; Villarroya, F.; Guilera, C.; Ortega, F.J.; Naudí, A.; Pamplona, R.; Gimeno, R.; et al. Altered glycolipid and glycerophospholipid signaling drive inflammatory cascades in adrenomyeloneuropathy. Hum. Mol. Genet. 2015, 24, 6861–6876. [Google Scholar] [CrossRef]
- López-Erauskin, J.; Fourcade, S.; Galino, J.; Ruiz, M.; Schlüter, A.; Naudi, A.; Jove, M.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann. Neurol. 2011, 70, 84–92. [Google Scholar] [CrossRef]
- Launay, N.; Ruiz, M.; Fourcade, S.; Schlüter, A.; Guilera, C.; Ferrer, I.; Knecht, E.; Pujol, A. Oxidative stress regulates the ubiquitin–proteasome system and immunoproteasome functioning in a mouse model of X-adrenoleukodystrophy. Brain 2013, 136, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Erauskin, J.; Galino, J.; Bianchi, P.; Fourcade, S.; Andreu, A.L.; Ferrer, I.; Munoz-Pinedo, C.; Pujol, A. Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain 2012, 135, 3584–3598. [Google Scholar] [CrossRef] [PubMed]
- Galea, E.; Launay, N.; Portero-Otin, M.; Ruiz, M.; Pamplona, R.; Aubourg, P.; Ferrer, I.; Pujol, A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: A paradigm for multifactorial neurodegenerative diseases? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Galino, J.; Ruiz, M.; Fourcade, S.; Schlüter, A.; López-Erauskin, J.; Guilera, C.; Jove, M.; Naudi, A.; García-Arumí, E.; Andreu, A.L.; et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of X-adrenoleukodystrophy. Antioxid. Redox Signal 2011, 15, 2095–2107. [Google Scholar] [CrossRef]
- Kruska, N.; Schönfeld, P.; Pujol, A.; Reiser, G. Astrocytes and mitochondria from adrenoleukodystrophy protein (ABCD1)-deficient mice reveal that the adrenoleukodystrophy-associated very long-chain fatty acids target several cellular energy-dependent functions. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 925–936. [Google Scholar] [CrossRef]
- Pujol, A.; Ferrer, I.; Camps, C.; Metzger, E.; Hindelang, C.; Callizot, N.; Ruiz, M.; Pàmpols, T.; Giròs, M.; Mandel, J.L. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: A therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004, 13, 2997–3006. [Google Scholar] [CrossRef]
- Strachan, L.R.; Stevenson, T.J.; Freshner, B.; Keefe, M.D.; Miranda Bowles, D.; Bonkowsky, J.L. A zebrafish model of X-linked adrenoleukodystrophy recapitulates key disease features and demonstrates a developmental requirement for abcd1 in oligodendrocyte patterning and myelination. Hum. Mol. Genet. 2017, 26, 3600–3614. [Google Scholar] [CrossRef]
- Bülow, M.H.; Parsons, B.D.; Di Cara, F. The Drosophila melanogaster as Genetic Model System to Dissect the Mechanisms of Disease that Lead to Neurodegeneration in Adrenoleukodystrophy. Peroxisome Biol. Exp. Models Peroxisomal Disord. Neurol. Dis. 2020, 1299, 145–159. [Google Scholar]
- Banerjee, S.; Bhat, M.A. Glial ensheathment of peripheral axons in Drosophila. J. Neurosci. Res. 2008, 86, 1189–1198. [Google Scholar] [CrossRef]
- Manor, J.; Jangam, S.V.; Chung, H.-l.; Bhagwat, P.; Andrews, J.; Chester, H.; Kondo, S.; Srivastav, S.; Botas, J.; Moser, A.B. Genetic analysis of the X-linked Adrenoleukodystrophy ABCD1 gene in Drosophila uncovers a role in Peroxisomal dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Coppa, A.; Guha, S.; Fourcade, S.; Parameswaran, J.; Ruiz, M.; Moser, A.B.; Schlüter, A.; Murphy, M.P.; Lizcano, J.M.; Miranda-Vizuete, A. The peroxisomal fatty acid transporter ABCD1/PMP-4 is required in the C. elegans hypodermis for axonal maintenance: A worm model for adrenoleukodystrophy. Free Radic. Biol. Med. 2020, 152, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Kapfhammer, J.P.; Hindelang, C.; Kemp, S.; Troffer-Charlier, N.; Broccoli, V.; Callyzot, N.; Mooyer, P.; Selhorst, J.; Vreken, P. Inactivation of the peroxisomal ABCD2 transporter in the mouse leads to late-onset ataxia involving mitochondria, Golgi and endoplasmic reticulum damage. Hum. Mol. Genet. 2005, 14, 3565–3577. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef]
- Simons, M.; Gibson, E.M.; Nave, K.A. Oligodendrocytes: Myelination, Plasticity, and Axonal Support. Cold Spring Harb. Perspect. Biol. 2024, 16, a041359. [Google Scholar] [CrossRef] [PubMed]
- Ferrari Bardile, C.; Garcia-Miralles, M.; Caron, N.S.; Rayan, N.A.; Langley, S.R.; Harmston, N.; Rondelli, A.M.; Teo, R.T.Y.; Waltl, S.; Anderson, L.M.; et al. Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proc. Natl. Acad. Sci. USA 2019, 116, 9622–9627. [Google Scholar] [CrossRef]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Mendanha Falcão, A.; Xiao, L.; Li, H.; Häring, M.; Hochgerner, H.; Romanov, R.A.; et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef]
- van de Beek, M.C.; Dijkstra, I.M.; van Lenthe, H.; Ofman, R.; Goldhaber-Pasillas, D.; Schauer, N.; Schackmann, M.; Engelen-Lee, J.Y.; Vaz, F.M.; Kulik, W.; et al. C26:0-Carnitine Is a New Biomarker for X-Linked Adrenoleukodystrophy in Mice and Man. PLoS ONE 2016, 11, e0154597. [Google Scholar] [CrossRef]
- Denic, V.; Weissman, J.S. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 2007, 130, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Tvrdik, P.; Westerberg, R.; Silve, S.; Asadi, A.; Jakobsson, A.; Cannon, B.; Loison, G.; Jacobsson, A. Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J. Cell Biol. 2000, 149, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444. [Google Scholar] [CrossRef] [PubMed]
- Ofman, R.; Dijkstra, I.M.; van Roermund, C.W.; Burger, N.; Turkenburg, M.; van Cruchten, A.; van Engen, C.E.; Wanders, R.J.; Kemp, S. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol. Med. 2010, 2, 90–97. [Google Scholar] [CrossRef]
- Khandker, L.; Jeffries, M.A.; Chang, Y.-J.; Mather, M.L.; Evangelou, A.V.; Bourne, J.N.; Tafreshi, A.K.; Ornelas, I.M.; Bozdagi-Gunal, O.; Macklin, W.B. Cholesterol biosynthesis defines oligodendrocyte precursor heterogeneity between brain and spinal cord. Cell Rep. 2022, 38, 10423. [Google Scholar] [CrossRef]
- Williams, K.A.; Deber, C.M.; Klrschner, O. The structure and function of central nervous system myelin. Crit. Rev. Clin. Lab. Sci. 1993, 30, 29–64. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [CrossRef]
- Norton, W.; Poduslo, S.E. Myelination in rat brain: Method of myelin isolation 1. J. Neurochem. 1973, 21, 749–757. [Google Scholar] [CrossRef]
- Sassa, T.; Kihara, A. Metabolism of very long-chain fatty acids: Genes and pathophysiology. Biomol. Ther. 2014, 22, 83. [Google Scholar] [CrossRef]
- Sassa, T.; Suto, S.; Okayasu, Y.; Kihara, A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 1031–1037. [Google Scholar] [CrossRef]
- Imgrund, S.; Hartmann, D.; Farwanah, H.; Eckhardt, M.; Sandhoff, R.; Degen, J.; Gieselmann, V.; Sandhoff, K.; Willecke, K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 2009, 284, 33549–33560. [Google Scholar] [CrossRef]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H.; Futerman, A.H. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.-A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef]
- Honda, A.; Nozumi, M.; Ito, Y.; Natsume, R.; Kawasaki, A.; Nakatsu, F.; Abe, M.; Uchino, H.; Matsushita, N.; Ikeda, K. Very-long-chain fatty acids are crucial to neuronal polarity by providing sphingolipids to lipid rafts. Cell Rep. 2023, 42, 113195. [Google Scholar] [CrossRef] [PubMed]
- Floriddia, E. New myelin for old oligodendrocytes. Nat. Neurosci. 2022, 25, 404. [Google Scholar] [CrossRef]
- Meschkat, M.; Steyer, A.M.; Weil, M.-T.; Kusch, K.; Jahn, O.; Piepkorn, L.; Agüi-Gonzalez, P.; Phan, N.T.N.; Ruhwedel, T.; Sadowski, B. White matter integrity in mice requires continuous myelin synthesis at the inner tongue. Nat. Commun. 2022, 13, 1163. [Google Scholar] [CrossRef]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.-J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef]
- Xiao, L.; Ohayon, D.; McKenzie, I.A.; Sinclair-Wilson, A.; Wright, J.L.; Fudge, A.D.; Emery, B.; Li, H.; Richardson, W.D. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 2016, 19, 1210–1217. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; Paes de Faria, J.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- de Faria, O., Jr.; Pivonkova, H.; Varga, B.; Timmler, S.; Evans, K.A.; Káradóttir, R.T. Periods of synchronized myelin changes shape brain function and plasticity. Nat. Neurosci. 2021, 24, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Mayoral, S.R.; Choi, H.S.; Chan, J.R.; Kheirbek, M.A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 2020, 23, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience–dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.L.; Weigel, T.K.; Elkahloun, A.G.; Herkenham, M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci. Rep. 2017, 7, 46548. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef]
- Abraham, M.; Peterburs, J.; Mundorf, A. Oligodendrocytes matter: A review of animal studies on early adversity. J. Neural Transm. 2023, 130, 1177–1185. [Google Scholar] [CrossRef]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; Andrade da Costa, B.L.; De Stasi, A.M.; Bacci, A.; Imamura Kawasawa, Y.; Vaidya, V.A.; Gaspar, P. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef]
- Sams, E.C. Oligodendrocytes in the aging brain. Neuronal Signal. 2021, 5, NS20210008. [Google Scholar] [CrossRef] [PubMed]
- Cohn, E.F.; Clayton, B.L.; Madhavan, M.; Lee, K.A.; Yacoub, S.; Fedorov, Y.; Scavuzzo, M.A.; Paul Friedman, K.; Shafer, T.J.; Tesar, P.J. Pervasive environmental chemicals impair oligodendrocyte development. Nat. Neurosci. 2024, 27, 836–845. [Google Scholar] [CrossRef]
- Nicholson, M.; Wood, R.J.; Gonsalvez, D.G.; Hannan, A.J.; Fletcher, J.L.; Xiao, J.; Murray, S.S. Remodelling of myelinated axons and oligodendrocyte differentiation is stimulated by environmental enrichment in the young adult brain. Eur. J. Neurosci. 2022, 56, 6099–6114. [Google Scholar] [CrossRef]
- Forbes, T.A.; Gallo, V. All wrapped up: Environmental effects on myelination. Trends Neurosci. 2017, 40, 572–587. [Google Scholar] [CrossRef]
- SMITH, M.E. The metabolism of myelin lipids. Adv. Lipid Res. 1967, 5, 241–278. [Google Scholar] [PubMed]
- Asadollahi, E.; Nave, K.-A. Myelin lipid metabolism can provide energy for starved axons. Nat. Neurosci. 2024, 27, 1862–1863. [Google Scholar]
- Aber, E.R.; Griffey, C.J.; Davies, T.; Li, A.M.; Yang, Y.J.; Croce, K.R.; Goldman, J.E.; Grutzendler, J.; Canman, J.C.; Yamamoto, A. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 2022, 41, 111480. [Google Scholar] [CrossRef]
- Farré, J.C.; Mahalingam, S.S.; Proietto, M.; Subramani, S. Peroxisome biogenesis, membrane contact sites, and quality control. EMBO Rep. 2019, 20, e46864. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, I.; Chang, C.-L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid droplets in the nervous system. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef] [PubMed]
- Smolič, T.; Zorec, R.; Vardjan, N. Pathophysiology of lipid droplets in neuroglia. Antioxidants 2021, 11, 22. [Google Scholar] [CrossRef]
- Schuldiner, M.; Bohnert, M. A different kind of love–lipid droplet contact sites. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1188–1196. [Google Scholar] [CrossRef]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018, 37, e98947. [Google Scholar] [CrossRef]
- Wanders, R.J. Peroxisomes in human health and disease: Metabolic pathways, metabolite transport, interplay with other organelles and signal transduction. In Peroxisomes and their Key Role in Cellular Signaling and Metabolism; Springer: Berlin/Heidelberg, Germany, 2013; pp. 23–44. [Google Scholar]
- Lodhi, I.J.; Semenkovich, C.F. Peroxisomes: A nexus for lipid metabolism and cellular signaling. Cell Metab. 2014, 19, 380–392. [Google Scholar] [CrossRef]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kislinger, G.; Duschek, J.; Durmaz, A.D.; Wefers, B.; Feng, R.; Nalbach, K.; Wurst, W.; Behrends, C.; Schifferer, M. Nonvesicular lipid transfer drives myelin growth in the central nervous system. Nat. Commun. 2024, 15, 9756. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; Gross, S.P.; Parton, R.G. Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef]
- Hashemi, H.F.; Goodman, J.M. The life cycle of lipid droplets. Curr. Opin. Cell Biol. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Neubert, I.; Kovacs, W.J.; Baumgart-Vogt, E. Differential expression of peroxisomal matrix and membrane proteins during postnatal development of mouse brain. J. Comp. Neurol. 2007, 505, 1–17. [Google Scholar] [CrossRef]
- Kleinecke, S.; Richert, S.; de Hoz, L.; Brügger, B.; Kungl, T.; Asadollahi, E.; Quintes, S.; Blanz, J.; McGonigal, R.; Naseri, K. Peroxisomal dysfunctions cause lysosomal storage and axonal Kv1 channel redistribution in peripheral neuropathy. Elife 2017, 6, e23332. [Google Scholar] [CrossRef]
- Kassmann, C.M. Myelin peroxisomes–Essential organelles for the maintenance of white matter in the nervous system. Biochimie 2014, 98, 111–118. [Google Scholar] [CrossRef]
- Kassmann, C.M.; Quintes, S.; Rietdorf, J.; Möbius, W.; Sereda, M.W.; Nientiedt, T.; Saher, G.; Baes, M.; Nave, K.-A. A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS Lett. 2011, 585, 2205–2211. [Google Scholar] [CrossRef]
- Holtzman, E.; Teichberg, S.; Abrahams, S.J.; Citkowitz, E.; Crain, S.M.; Kawai, N.; Peterson, E.R. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J. Histochem. Cytochem. 1973, 21, 349–385. [Google Scholar] [CrossRef]
- Germain, K.; So, R.W.; DiGiovanni, L.F.; Watts, J.C.; Bandsma, R.H.; Kim, P.K. Upregulated pexophagy limits the capacity of selective autophagy. Nat. Commun. 2024, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, N.; Lu, B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci. Ther. 2019, 25, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Bajdzienko, J.; Bremm, A. Mammalian pexophagy at a glance. J. Cell Sci. 2024, 137, jcs259775. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Baarine, M.; Ragot, K.; Athias, A.; Nury, T.; Kattan, Z.; Genin, E.C.; Andreoletti, P.; Ménétrier, F.; Riedinger, J.M.; Bardou, M.; et al. Incidence of Abcd1 level on the induction of cell death and organelle dysfunctions triggered by very long chain fatty acids and TNF-α on oligodendrocytes and astrocytes. Neurotoxicology 2012, 33, 212–228. [Google Scholar] [CrossRef]
- Khan, M.; Singh, J.; Gilg, A.G.; Uto, T.; Singh, I. Very long-chain fatty acid accumulation causes lipotoxic response via 5-lipoxygenase in cerebral adrenoleukodystrophy. J. Lipid Res. 2010, 51, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Brites, P.; Mooyer, P.A.; El Mrabet, L.; Waterham, H.R.; Wanders, R.J. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain 2009, 132, 482–492. [Google Scholar] [CrossRef]
- Malheiro, A.R.; Correia, B.; Ferreira da Silva, T.; Bessa-Neto, D.; Van Veldhoven, P.P.; Brites, P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain Pathol. 2019, 29, 622–639. [Google Scholar] [CrossRef]
- Bottelbergs, A.; Verheijden, S.; Hulshagen, L.; Gutmann, D.H.; Goebbels, S.; Nave, K.A.; Kassmann, C.; Baes, M. Axonal integrity in the absence of functional peroxisomes from projection neurons and astrocytes. Glia 2010, 58, 1532–1543. [Google Scholar] [CrossRef]
- Hulshagen, L.; Krysko, O.; Bottelbergs, A.; Huyghe, S.; Klein, R.; Van Veldhoven, P.P.; De Deyn, P.P.; d’Hooge, R.; Hartmann, D.; Baes, M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J. Neurosci. 2008, 28, 4015–4027. [Google Scholar] [CrossRef]
- Krysko, O.; Hulshagen, L.; Janssen, A.; Schütz, G.; Klein, R.; De Bruycker, M.; Espeel, M.; Gressens, P.; Baes, M. Neocortical and cerebellar developmental abnormalities in conditions of selective elimination of peroxisomes from brain or from liver. J. Neurosci. Res. 2007, 85, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-Y.; Pan, J.; Chu, R.; Lee, D.; Kluckman, K.D.; Usuda, N.; Singh, I.; Yeldandi, A.V.; Rao, M.S.; Maeda, N. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J. Biol. Chem. 1996, 271, 24698–24710. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, M.E.; Giles, D.A.; Stankiewicz, T.E.; Sheridan, R.; Karns, R.; Cappelletti, M.; Lampe, K.; Mukherjee, R.; Sina, C.; Sallese, A. Peroxisomal β-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease. JCI Insight 2018, 3, e93626. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Hogenhout, E.M.; Koster, J.; Van Roermund, C.W.; IJlst, L.; Moser, A.B.; Wanders, R.J.; Waterham, H.R. Clinical, biochemical, and mutational spectrum of peroxisomal acyl–coenzyme A oxidase deficiency. Hum. Mutat. 2007, 28, 904–912. [Google Scholar] [CrossRef]
- Chung, H.-l.; Wangler, M.F.; Marcogliese, P.C.; Jo, J.; Ravenscroft, T.A.; Zuo, Z.; Duraine, L.; Sadeghzadeh, S.; Li-Kroeger, D.; Schmidt, R.E. Loss-or gain-of-function mutations in ACOX1 cause axonal loss via different mechanisms. Neuron 2020, 106, 589–606.e6. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Denis, S.; Overmars, H.; Van Eeckhoudt, L.; Van Veldhoven, P.P.; Duran, M.; Wanders, R.J.; Baes, M. Developmental changes of bile acid composition and conjugation in L- and D-bifunctional protein single and double knockout mice. J. Biol. Chem. 2005, 280, 18658–18666. [Google Scholar] [CrossRef]
- Verheijden, S.; Bottelbergs, A.; Krysko, O.; Krysko, D.V.; Beckers, L.; De Munter, S.; Van Veldhoven, P.P.; Wyns, S.; Kulik, W.; Nave, K.-A. Peroxisomal multifunctional protein-2 deficiency causes neuroinflammation and degeneration of Purkinje cells independent of very long chain fatty acid accumulation. Neurobiol. Dis. 2013, 58, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-l.; Ye, Q.; Park, Y.-J.; Zuo, Z.; Mok, J.-W.; Kanca, O.; Tattikota, S.G.; Lu, S.; Perrimon, N.; Lee, H.K. Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation. Cell Metab. 2023, 35, 855–874.e5. [Google Scholar] [CrossRef]
- Maharaj, A.; Williams, J.; Bradshaw, T.; Güran, T.; Braslavsky, D.; Casas, J.; Chan, L.; Metherell, L.; Prasad, R. Sphingosine-1-phosphate lyase (SGPL1) deficiency is associated with mitochondrial dysfunction. J. Steroid Biochem. Mol. Biol. 2020, 202, 105730. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Differential expression of (dihydro) ceramide synthases in mouse brain: Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 2008, 129, 233–241. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Ebrahimi, R.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 2024, 27, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Späte, E.; Zhou, B.; Sun, T.; Kusch, K.; Asadollahi, E.; Siems, S.B.; Depp, C.; Werner, H.B.; Saher, G.; Hirrlinger, J. Downregulated expression of lactate dehydrogenase in adult oligodendrocytes and its implication for the transfer of glycolysis products to axons. Glia 2024, 72, 1374–1391. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Booth, F.W. Eating, exercise, and “thrifty” genotypes: Connecting the dots toward an evolutionary understanding of modern chronic diseases. J. Appl. Physiol. 2004, 96, 3–10. [Google Scholar] [CrossRef]
- Freese, J.; Klement, R.J.; Ruiz-Núñez, B.; Schwarz, S.; Lötzerich, H. The sedentary (r) evolution: Have we lost our metabolic flexibility? F1000Research 2018, 6, 1787. [Google Scholar] [CrossRef]
- Harris, J.J.; Attwell, D. The energetics of CNS white matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.; Amphornrat, J.; Thilemann, S. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte–Neuron Communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Forss-Petter, S.; Eichler, F. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 2014, 98, 135–142. [Google Scholar] [CrossRef]
- von Jonquieres, G.; Fröhlich, D.; Klugmann, C.B.; Wen, X.; Harasta, A.E.; Ramkumar, R.; Spencer, Z.H.; Housley, G.D.; Klugmann, M. Recombinant Human Myelin-Associated Glycoprotein Promoter Drives Selective AAV-Mediated Transgene Expression in Oligodendrocytes. Front. Mol. Neurosci. 2016, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Berenson, A.; Laheji, F.; Gao, G.; Wang, D.; Ng, C.; Volak, A.; Kok, R.; Kreouzis, V.; Dijkstra, I.M.; et al. Intrathecal Adeno-Associated Viral Vector-Mediated Gene Delivery for Adrenomyeloneuropathy. Hum. Gene Ther. 2019, 30, 544–555. [Google Scholar] [CrossRef]
- Gong, Y.; Mu, D.; Prabhakar, S.; Moser, A.; Musolino, P.; Ren, J.; Breakefield, X.O.; Maguire, C.A.; Eichler, F.S. Adenoassociated virus serotype 9-mediated gene therapy for x-linked adrenoleukodystrophy. Mol. Ther. 2015, 23, 824–834. [Google Scholar] [CrossRef]
- Covill-Cooke, C.; Toncheva, V.S.; Kittler, J.T. Regulation of peroxisomal trafficking and distribution. Cell. Mol. Life Sci. 2021, 78, 1929–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Metz, J.; Costello, J.L.; Passmore, J.; Schrader, M.; Schultz, C.; Islinger, M. Intracellular redistribution of neuronal peroxisomes in response to ACBD5 expression. PLoS ONE 2018, 13, e0209507. [Google Scholar] [CrossRef]
- Ferrer, I.; Aubourg, P.; Pujol, A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010, 20, 817–830. [Google Scholar] [CrossRef]
- Yska, H.A.; Engelen, M.; Bugiani, M. The pathology of X-linked adrenoleukodystrophy: Tissue specific changes as a clue to pathophysiology. Orphanet J. Rare Dis. 2024, 19, 138. [Google Scholar] [CrossRef]
- Parasar, P.; Kaur, N.; Singh, J. Pathophysiology of X-Linked Adrenoleukodystrophy: Updates on Molecular Mechanisms. J. Biotechnol. Biomed. 2024, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Bergner, C.G.; Genc, N.; Hametner, S.; Franz, J.; van der Meer, F.; Mitkovski, M.; Weber, M.S.; Stoltenburg-Didinger, G.; Kühl, J.S.; Köhler, W. Concurrent axon and myelin destruction differentiates X-linked adrenoleukodystrophy from multiple sclerosis. Glia 2021, 69, 2362–2377. [Google Scholar] [CrossRef]
- Yaron, A.; Schuldiner, O. Common and divergent mechanisms in developmental neuronal remodeling and dying back neurodegeneration. Curr. Biol. 2016, 26, R628–R639. [Google Scholar] [CrossRef]
- Wang, J.T.; Medress, Z.A.; Barres, B.A. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J. Cell Biol. 2012, 196, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; Przedborski, S. Mitochondria: The next (neurode) generation. Neuron 2011, 70, 1033–1053. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef]
- Nave, K.-A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef]
- Tepavčević, V. Oligodendroglial energy metabolism and (re) myelination. Life 2021, 11, 238. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef]
- Vasireddy, V.; Maguire, C.A.; Anderson, D.W.; Ng, C.; Gong, Y.; Eichler, F.; Fourcade, S.; Guilera, C.; Onieva, A.; Sanchez, A. An in vitro and in vivo efficacy evaluation of gene therapy candidate SBT101 in mouse models of adrenomyeloneuropathy and in NHPs. Mol. Ther. Methods Clin. Dev. 2024, 32, 101354. [Google Scholar] [CrossRef]
- Mittelbronn, M.; Dietz, K.; Schluesener, H.; Meyermann, R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001, 101, 249–255. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Jung, S.; Priller, J. Microglia biology: One century of evolving concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Sierra, A.; Miron, V.E.; Paolicelli, R.C.; Ransohoff, R.M. Microglia in Health and Diseases: Integrative Hubs of the Central Nervous System (CNS). Cold Spring Harb. Perspect. Biol. 2024, 16, a041366. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- de Soysa, T.Y.; Therrien, M.; Walker, A.C.; Stevens, B. Redefining microglia states: Lessons and limits of human and mouse models to study microglia states in neurodegenerative diseases. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2022; p. 101651. [Google Scholar]
- Füger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.-C.; Schlosser, C.; Obermüller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Boda, E. Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci. 2021, 53, 281–297. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Süß, P.; Diebold, M.; Sankowski, R. Advances in understanding the immunity of the brain and its borders: Focus on brain macrophages. Clin. Transl. Med. 2024, 14, e70014. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; de Castro, F.; del Río-Hortega, J.; Rafael Iglesias-Rozas, J.; Garrosa, M.; Kettenmann, H. The “Big-Bang” for modern glial biology: Translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia 2016, 64, 1801–1840. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.B.; Munro, D.A.; Bestard-Cuche, N.; Uyeda, A.; Bogie, J.F.; Hoffmann, A.; Holloway, R.K.; Molina-Gonzalez, I.; Askew, K.E.; Mitchell, S. Microglia regulate central nervous system myelin growth and integrity. Nature 2023, 613, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, S.; Wu, J.; Chen, C.; Li, X.; Liu, K.; Qu, J.Y. Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window. Nat. Commun. 2022, 13, 1959. [Google Scholar] [CrossRef]
- Hildebrand, C.; Remahl, S.; Persson, H.; Bjartmar, C. Myelinated nerve fibres in the CNS. Prog. Neurobiol. 1993, 40, 319–384. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Askew, K.; Li, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Hammond, T.R.; Robinton, D.; Stevens, B. Microglia and the brain: Complementary partners in development and disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 523–544. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Friedman, B.A.; Dejanovic, B.; Sheng, M. Microglia in brain development, homeostasis, and neurodegeneration. Annu. Rev. Genet. 2019, 53, 263–288. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Safaiyan, S.; Besson-Girard, S.; Kaya, T.; Cantuti-Castelvetri, L.; Liu, L.; Ji, H.; Schifferer, M.; Gouna, G.; Usifo, F.; Kannaiyan, N. White matter aging drives microglial diversity. Neuron 2021, 109, 1100–1117.e10. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; McNamara, N.B.; Gerrits, E.; Marzin, M.C.; Kooistra, S.M.; Miron, V.E.; Nutma, E. White matter microglia heterogeneity in the CNS. Acta Neuropathol. 2022, 143, 125–141. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223.e210. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar, N.; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef]
- Priller, J.; Prinz, M. Targeting microglia in brain disorders. Science 2019, 365, 32–33. [Google Scholar] [CrossRef]
- Nugent, A.A.; Lin, K.; Van Lengerich, B.; Lianoglou, S.; Przybyla, L.; Davis, S.S.; Llapashtica, C.; Wang, J.; Xia, D.; Lucas, A. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 2020, 105, 837–854.e9. [Google Scholar] [CrossRef] [PubMed]
- Parakalan, R.; Jiang, B.; Nimmi, B.; Janani, M.; Jayapal, M.; Lu, J.; Tay, S.S.; Ling, E.-A.; Dheen, S.T. Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neurosci. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS pro-inflammatory response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-associated microglia: A universal immune sensor of neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Rathakrishnan, P.; Xiao, H.; Gao, T.; Duong, D.M.; Pennington, M.W.; Lah, J.J.; Seyfried, N.T. Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 24. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef]
- Shemer, A.; Erny, D.; Jung, S.; Prinz, M. Microglia plasticity during health and disease: An immunological perspective. Trends Immunol. 2015, 36, 614–624. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef] [PubMed]
- Raas, Q.; Gondcaille, C.; Hamon, Y.; Leoni, V.; Caccia, C.; Ménétrier, F.; Lizard, G.; Trompier, D.; Savary, S. CRISPR/Cas9-mediated knockout of Abcd1 and Abcd2 genes in BV-2 cells: Novel microglial models for X-linked Adrenoleukodystrophy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 704–714. [Google Scholar] [CrossRef]
- Raas, Q.; Tawbeh, A.; Tahri-Joutey, M.; Gondcaille, C.; Keime, C.; Kaiser, R.; Trompier, D.; Nasser, B.; Leoni, V.; Bellanger, E. Peroxisomal defects in microglial cells induce a disease-associated microglial signature. Front. Mol. Neurosci. 2023, 16, 1170313. [Google Scholar] [CrossRef] [PubMed]
- Tawbeh, A.; Gondcaille, C.; Saih, F.-E.; Raas, Q.; Loichot, D.; Hamon, Y.; Keime, C.; Benani, A.; Di Cara, F.; Cherkaoui-Malki, M. Impaired peroxisomal beta-oxidation in microglia triggers oxidative stress and impacts neurons and oligodendrocytes. Front. Mol. Neurosci. 2025, 18, 1542938. [Google Scholar] [CrossRef]
- Tawbeh, A.; Raas, Q.; Tahri-Joutey, M.; Keime, C.; Kaiser, R.; Trompier, D.; Nasser, B.; Bellanger, E.; Dessard, M.; Hamon, Y. Immune response of BV-2 microglial cells is impacted by peroxisomal beta-oxidation. Front. Mol. Neurosci. 2023, 16, 1299314. [Google Scholar] [CrossRef]
- Eichler, F.S.; Ren, J.Q.; Cossoy, M.; Rietsch, A.M.; Nagpal, S.; Moser, A.B.; Frosch, M.P.; Ransohoff, R.M. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann. Neurol. 2008, 63, 729–742. [Google Scholar] [CrossRef]
- Bergner, C.G.; van der Meer, F.; Winkler, A.; Wrzos, C.; Türkmen, M.; Valizada, E.; Fitzner, D.; Hametner, S.; Hartmann, C.; Pfeifenbring, S.; et al. Microglia damage precedes major myelin breakdown in X-linked adrenoleukodystrophy and metachromatic leukodystrophy. Glia 2019, 67, 1196–1209. [Google Scholar] [CrossRef]
- Weinhofer, I.; Zierfuss, B.; Hametner, S.; Wagner, M.; Popitsch, N.; Machacek, C.; Bartolini, B.; Zlabinger, G.; Ohradanova-Repic, A.; Stockinger, H. Impaired plasticity of macrophages in X-linked adrenoleukodystrophy. Brain 2018, 141, 2329–2342. [Google Scholar] [CrossRef]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef]
- Hickey, W.F.; Kimura, H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 1988, 239, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Benhamida, S.; Pflumio, F.; Dubart-Kupperschmitt, A.; Zhao-Emonet, J.C.; Cavazzana-Calvo, M.; Rocchiccioli, F.; Fichelson, S.; Aubourg, P.; Charneau, P.; Cartier, N. Transduced CD34+ cells from adrenoleukodystrophy patients with HIV-derived vector mediate long-term engraftment of NOD/SCID mice. Mol. Ther. 2003, 7, 317–324. [Google Scholar] [CrossRef] [PubMed]
- van Geel, B.M.; Poll-The, B.T.; Verrips, A.; Boelens, J.J.; Kemp, S.; Engelen, M. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: A retrospective study. J. Inherit. Metab. Dis. 2015, 38, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Schulz, C.; Geissmann, F. Development and homeostasis of “resident” myeloid cells: The case of the microglia. Glia 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Faraco, G.; Park, L.; Anrather, J.; Iadecola, C. Brain perivascular macrophages: Characterization and functional roles in health and disease. J. Mol. Med. 2017, 95, 1143–1152. [Google Scholar] [CrossRef]
- Masuda, T.; Amann, L.; Monaco, G.; Sankowski, R.; Staszewski, O.; Krueger, M.; Del Gaudio, F.; He, L.; Paterson, N.; Nent, E. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 2022, 604, 740–748. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Jordão, M.J.C.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef]
- Van Hove, H.; Martens, L.; Scheyltjens, I.; De Vlaminck, K.; Pombo Antunes, A.R.; De Prijck, S.; Vandamme, N.; De Schepper, S.; Van Isterdael, G.; Scott, C.L. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef]
- Hiraga, S.i.; Masuda, T. Common principles of macrophage biology in blood–tissue barriers. Clin. Exp. Neuroimmunol. 2024, 15, 203–214. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 2022, 612, 417–429. [Google Scholar] [CrossRef]
- Kierdorf, K.; Masuda, T.; Jordão, M.J.C.; Prinz, M. Macrophages at CNS interfaces: Ontogeny and function in health and disease. Nat. Rev. Neurosci. 2019, 20, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 2018, 48, 380–395.e6. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Ahmari, J.; Mezö, C.; Hrabě de Angelis, A.L.; Fuchs, V.; Utermöhlen, O.; Buch, T.; Blank, T.; Gomez de Agüero, M.; Macpherson, A.J. Commensal microbiota divergently affect myeloid subsets in the mammalian central nervous system during homeostasis and disease. EMBO J. 2021, 40, e108605. [Google Scholar] [CrossRef]
- Faraco, G.; Sugiyama, Y.; Lane, D.; Garcia-Bonilla, L.; Chang, H.; Santisteban, M.M.; Racchumi, G.; Murphy, M.; Van Rooijen, N.; Anrather, J. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Investig. 2016, 126, 4674–4689. [Google Scholar] [CrossRef]
- Ajami, B.; Samusik, N.; Wieghofer, P.; Ho, P.P.; Crotti, A.; Bjornson, Z.; Prinz, M.; Fantl, W.J.; Nolan, G.P.; Steinman, L. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 2018, 21, 541–551. [Google Scholar] [CrossRef]
- Jordão, M.J.C.; Sankowski, R.; Brendecke, S.M.; Sagar; Locatelli, G.; Tai, Y.-H.; Tay, T.L.; Schramm, E.; Armbruster, S.; Hagemeyer, N. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019, 363, eaat7554. [Google Scholar] [CrossRef]
- Kearns, N.A.; Iatrou, A.; Flood, D.J.; De Tissera, S.; Mullaney, Z.M.; Xu, J.; Gaiteri, C.; Bennett, D.A.; Wang, Y. Dissecting the human leptomeninges at single-cell resolution. Nat. Commun. 2023, 14, 7036. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Süß, P.; Benkendorff, A.; Böttcher, C.; Fernandez-Zapata, C.; Chhatbar, C.; Cahueau, J.; Monaco, G.; Gasull, A.D.; Khavaran, A. Multiomic spatial landscape of innate immune cells at human central nervous system borders. Nat. Med. 2024, 30, 186–198. [Google Scholar] [CrossRef]
- Schonhoff, A.; Figge, D.; Williams, G.; Jurkuvenaite, A.; Gallups, N.; Childers, G.; Webster, J.; Standaert, D.; Goldman, J.; Harms, A. Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nat. Commun. 2023, 14, 3754. [Google Scholar] [CrossRef]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef]
- Dalmau Gasull, A.; Glavan, M.; Samawar, S.K.R.; Kapupara, K.; Kelk, J.; Rubio, M.; Fumagalli, S.; Sorokin, L.; Vivien, D.; Prinz, M. The niche matters: Origin, function and fate of CNS-associated macrophages during health and disease. Acta Neuropathol. 2024, 147, 37. [Google Scholar] [CrossRef] [PubMed]
- Amann, L.; Masuda, T.; Prinz, M. Mechanisms of myeloid cell entry to the healthy and diseased central nervous system. Nat. Immunol. 2023, 24, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and central nervous system–associated macrophages—From origin to disease modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Weller, R.O.; Sharp, M.M.; Christodoulides, M.; Carare, R.O.; Møllgård, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018, 135, 363–385. [Google Scholar] [CrossRef]
- Drieu, A.; Du, S.; Storck, S.E.; Rustenhoven, J.; Papadopoulos, Z.; Dykstra, T.; Zhong, F.; Kim, K.; Blackburn, S.; Mamuladze, T. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 2022, 611, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef]
- Johnston, J.B.; Silva, C.; Holden, J.; Warren, K.G.; Clark, A.W.; Power, C. Monocyte activation and differentiation augment human endogenous retrovirus expression: Implications for inflammatory brain diseases. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 2001, 50, 434–442. [Google Scholar] [CrossRef]
- Silvin, A.; Qian, J.; Ginhoux, F. Brain macrophage development, diversity and dysregulation in health and disease. Cell. Mol. Immunol. 2023, 20, 1277–1289. [Google Scholar] [CrossRef]
- Depreter, M.; Espeel, M.; Roels, F. Human peroxisomal disorders. Microsc. Res. Tech. 2003, 61, 203–223. [Google Scholar] [CrossRef]
- Gilchrist, K.W.; Gilbert, E.F.; Goldfarb, S.; Goll, U.; Spranger, J.W.; Opitz, J.M. Studies of malformation syndromes of man XIB: The cerebro-hepato-renal syndrome of Zellweger: Comparative pathology. Eur. J. Pediatr. 1976, 121, 99–118. [Google Scholar] [CrossRef]
- Mazzitelli, J.A.; Pulous, F.E.; Smyth, L.C.; Kaya, Z.; Rustenhoven, J.; Moskowitz, M.A.; Kipnis, J.; Nahrendorf, M. Skull bone marrow channels as immune gateways to the central nervous system. Nat. Neurosci. 2023, 26, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.; Frodermann, V.; Courties, G.; Rohde, D.; Sun, Y.; Vandoorne, K.; Wojtkiewicz, G.R.; Masson, G.S.; Vinegoni, C.; Kim, J. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 2018, 21, 1209–1217. [Google Scholar] [CrossRef]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Weinhofer, I.; Buda, A.; Kunze, M.; Palfi, Z.; Traunfellner, M.; Hesse, S.; Villoria-Gonzalez, A.; Hofmann, J.; Hametner, S.; Regelsberger, G. Peroxisomal very long-chain fatty acid transport is targeted by herpesviruses and the antiviral host response. Commun. Biol. 2022, 5, 944. [Google Scholar] [CrossRef]
- Lund, T.C.; Ng, M.; Orchard, P.J.; Loes, D.J.; Raymond, G.V.; Gupta, A.; Kenny-Jung, D.; Nascene, D.R. Volume of gadolinium enhancement and successful repair of the blood-brain barrier in cerebral adrenoleukodystrophy. Biol. Blood Marrow Transplant. 2020, 26, 1894–1899. [Google Scholar] [CrossRef]
- Lauer, A.; Speroni, S.L.; Choi, M.; Da, X.; Duncan, C.; McCarthy, S.; Krishnan, V.; Lusk, C.A.; Rohde, D.; Hansen, M.B. Hematopoietic stem-cell gene therapy is associated with restored white matter microvascular function in cerebral adrenoleukodystrophy. Nat. Commun. 2023, 14, 1900. [Google Scholar] [CrossRef]
- Bougnères, P.; Hacein-Bey-Abina, S.; Labik, I.; Adamsbaum, C.; Castaignède, C.; Bellesme, C.; Schmidt, M. Long-Term Follow-Up of Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. Hum. Gene Ther. 2021, 32, 1260–1269. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Tian, G.-F.; Han, X.; Peng, W.; Takano, T.; Ransom, B.; Nedergaard, M. Loss of astrocytic domain organization in the epileptic brain. J. Neurosci. 2008, 28, 3264–3276. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.-H.; Haydon, P.G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007, 27, 6473–6477. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Barros, L.F. The astrocyte: Powerhouse and recycling center. Cold Spring Harb. Perspect. Biol. 2015, 7, a020396. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Olsen, M.L.; Sofroniew, M.V. Reactive astrocytes and emerging roles in central nervous system (CNS) disorders. Cold Spring Harb. Perspect. Biol. 2024, 16, a041356. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Lu, N.; Gin, T.; Cheng, C.H.; Chan, M.T. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS ONE 2013, 8, e75804. [Google Scholar] [CrossRef]
- Stöberl, N.; Maguire, E.; Salis, E.; Shaw, B.; Hall-Roberts, H. Human iPSC-derived glia models for the study of neuroinflammation. J. Neuroinflammation 2023, 20, 231. [Google Scholar] [CrossRef]
- Lee, H.-G.; Rone, J.M.; Li, Z.; Akl, C.F.; Shin, S.W.; Lee, J.-H.; Flausino, L.E.; Pernin, F.; Chao, C.-C.; Kleemann, K.L. Disease-associated astrocyte epigenetic memory promotes CNS pathology. Nature 2024, 627, 865–872. [Google Scholar] [CrossRef]
- Lee, H.G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and microglia: In sickness and in health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key regulators of neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed]
- Hasel, P.; Rose, I.V.L.; Sadick, J.S.; Kim, R.D.; Liddelow, S.A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 2021, 24, 1475–1487. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. Control of autoimmune CNS inflammation by astrocytes. Semin. Immunopathol. 2015, 37, 625–638. [Google Scholar] [CrossRef]
- McKenna, O.; Arnold, G.; Holtzman, E. Microperoxisome distribution in the central nervous system of the rat. Brain Res. 1976, 117, 181–194. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Troffer-Charlier, N.; Doerflinger, N.; Metzger, E.; Fouquet, F.; Mandel, J.L.; Aubourg, P. Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur. J. Cell Biol. 1998, 75, 254–264. [Google Scholar] [CrossRef]
- Musolino, P.L.; Gong, Y.; Snyder, J.M.; Jimenez, S.; Lok, J.; Lo, E.H.; Moser, A.B.; Grabowski, E.F.; Frosch, M.P.; Eichler, F.S. Brain endothelial dysfunction in cerebral adrenoleukodystrophy. Brain 2015, 138, 3206–3220. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.; Da, X.; Hansen, M.B.; Boulouis, G.; Ou, Y.; Cai, X.; Liberato Celso Pedrotti, A.; Kalpathy-Cramer, J.; Caruso, P.; Hayden, D.L. ABCD1 dysfunction alters white matter microvascular perfusion. Brain 2017, 140, 3139–3152. [Google Scholar] [CrossRef]
- Musolino, P.L.; Rapalino, O.; Caruso, P.; Caviness, V.S.; Eichler, F.S. Hypoperfusion predicts lesion progression in cerebral X-linked adrenoleukodystrophy. Brain 2012, 135, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Weinhofer, I.; Kunze, M.; Forss-Petter, S.; Berger, J. Involvement of human peroxisomes in biosynthesis and signaling of steroid and peptide hormones. In Peroxisomes and Their Key Role in Cellular Signaling and Metabolism; Springer: Berlin/Heidelberg, Germany, 2013; pp. 101–110. [Google Scholar]
- Braverman, N.E.; Raymond, G.V.; Rizzo, W.B.; Moser, A.B.; Wilkinson, M.E.; Stone, E.M.; Steinberg, S.J.; Wangler, M.F.; Rush, E.T.; Hacia, J.G. Peroxisome biogenesis disorders in the Zellweger spectrum: An overview of current diagnosis, clinical manifestations, and treatment guidelines. Mol. Genet. Metab. 2016, 117, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cappa, M.; Todisco, T.; Bizzarri, C. X-linked adrenoleukodystrophy and primary adrenal insufficiency. Front. Endocrinol. 2023, 14, 1309053. [Google Scholar] [CrossRef]
- Powers, J.; Schaumburg, H.; Johnson, A.; Raine, C. A correlative study of the adrenal cortex in adreno-leukodystrophy--evidence for a fatal intoxication with very long chain saturated fatty acids. Investig. Cell Pathol. 1980, 3, 353–376. [Google Scholar]
- Powers, J.M.; Moser, H.W.; Moser, A.B.; Schaumburg, H.H. Fetal adrenoleukodystrophy: The significance of pathologic lesions in adrenal gland and testis. Hum. Pathol. 1982, 13, 1013–1019. [Google Scholar] [CrossRef]
- Whitcomb, R.; Linehan, W.; Knazek, R. Effects of long-chain, saturated fatty acids on membrane microviscosity and adrenocorticotropin responsiveness of human adrenocortical cells in vitro. J. Clin. Investig. 1988, 81, 185–188. [Google Scholar] [CrossRef]
- Buda, A.; Forss-Petter, S.; Hua, R.; Jaspers, Y.; Lassnig, M.; Waidhofer-Söllner, P.; Kemp, S.; Kim, P.; Weinhofer, I.; Berger, J. ABCD1 transporter deficiency results in altered cholesterol homeostasis. Biomolecules 2023, 13, 1333. [Google Scholar] [CrossRef]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef]
- Huffnagel, I.C.; Laheji, F.K.; Aziz-Bose, R.; Tritos, N.A.; Marino, R.; Linthorst, G.E.; Kemp, S.; Engelen, M.; Eichler, F. The natural history of adrenal insufficiency in X-linked adrenoleukodystrophy: An international collaboration. J. Clin. Endocrinol. Metab. 2019, 104, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Vieyres, G.; Beller, M.; Krahmer, N.; Bohnert, M. Lipid droplet contact sites in health and disease. Trends Cell Biol. 2021, 31, 345–358. [Google Scholar] [CrossRef]
- Chang, C.-L.; Weigel, A.V.; Ioannou, M.S.; Pasolli, H.A.; Xu, C.S.; Peale, D.R.; Shtengel, G.; Freeman, M.; Hess, H.F.; Blackstone, C. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J. Cell Biol. 2019, 218, 2583–2599. [Google Scholar] [CrossRef]
- Perdomini, M.; Dos Santos, C.; Goumeaux, C.; Blouin, V.; Bougnères, P. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21−/− mouse model. Gene Ther. 2017, 24, 275–281. [Google Scholar] [CrossRef]
- Tsuji, S.; Sano, T.; Ariga, T.; Miyatake, T. Increased synthesis of hexacosanoic acid (C26: 0) by cultured skin fibroblasts from patients with adrenoleukodystrophy (ALD) and adrenomyeloneuropathy (AMN). J. Biochem. 1981, 90, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Moser, A.E.; Moser, H.W.; Kishimoto, Y. Adrenoleukodystrophy: Impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr. Res. 1984, 18, 286–290. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B.; Kawamura, N.; Murphy, J.; Suzuki, K.; Schaumburg, H.; Kishimoto, Y. Adrenoleukodystrophy: Elevated C26 fatty acid in cultured skin fibroblasts. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 1980, 7, 542–549. [Google Scholar] [CrossRef]
- Wilson, R.; Tocher, D.R.; Sargent, J.R. Effects of exogenous monounsaturated fatty acids on fatty acid metabolism in cultured skin fibroblasts from adrenoleukodystrophy patients. J. Neurol. Sci. 1992, 109, 207–214. [Google Scholar] [CrossRef]
- Cartier, N.; Lopez, J.; Moullier, P.; Rocchiccioli, F.; Rolland, M.-O.; Jorge, P.; Mosser, J.; Mandel, J.-L.; Bougneres, P.-F.; Danos, O. Retroviral-mediated gene transfer corrects very-long-chain fatty acid metabolism in adrenoleukodystrophy fibroblasts. Proc. Natl. Acad. Sci. USA 1995, 92, 1674–1678. [Google Scholar] [CrossRef]
- Engelen, M.; Ofman, R.; Mooijer, P.; Poll-The, B.; Wanders, R.; Kemp, S. Cholesterol-deprivation increases mono-unsaturated very long-chain fatty acids in skin fibroblasts from patients with X-linked adrenoleukodystrophy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2008, 1781, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, W.B.; Avigan, J.; Chemke, J.; Schulman, J.D. Adrenoleukodystrophy: Very long-chain fatty acid metabolism in fibroblasts. Neurology 1984, 34, 163–169. [Google Scholar] [CrossRef]
- Singh, I.; Khan, M.; Key, L.; Pai, S. Lovastatin for X-linked adrenoleukodystrophy. N. Engl. J. Med. 1998, 339, 702–703. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hama, K.; Shimozawa, N.; Yokoyama, K. Glycosphingolipids with very long-chain fatty acids accumulate in fibroblasts from adrenoleukodystrophy patients. Int. J. Mol. Sci. 2021, 22, 8645. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.; Pras-Raves, M.L.; Ferdinandusse, S.; Vervaart, M.A.; Luyf, A.C.; van Kampen, A.H.; Wanders, R.J.; Waterham, H.R.; Vaz, F.M. Functional characterisation of peroxisomal β-oxidation disorders in fibroblasts using lipidomics. J. Inherit. Metab. Dis. 2018, 41, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, N.; Moser, A.B.; Moser, H.W.; Ogino, T.; Suzuki, K.; Schaumburg, H.; Milunsky, A.; Murphy, J.; Kishimoto, Y. High concentration of hexacosanoate in cultured skin fibroblast lipids from adrenoleukodystrophy patients. Biochem. Biophys. Res. Commun. 1978, 82, 114–120. [Google Scholar] [CrossRef]
- Flavigny, E.; Sanhaj, A.; Aubourg, P.; Cartier, N. Retroviral-mediated adrenoleukodystrophy-related gene transfer corrects very long chain fatty acid metabolism in adrenoleukodystrophy fibroblasts: Implications for therapy. FEBS Lett. 1999, 448, 261–264. [Google Scholar] [CrossRef]
- Engelen, M.; Schackmann, M.J.; Ofman, R.; Sanders, R.-J.; Dijkstra, I.M.; Houten, S.M.; Fourcade, S.; Pujol, A.; Poll-The, B.T.; Wanders, R.J. Bezafibrate lowers very long-chain fatty acids in X-linked adrenoleukodystrophy fibroblasts by inhibiting fatty acid elongation. J. Inherit. Metab. Dis. 2012, 35, 1137–1145. [Google Scholar] [CrossRef]
- van Geel, B.M.; Assies, J.; Wanders, R.J.; Barth, P.G. X linked adrenoleukodystrophy: Clinical presentation, diagnosis, and therapy. J. Neurol. Neurosurg. Psychiatry 1997, 63, 4–14. [Google Scholar] [CrossRef]
- Wanders, R.; Van Roermund, C.; Van Wijland, M.; Nijenhuis, A.; Tromp, A.; Schutgens, R.; Brouwer-Kelder, E.; Schram, A.; Tager, J.; Van den Bosch, H. X-linked adrenoleukodystrophy: Defective peroxisomal oxidation of very long chain fatty acids but not of very long chain fatty acyl-CoA esters. Clin. Chim. Acta 1987, 165, 321–329. [Google Scholar] [CrossRef]
- Feigenbaum, V.; Gélot, A.; Casanova, P.; Daumas-Duport, C.; Aubourg, P.; Dubois-Dalcq, M. Apoptosis in the central nervous system of cerebral adrenoleukodystrophy patients. Neurobiol. Dis. 2000, 7, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, P.; Dubois-Dalcq, M. X-linked adrenoleukodystrophy enigma: How does the ALD peroxisomal transporter mutation affect CNS glia? Glia 2000, 29, 186–190. [Google Scholar] [CrossRef]
- Igarashi, M.; Belchjs, D.; Suzuki, K. Brain gangliosides in adrenoleukodystrophy 1. J. Neurochem. 1976, 27, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Nudelman, E.; Hakomori, S.-I. Possible role of ceramide in defining structure and function of membrane glycolipids. Proc. Natl. Acad. Sci. USA 1982, 79, 3470–3474. [Google Scholar] [CrossRef]
- Kemp, S.; Wanders, R. Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathol. 2010, 20, 831–837. [Google Scholar] [CrossRef]
- Schlüter, A.; Sandoval, J.; Fourcade, S.; Díaz-Lagares, A.; Ruiz, M.; Casaccia, P.; Esteller, M.; Pujol, A. Epigenomic signature of adrenoleukodystrophy predicts compromised oligodendrocyte differentiation. Brain Pathol. 2018, 28, 902–919. [Google Scholar] [CrossRef] [PubMed]
- Che-Castaldo, J.; Havercamp, K.; Watanuki, K.; Matsuzawa, T.; Hirata, S.; Ross, S.R. Comparative survival analyses among captive chimpanzees (Pan troglodytes) in America and Japan. PeerJ 2021, 9, e11913. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Rodríguez-Pascau, L.; Vilalta, A.; Cerrada, M.; Traver, E.; Forss-Petter, S.; Weinhofer, I.; Bauer, J.; Kemp, S.; Pina, G.; Pascual, S. The brain penetrant PPARγ agonist leriglitazone restores multiple altered pathways in models of X-linked adrenoleukodystrophy. Sci. Transl. Med. 2021, 13, eabc0555. [Google Scholar] [CrossRef]
- Düsedau, H.P.; Steffen, J.; Figueiredo, C.A.; Boehme, J.D.; Schultz, K.; Erck, C.; Korte, M.; Faber-Zuschratter, H.; Smalla, K.-H.; Dieterich, D. Influenza A virus (H1N1) infection induces microglial activation and temporal dysbalance in glutamatergic synaptic transmission. MBio 2021, 12. [Google Scholar] [CrossRef]
- Ismail, F.S.; Faustmann, T.J.; Faustmann, P.M.; Corvace, F. Microglia as potential key regulators in viral-induced neuroinflammation. Front. Cell. Neurosci. 2024, 18, 1426079. [Google Scholar] [CrossRef] [PubMed]
- Sadasivan, S.; Zanin, M.; O’Brien, K.; Schultz-Cherry, S.; Smeyne, R.J. Induction of microglia activation after infection with the non-neurotropic A/CA/04/2009 H1N1 influenza virus. PLoS ONE 2015, 10, e0124047. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef]
- Jeong, G.U.; Lyu, J.; Kim, K.-D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.-H.; Ko, J.; Lee, J.-Y. SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol. Spectr. 2022, 10, e01091-22. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. The link between microglia and the severity of COVID-19: The “two-hit” hypothesis. J. Med. Virol. 2021, 93, 4111. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, E.A.; Amarilla, A.A.; Modhiran, N.; Parker, S.; Li, X.X.; Wijesundara, D.K.; Aguado, J.; Zamora, A.P.; McMillan, C.L.; Liang, B. SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol. Psychiatry 2023, 28, 2878–2893. [Google Scholar] [CrossRef]
- Matschke, J.; Lahann, H.; Krasemann, S.; Altmeppen, H.; Pfefferle, S.; Galliciotti, G.; Fitzek, A.; Sperhake, J.-P.; Ondruschka, B.; Busch, M. Young COVID-19 patients show a higher degree of microglial activation when compared to controls. Front. Neurol. 2022, 13, 908081. [Google Scholar] [CrossRef]
- McMillan, R.E.; Wang, E.; Carlin, A.F.; Coufal, N.G. Human microglial models to study host–virus interactions. Exp. Neurol. 2023, 363, 114375. [Google Scholar] [CrossRef]
- Golse, M.; Weinhofer, I.; Blanco, B.; Barbier, M.; Yazbeck, E.; Huiban, C.; Chaumette, B.; Pichon, B.; Fatemi, A.; Pascual, S. Leriglitazone halts disease progression in adult patients with early cerebral adrenoleukodystrophy. Brain 2024, 147, 3344–3351. [Google Scholar] [CrossRef]
- Chataway, J.; Wade, C.; Murphy, E.; Lynch, D.S. An alternative therapeutic approach to haematopoetic stem cell transplantation in early cerebral adrenoleukodystrophy. Brain 2024, 147, 3271–3273. [Google Scholar] [CrossRef]
- Köhler, W.; Engelen, M.; Eichler, F.; Lachmann, R.; Fatemi, A.; Sampson, J.; Salsano, E.; Gamez, J.; Molnar, M.J.; Pascual, S.; et al. Safety and efficacy of leriglitazone for preventing disease progression in men with adrenomyeloneuropathy (ADVANCE): A randomised, double-blind, multi-centre, placebo-controlled phase 2-3 trial. Lancet Neurol. 2023, 22, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Schöls, L. Leriglitazone: Frustration and hope in adrenoleukodystrophy. Lancet Neurol. 2023, 22, 103–105. [Google Scholar] [CrossRef]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A comprehensive atlas of immunological differences between humans, mice, and non-human primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef] [PubMed]
- Simões-Abade, M.B.; Patterer, M.; Nicaise, A.M.; Pluchino, S. Brain organoid methodologies to explore mechanisms of disease in progressive multiple sclerosis. Front. Cell. Neurosci. 2024, 18, 1488691. [Google Scholar] [CrossRef] [PubMed]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Okano, H.; Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 2022, 29, 189–208. [Google Scholar] [CrossRef]
- Scesa, G.; Adami, R.; Bottai, D. iPSC preparation and epigenetic memory: Does the tissue origin matter? Cells 2021, 10, 1470. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Q.; Zhou, J.; Bartlett, A.; Wang, B.-A.; Berube, P.; Tian, W.; Kenworthy, M.; Altshul, J.; Nery, J.R. Single-cell DNA methylome and 3D multi-omic atlas of the adult mouse brain. Nature 2023, 624, 366–377. [Google Scholar] [CrossRef]
- Tian, W.; Zhou, J.; Bartlett, A.; Zeng, Q.; Liu, H.; Castanon, R.; Kenworthy, M.; Altshul, J.; Valadon, C.; Aldridge, A. Single-cell DNA methylation and 3D genome architecture in the human brain. Science 2023, 382, eadf5357. [Google Scholar] [CrossRef]
- Baarine, M.; Khan, M.; Singh, A.; Singh, I. Functional characterization of IPSC-derived brain cells as a model for X-linked adrenoleukodystrophy. PLoS ONE 2015, 10, e0143238. [Google Scholar] [CrossRef]
- Wang, X.-M.; Yik, W.Y.; Zhang, P.; Lu, W.; Dranchak, P.K.; Shibata, D.; Steinberg, S.J.; Hacia, J.G. The gene expression profiles of induced pluripotent stem cells from individuals with childhood cerebral adrenoleukodystrophy are consistent with proposed mechanisms of pathogenesis. Stem Cell Res. Ther. 2012, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kang, H.C.; Kim, H.S.; Kim, J.Y.; Huh, Y.J.; Kim, D.S.; Yoo, J.E.; Lee, J.A.; Lim, B.; Lee, J. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann. Neurol. 2011, 70, 402–409. [Google Scholar] [CrossRef]
- Franklin, H.; Clarke, B.E.; Patani, R. Astrocytes and microglia in neurodegenerative diseases: Lessons from human in vitro models. Prog. Neurobiol. 2021, 200, 101973. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.A.; Fagiani, F.; Rowitch, D.H.; Absinta, M.; Reich, D.S. Novel human iPSC models of neuroinflammation in neurodegenerative disease and regenerative medicine. Trends Immunol. 2024, 45, 799–813. [Google Scholar] [CrossRef]
- Lanciotti, A.; Brignone, M.S.; Macioce, P.; Visentin, S.; Ambrosini, E. Human iPSC-derived astrocytes: A powerful tool to study primary astrocyte dysfunction in the pathogenesis of rare leukodystrophies. Int. J. Mol. Sci. 2021, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, J. Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes. Int. J. Mol. Sci. 2025, 26, 1576. [Google Scholar] [CrossRef]
- Lee, C.Z.; Kozaki, T.; Ginhoux, F. Studying tissue macrophages in vitro: Are iPSC-derived cells the answer? Nat. Rev. Immunol. 2018, 18, 716–725. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, C.; Shah, R.; Bermingham, K.; Hinkle, C.C.; Li, W.; Rodrigues, A.; Tabita-Martinez, J.; Millar, J.S.; Cuchel, M. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ. Res. 2015, 117, 17–28. [Google Scholar] [CrossRef]
- Gutbier, S.; Wanke, F.; Dahm, N.; Rümmelin, A.; Zimmermann, S.; Christensen, K.; Köchl, F.; Rautanen, A.; Hatje, K.; Geering, B. Large-scale production of human iPSC-derived macrophages for drug screening. Int. J. Mol. Sci. 2020, 21, 4808. [Google Scholar] [CrossRef] [PubMed]
- Poulis, N.; Martin, M.; Hoerstrup, S.P.; Emmert, M.Y.; Fioretta, E.S. Development of an iPSC-derived tissue-resident macrophage-based platform for the in vitro immunocompatibility assessment of human tissue engineered matrices. Sci. Rep. 2024, 14, 12171. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kozaki, T.; Lee, C.Z.W.; Thion, M.S.; Otsuka, M.; Lim, S.; Utami, K.H.; Fidan, K.; Park, D.S.; Malleret, B. Induced-pluripotent-stem-cell-derived primitive macrophages provide a platform for modeling tissue-resident macrophage differentiation and function. Immunity 2017, 47, 183–198.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Seo, H.S.; Armien, A.G.; Bates, F.S.; Tolar, J.; Azarin, S.M. Modeling and rescue of defective blood–brain barrier function of induced brain microvascular endothelial cells from childhood cerebral adrenoleukodystrophy patients. Fluids Barriers CNS 2018, 15, 9. [Google Scholar] [CrossRef]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef]
- Piwecka, M.; Rajewsky, N.; Rybak-Wolf, A. Single-cell and spatial transcriptomics: Deciphering brain complexity in health and disease. Nat. Rev. Neurol. 2023, 19, 346–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Wang, G.; Ma, Y. Advancements in Single-Cell RNA Sequencing and Spatial Transcriptomics for Central Nervous System Disease. Cell. Mol. Neurobiol. 2024, 44, 65. [Google Scholar] [CrossRef]
- Poisson, L.M.; Kaur, N.; Felicella, M.M.; Singh, J. System-based integrated metabolomics and microRNA analysis identifies potential molecular alterations in human X-linked cerebral adrenoleukodystrophy brain. Hum. Mol. Genet. 2023, 32, 3249–3262. [Google Scholar] [CrossRef]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef]
- Koerte, I.K.; Lin, A.P.; Willems, A.; Muehlmann, M.; Hufschmidt, J.; Coleman, M.J.; Green, I.; Liao, H.; Tate, D.F.; Wilde, E.A.; et al. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015, 25, 318–349. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Velayudhan, P.S.; Schwab, N.; Hazrati, L.N.; Wheeler, A.L. Temporal patterns of microglial activation in white matter following experimental mild traumatic brain injury: A systematic literature review. Acta Neuropathol. Commun. 2021, 9, 197. [Google Scholar] [CrossRef]
- Pybus, A.F.; Bitarafan, S.; Brothers, R.O.; Rohrer, A.; Khaitan, A.; Moctezuma, F.R.; Udeshi, K.; Davies, B.; Triplett, S.; Griffin, M.N.; et al. Profiling the neuroimmune cascade in 3xTg-AD mice exposed to successive mild traumatic brain injuries. J. Neuroinflammation 2024, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Morganti-Kossman, M.C.; Lenzlinger, P.M.; Hans, V.; Stahel, P.; Csuka, E.; Ammann, E.; Stocker, R.; Trentz, O.; Kossmann, T. Production of cytokines following brain injury: Beneficial and deleterious for the damaged tissue. Mol. Psychiatry 1997, 2, 133–136. [Google Scholar] [CrossRef]
- Gihring, A.; Gärtner, F.; Schirmer, M.; Wabitsch, M.; Knippschild, U. Recent Developments in Mouse Trauma Research Models: A Mini-Review. Front. Physiol. 2022, 13, 866617. [Google Scholar] [CrossRef]
- Kahriman, A.; Bouley, J.; Smith, T.W.; Bosco, D.A.; Woerman, A.L.; Henninger, N. Mouse closed head traumatic brain injury replicates the histological tau pathology pattern of human disease: Characterization of a novel model and systematic review of the literature. Acta Neuropathol. Commun. 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.; Harding, A.T. Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses. Viruses 2023, 15, 261. [Google Scholar] [CrossRef]
- Feng, G.; Jensen, F.E.; Greely, H.T.; Okano, H.; Treue, S.; Roberts, A.C.; Fox, J.G.; Caddick, S.; Poo, M.M.; Newsome, W.T.; et al. Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proc. Natl. Acad. Sci. USA 2020, 117, 24022–24031. [Google Scholar] [CrossRef]
- Okano, H. Current status of and perspectives on the application of marmosets in neurobiology. Annu. Rev. Neurosci. 2021, 44, 27–48. [Google Scholar] [CrossRef]
- Boche, D.; Gerhard, A.; Rodriguez-Vieitez, E.; Faculty, M. Prospects and challenges of imaging neuroinflammation beyond TSPO in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2831–2847. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, R.; Cerdán Cerdá, A.; Trouve Carpena, A.; Drakesmith, M.; Koller, K.; Jones, D.K.; Canals, S.; De Santis, S. Mapping microglia and astrocyte activation in vivo using diffusion MRI. Sci. Adv. 2022, 8, eabq2923. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Z.; Wu, Y.; Zheng, N.; Liu, X.; Cai, A.; Zheng, D.; Zhu, J.; Wu, J.; Xu, L. In vivo imaging of astrocytes in the whole brain with engineered AAVs and diffusion-weighted magnetic resonance imaging. Mol. Psychiatry 2024, 29, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ligneul, C.; Palombo, M.; Hernández-Garzón, E.; Carrillo-de Sauvage, M.-A.; Flament, J.; Hantraye, P.; Brouillet, E.; Bonvento, G.; Escartin, C.; Valette, J. Diffusion-weighted magnetic resonance spectroscopy enables cell-specific monitoring of astrocyte reactivity in vivo. NeuroImage 2019, 191, 457–469. [Google Scholar] [CrossRef]
- Genovese, G.; Palombo, M.; Santin, M.D.; Valette, J.; Ligneul, C.; Aigrot, M.S.; Abdoulkader, N.; Langui, D.; Millecamps, A.; Baron-Van Evercooren, A. Inflammation-driven glial alterations in the cuprizone mouse model probed with diffusion-weighted magnetic resonance spectroscopy at 11.7 T. NMR Biomed. 2021, 34, e4480. [Google Scholar] [CrossRef]
- De Marco, R.; Ronen, I.; Branzoli, F.; Amato, M.L.; Asllani, I.; Colasanti, A.; Harrison, N.A.; Cercignani, M. Diffusion-weighted MR spectroscopy (DW-MRS) is sensitive to LPS-induced changes in human glial morphometry: A preliminary study. Brain Behav. Immun. 2022, 99, 256–265. [Google Scholar] [CrossRef]
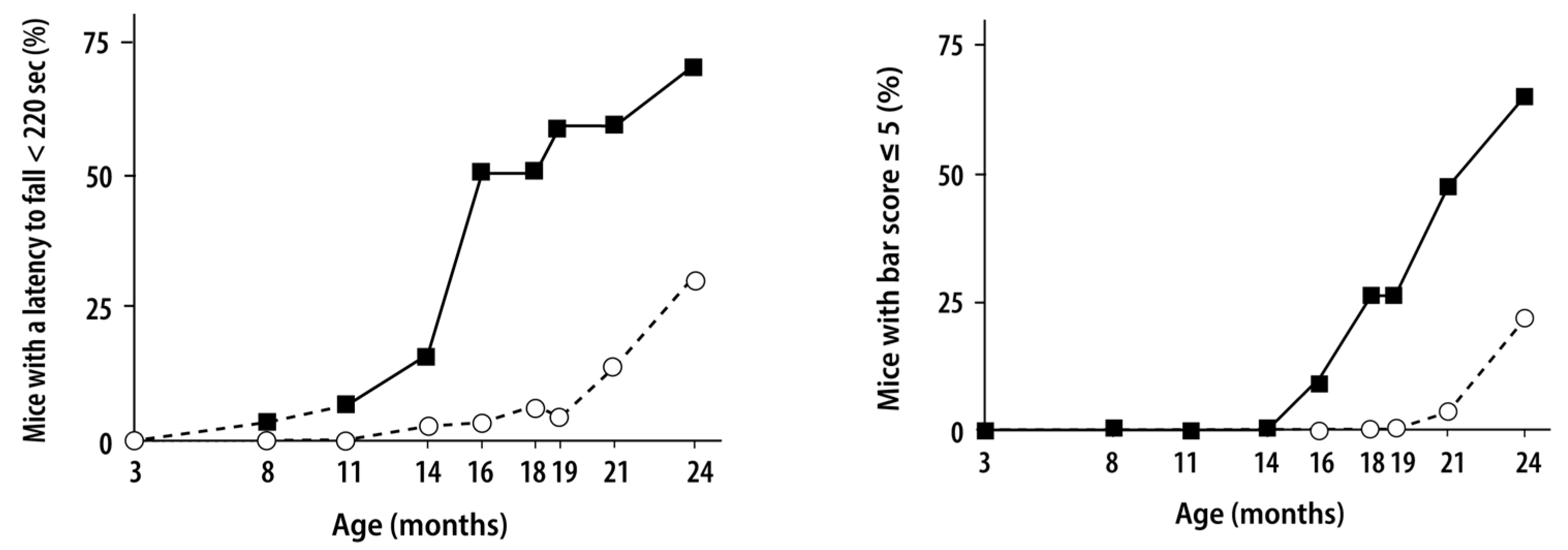
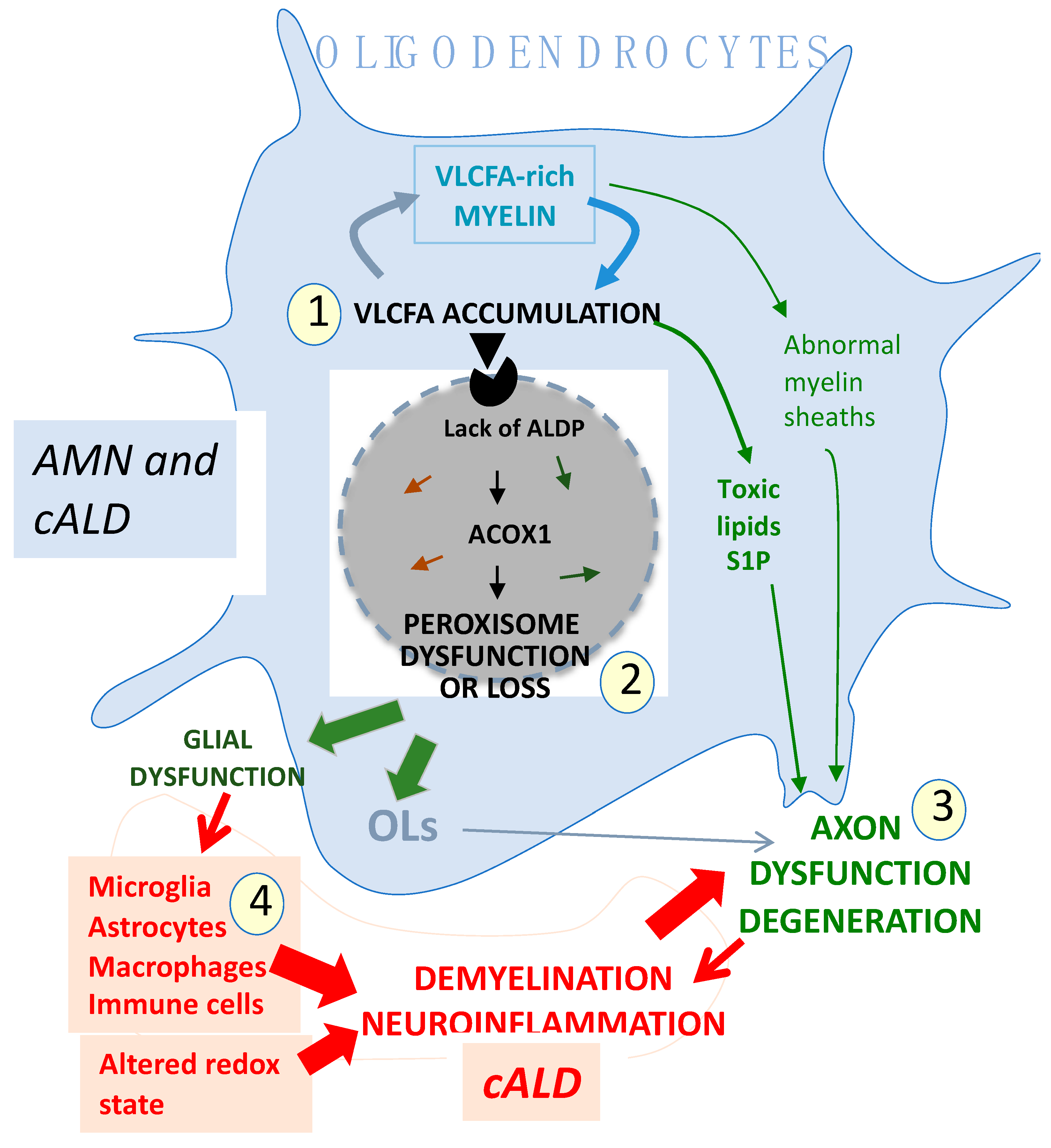
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougnères, P.; Le Stunff, C. Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy. Genes 2025, 16, 590. https://doi.org/10.3390/genes16050590
Bougnères P, Le Stunff C. Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy. Genes. 2025; 16(5):590. https://doi.org/10.3390/genes16050590
Chicago/Turabian StyleBougnères, Pierre, and Catherine Le Stunff. 2025. "Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy" Genes 16, no. 5: 590. https://doi.org/10.3390/genes16050590
APA StyleBougnères, P., & Le Stunff, C. (2025). Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy. Genes, 16(5), 590. https://doi.org/10.3390/genes16050590




