Genetic Analysis Reveals a Protective Effect of Sphingomyelin on Cholelithiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Genetic Instrument Selection
2.4. TSMR and MVMR Analyses
2.5. Reverse and Mediated Mendelian Randomization Analysis
2.6. LDSC and Colocalization Analysis
2.7. Gene Enrichment Analysis
3. Results
3.1. Significant Signals Were Identified for Causal Relationship Between Sphingomyelin and Cholelithiasis
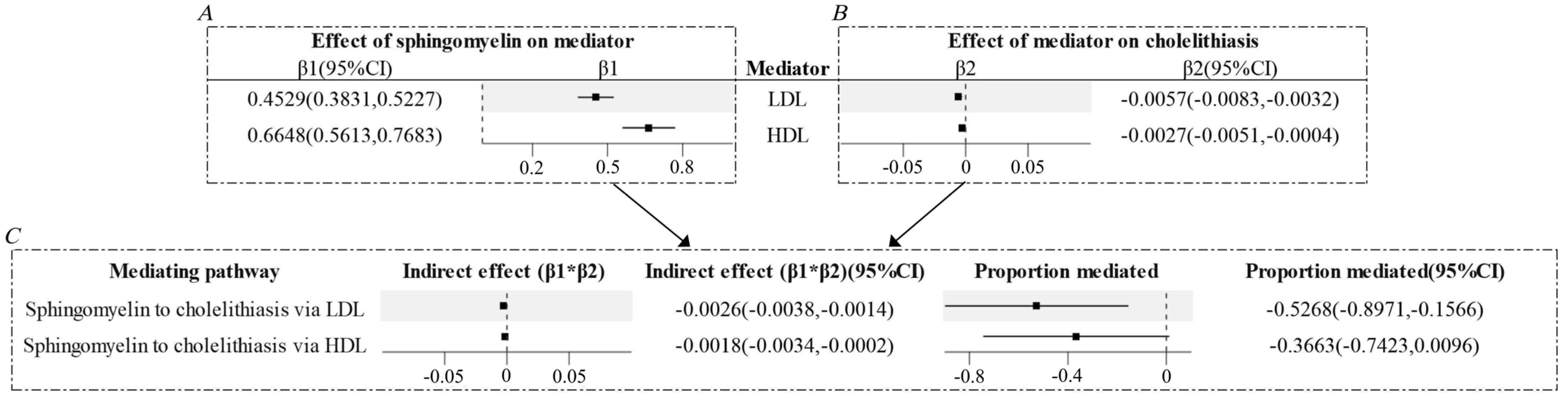
3.2. Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) Might Mediate Part of the Causal Relationship Between Sphingomyelin and Cholelithiasis
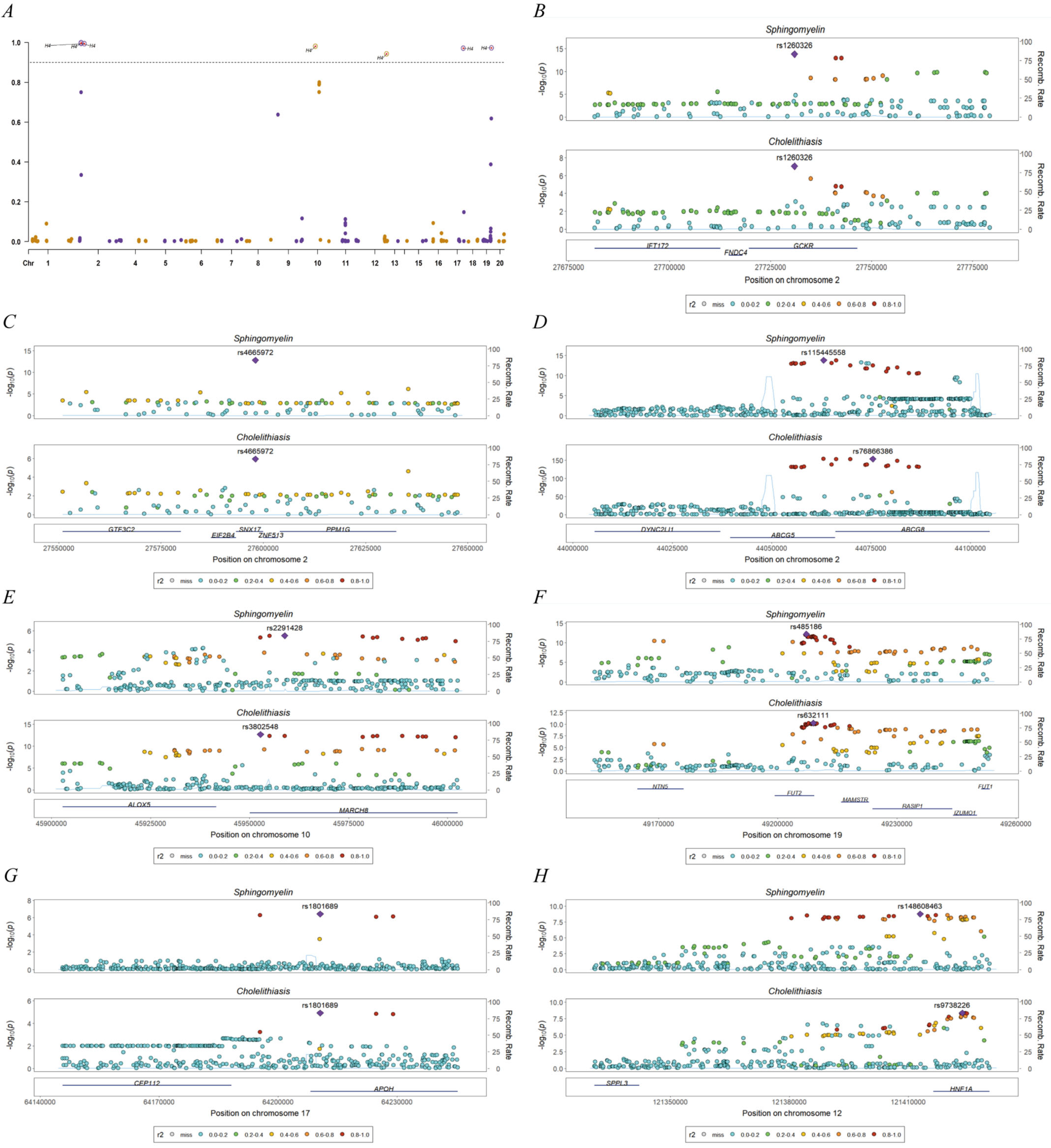
3.3. Bioinformatics Evidence for Unraveling the Significant Causal Signal Between Sphingomyelin and Cholelithiasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MR | Mendelian randomization |
| SNPs | Single-nucleotide polymorphisms |
| GWAS | Genome-wide association studies |
| IVs | Instrumental variables |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| CAD | Coronary artery disease |
| LDSC | Linkage disequilibrium score regression |
| LD | Linkage disequilibrium |
| TSMR | Two-sample Mendelian randomization |
| MVMR | Multivariate Mendelian randomization |
| IVW | Inverse variance weighted |
| GCKR | Glucokinase regulatory protein |
| SNX17 | Sorting nexin-17 |
| ABCG8 | ATP-binding cassette sub-family G member 5 |
| MARCH8 | Membrane-associated ring-CH-type finger 8 |
| FUT2 | Fucosyltransferase 2 |
| APOH | Apolipoprotein H |
| HNF1A | Hepatocyte nuclear factor 1α |
References
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; Van Erpecum, K.J.; Van Laarhoven, C.J.; Wang, D.Q.H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, A.; Lenahan, C. Cholelithiasis: Presentation and Management. J. Midwifery Womens Health 2019, 64, 289–297. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.M.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Dietary choline—Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.R.; Jiang, L.J.; Liu, Z.Y.; Wu, X.; Zhao, N.; Wang, Y.Z.; Bai, X.Y. Identification of blood metabolites linked to the risk of cholelithiasis: A comprehensive Mendelian randomization study. Hepatol. Int. 2022, 16, 1484–1493. [Google Scholar] [CrossRef]
- Lin, J.F.; Zhou, J.W.; Xu, Y. Potential drug targets for multiple sclerosis identified through Mendelian randomization analysis. Brain 2023, 146, 3364–3372. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, Q.X.; Cao, Z.Q.; Wu, J.H. Multicenter proteome-wide Mendelian randomization study identifies causal plasma proteins in melanoma and non-melanoma skin cancers. Commun. Biol. 2024, 7, 857. [Google Scholar] [CrossRef]
- Li, H.R.; Du, S.; Dai, J.L.; Jiang, Y.K.; Li, Z.M.; Fan, Q.H.; Zhang, Y.X.; You, D.F.; Zhang, R.Y.; Zhao, Y.; et al. Proteome-wide Mendelian randomization identifies causal plasma proteins in lung cancer. Iscience 2024, 27, 108985. [Google Scholar] [CrossRef]
- Li, H.B.; Zhang, Z.; Qiu, Y.T.; Weng, H.Y.; Yuan, S.; Zhang, Y.X.; Zhang, Y.; Xi, L.F.; Xu, F.Y.; Ji, X.F.; et al. Proteome-wide mendelian randomization identifies causal plasma proteins in venous thromboembolism development. J. Hum. Genet. 2023, 68, 805–812. [Google Scholar] [CrossRef]
- Yeung, S.L.A.; Gill, D. Standardizing the reporting of Mendelian randomization studies. BMC Med. 2023, 21, 187. [Google Scholar] [CrossRef]
- Lyon, M.S.; Andrews, S.J.; Elsworth, B.; Gaunt, T.R.; Hemani, G.; Marcora, E. The variant call format provides efficient and robust storage of GWAS summary statistics. Genome Biol. 2021, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Smith, G.D.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef]
- Mbatchou, J.; Barnard, L.; Backman, J.; Marcketta, A.; Kosmicki, J.A.; Ziyatdinov, A.; Benner, C.; O’Dushlaine, C.; Barber, M.; Boutkov, B.; et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat. Genet. 2021, 53, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J.; et al. PhenoScanner: A Database of Human Genotype-Phenotype Associations. Bioinformatics 2016, 32, 3207–3209. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA-J. Am. Med. Assoc. 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Smith, G.D. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Smith, G.D.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Araujo, H.A.; Cooper, A.B.; Hassan, M.A.; Venditti, J. Estimating suspended sediment concentrations in areas with limited hydrological data using a mixed-effects model. Hydrol. Process. 2012, 26, 3678–3688. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Tilling, K.; Smith, G.D. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ-Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.Y.; Moser, G.; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Wray, N.R.; Lee, S. Estimation of Genetic Correlation via Linkage Disequilibrium Score Regression and Genomic Restricted Maximum Likelihood. Am. J. Hum. Genet. 2018, 102, 1185–1194. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M.; Schizophrenia Working, G. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.E.; Franke, L.; Hingorani, A.D.; Wallace, C.; Plagnol, V. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Jin, L.; Guo, T.T.; Li, Z.X.; Lei, Z.; Li, H.; Mao, Y.Q.; Wang, X.; Zhou, N.; Zhang, Y.Z.; Hu, R.B.; et al. Role of Glucokinase in the Subcellular Localization of Glucokinase Regulatory Protein. Int. J. Mol. Sci. 2015, 16, 7377–7393. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, F.N.; Tan, F.S.; Tang, L.; Du, Z.Y.; Mou, J.; Zhou, G.; Yuan, C.F. HNF1A-AS1: A Tumor-associated Long Non-coding RNA. Curr. Pharm. Des. 2022, 28, 1720–1729. [Google Scholar] [CrossRef]
- Fong, V.; Patel, S.B. Recent advances in ABCG5 and ABCG8 variants. Curr. Opin. Lipidol. 2021, 32, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Choi, H.S.; Jun, D.W.; Yoo, K.S.; Lee, J.; Yang, S.Y.; Kuver, R. ATP-Binding Cassette Sterol Transporters Are Differentially Expressed in Normal and Diseased Human Gallbladder. Dig. Dis. Sci. 2013, 58, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ellard, S.; Colclough, K. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha (HNF1A) and 4 alpha (HNF4A) in maturity-onset diabetes of the young. Hum. Mutat. 2006, 27, 854–869. [Google Scholar] [CrossRef]
- Bonetti, S.; Trombetta, M.; Boselli, M.L.; Turrini, F.; Malerba, G.; Trabetti, E.; Pignatti, P.F.; Bonora, E.; Bonadonna, R.C. Variants of GCKR Affect Both β-Cell and Kidney Function in Patients with Newly Diagnosed Type 2 Diabetes The Verona Newly Diagnosed Type 2 Diabetes Study 2. Diabetes Care 2011, 34, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Bloks, V.W.; Bakker-van Waarde, W.M.; Verkade, H.J.; Kema, I.P.; Wolters, H.; Vink, E.; Groen, A.K.; Kuipers, F. Down-regulation of hepatic and intestinal Abcg5 and Abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia 2004, 47, 104–112. [Google Scholar] [CrossRef]
- Katz, L.A.; Spiro, H.M. Gastrointestinal Manifestations of Diabetes. N. Engl. J. Med. 1966, 275, 1350–1361. [Google Scholar] [CrossRef]
- Lee, K.S.; Rim, J.H.; Lee, Y.H.; Lee, S.G.; Lim, J.B.; Kim, J.H. Association of circulating metabolites with incident type 2 diabetes in an obese population from a national cohort. Diabetes Res. Clin. Pract. 2021, 180, 109077. [Google Scholar] [CrossRef]
- Barlovic, D.P.; Harjutsalo, V.; Sandholm, N.; Forsblom, C.; Groop, P.H.; FinnDiane Study Group. Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 2020, 63, 1847–1856. [Google Scholar] [CrossRef]

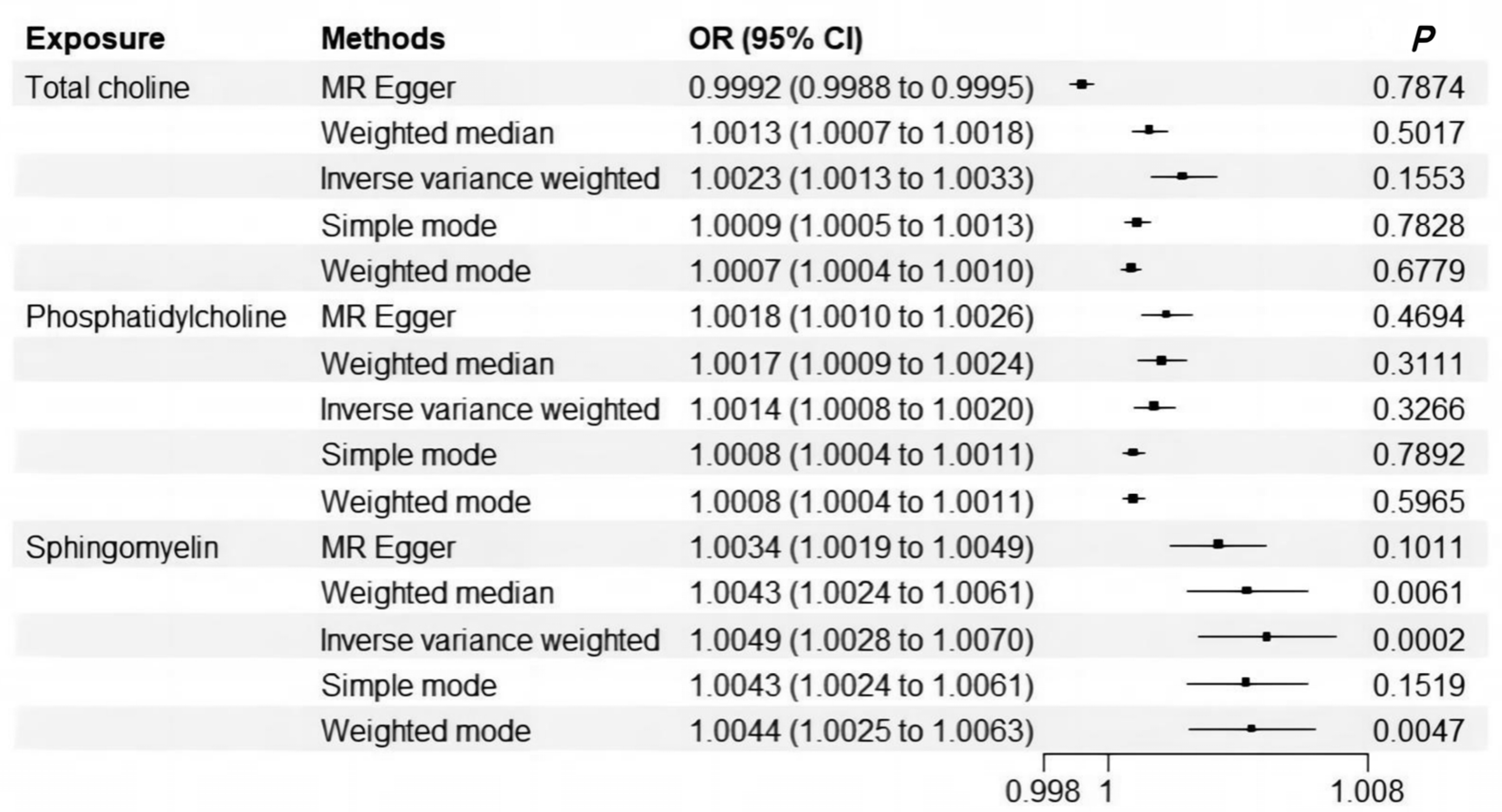
| Outcome | Exposure | nSNPs | MVMR-IVW | MVMR-Egger | p for MR-Egger Intercept | F-Value | ||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||||
| Cholelithiasis | HDL | 315 | 1.0000 (1.0000 to 1.0000) | 0.9990 | 1.0052 (1.0029 to 1.0074) | 0.0122 | 0.0002 | 17.6444 |
| Total choline | 0.9961 (0.9944 to 0.9978) | 0.0590 | 0.9956 (0.9937 to 0.9975) | 0.0308 | 11.2519 | |||
| LDL | 90 | 0.9931 (0.9901 to 0.9961) | 0.0671 | 0.9932 (0.9903 to 0.9962) | 0.1200 | 0.9559 | 52.8175 | |
| Total choline | 0.9977 (0.9967 to 0.9987) | 0.5898 | 0.9978 (0.9969 to 0.9988) | 0.6240 | 47.1951 | |||
| Triglyceride | 275 | 1.0002 (1.0001 to 1.0002) | 0.8990 | 0.9964 (0.9948 to 0.9979) | 0.0450 | 0.0039 | 116.1858 | |
| Total choline | 0.9984 (0.9978 to 0.9991) | 0.3330 | 0.9982 (0.9974 to 0.9990) | 0.2580 | 22.2594 | |||
| CAD | 84 | 0.9990 (0.9985 to 0.9994) | 0.4017 | 0.9976 (0.9965 to 0.9986) | 0.1762 | 0.2870 | 30.1093 | |
| Total choline | 0.9956 (0.9936 to 0.9975) | 0.0083 | 0.9951 (0.9930 to 0.9972) | 0.0047 | 78.4745 | |||
| HDL | 316 | 0.9994 (0.9991 to 0.9996) | 0.6820 | 1.0043 (1.0024 to 1.0061) | 0.0388 | 0.0004 | 20.3171 | |
| Phosphatidylcholine | 0.9976 (0.9965 to 0.9986) | 0.2170 | 0.9971 (0.9958 to 0.9984) | 0.1313 | 12.9910 | |||
| LDL | 91 | 0.9926 (0.9894 to 0.9958) | 0.0404 | 0.9924 (0.9891 to 0.9957) | 0.0779 | 0.9270 | 68.9316 | |
| Phosphatidylcholine | 0.9989 (0.9985 to 0.9994) | 0.7796 | 0.9988 (0.9983 to 0.9993) | 0.7696 | 58.3947 | |||
| Triglyceride | 277 | 1.0004 (1.0002 to 1.0005) | 0.7690 | 0.9964 (0.9949 to 0.9980) | 0.0502 | 0.0027 | 108.2092 | |
| Phosphatidylcholine | 0.9986 (0.9980 to 0.9992) | 0.3500 | 0.9984 (0.9977 to 0.9991) | 0.2956 | 25.0179 | |||
| CAD | 84 | 0.9988 (0.9982 to 0.9993) | 0.3477 | 0.9973 (0.9962 to 0.9985) | 0.1669 | 0.3105 | 29.6764 | |
| Phosphatidylcholine | 0.9971 (0.9958 to 0.9984) | 0.0868 | 0.9967 (0.9952 to 0.9981) | 0.0577 | 88.3187 | |||
| HDL | 309 | 1.0028 (1.0016 to 1.0040) | 0.3119 | 1.0096 (1.0054 to 1.0137) | 0.0051 | 0.0009 | 9.2775 | |
| Sphingomyelin | 0.9907 (0.9866 to 0.9947) | 0.0146 | 0.9901 (0.9858 to 0.9944) | 0.0089 | 6.8220 | |||
| LDL | 90 | 0.9945 (0.9921 to 0.9969) | 0.2890 | 0.9947 (0.9924 to 0.9970) | 0.3420 | 0.9368 | 23.9513 | |
| Sphingomyelin | 0.9969 (0.9956 to 0.9983) | 0.5530 | 0.9970 (0.9957 to 0.9983) | 0.5710 | 24.7277 | |||
| Triglyceride | 273 | 0.9989 (0.9984 to 0.9994) | 0.5529 | 0.9953 (0.9933 to 0.9973) | 0.0972 | 0.0895 | 66.8256 | |
| Sphingomyelin | 0.9943 (0.9919 to 0.9968) | 0.0297 | 0.9940 (0.9914 to 0.9966) | 0.0212 | 19.0968 | |||
| CAD | 90 | 0.9982 (0.9974 to 0.9990) | 0.4367 | 0.9983 (0.9976 to 0.9991) | 0.6246 | 0.9627 | 28.3273 | |
| Sphingomyelin | 0.9923 (0.9889 to 0.9957) | 0.0231 | 0.9923 (0.9890 to 0.9957) | 0.0273 | 67.9384 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, K.; Li, A.; Liu, H.; Gao, Y.; Wang, Z.; Wang, X.; Liu, S.; Gao, Z.; Quan, J.; Shao, M.; et al. Genetic Analysis Reveals a Protective Effect of Sphingomyelin on Cholelithiasis. Genes 2025, 16, 523. https://doi.org/10.3390/genes16050523
Mao K, Li A, Liu H, Gao Y, Wang Z, Wang X, Liu S, Gao Z, Quan J, Shao M, et al. Genetic Analysis Reveals a Protective Effect of Sphingomyelin on Cholelithiasis. Genes. 2025; 16(5):523. https://doi.org/10.3390/genes16050523
Chicago/Turabian StyleMao, Kun, Ang Li, Haochen Liu, Yuntong Gao, Ziyan Wang, Xisu Wang, Shixuan Liu, Ziyuan Gao, Jiaqi Quan, Moyan Shao, and et al. 2025. "Genetic Analysis Reveals a Protective Effect of Sphingomyelin on Cholelithiasis" Genes 16, no. 5: 523. https://doi.org/10.3390/genes16050523
APA StyleMao, K., Li, A., Liu, H., Gao, Y., Wang, Z., Wang, X., Liu, S., Gao, Z., Quan, J., Shao, M., Liu, Y., Shi, L., Zhang, B., & Zhang, T. (2025). Genetic Analysis Reveals a Protective Effect of Sphingomyelin on Cholelithiasis. Genes, 16(5), 523. https://doi.org/10.3390/genes16050523






