Identification and Functional Validation of ACSL1 and FABP3 as Muscle-Related Genes Screened by Transcriptomics in Crossbred Duroc × Berkshire × Diannan Small-Eared Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Materials
2.2. Section Preparation and HE Staining
2.3. ELISA
2.4. Cell Culture
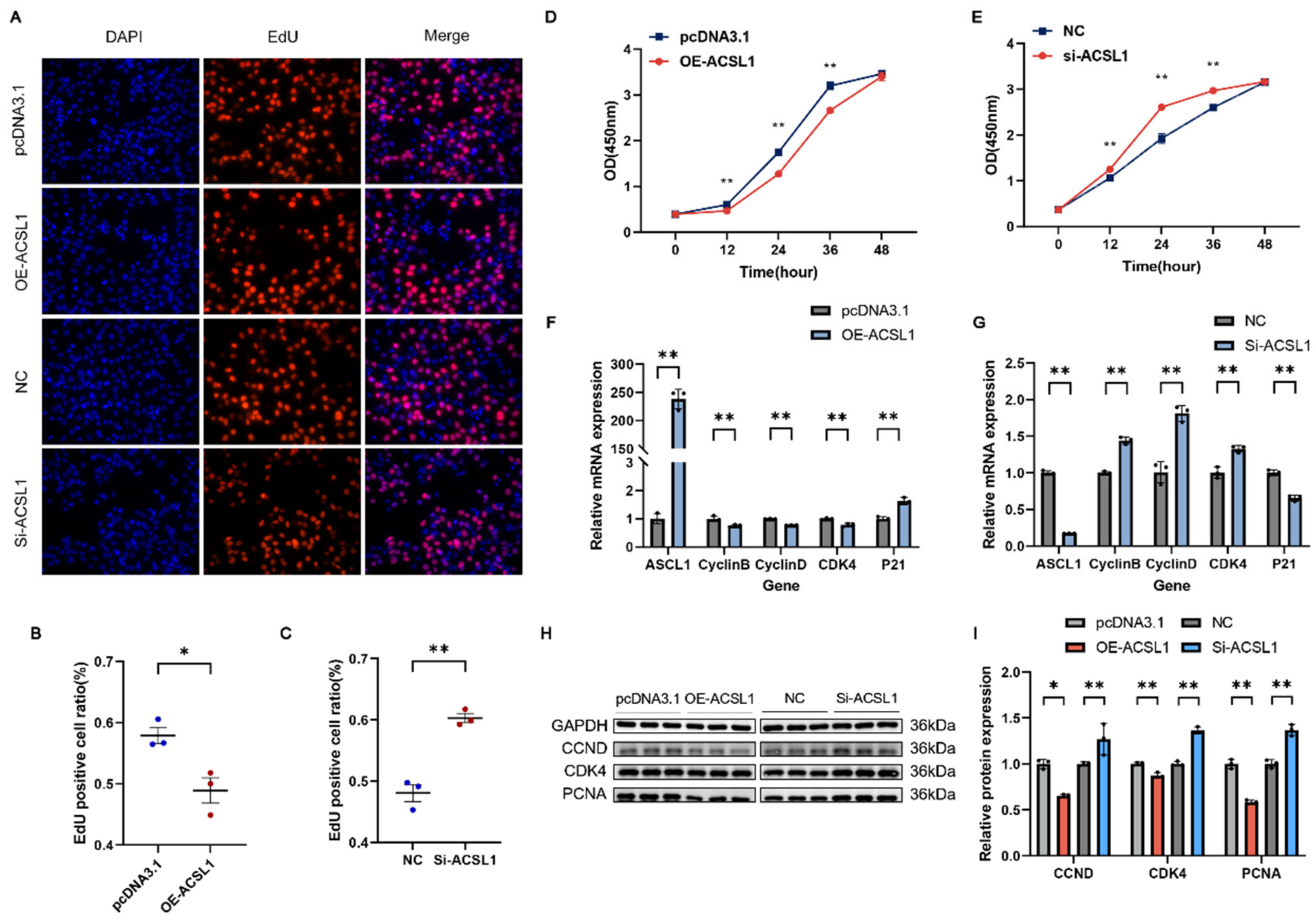
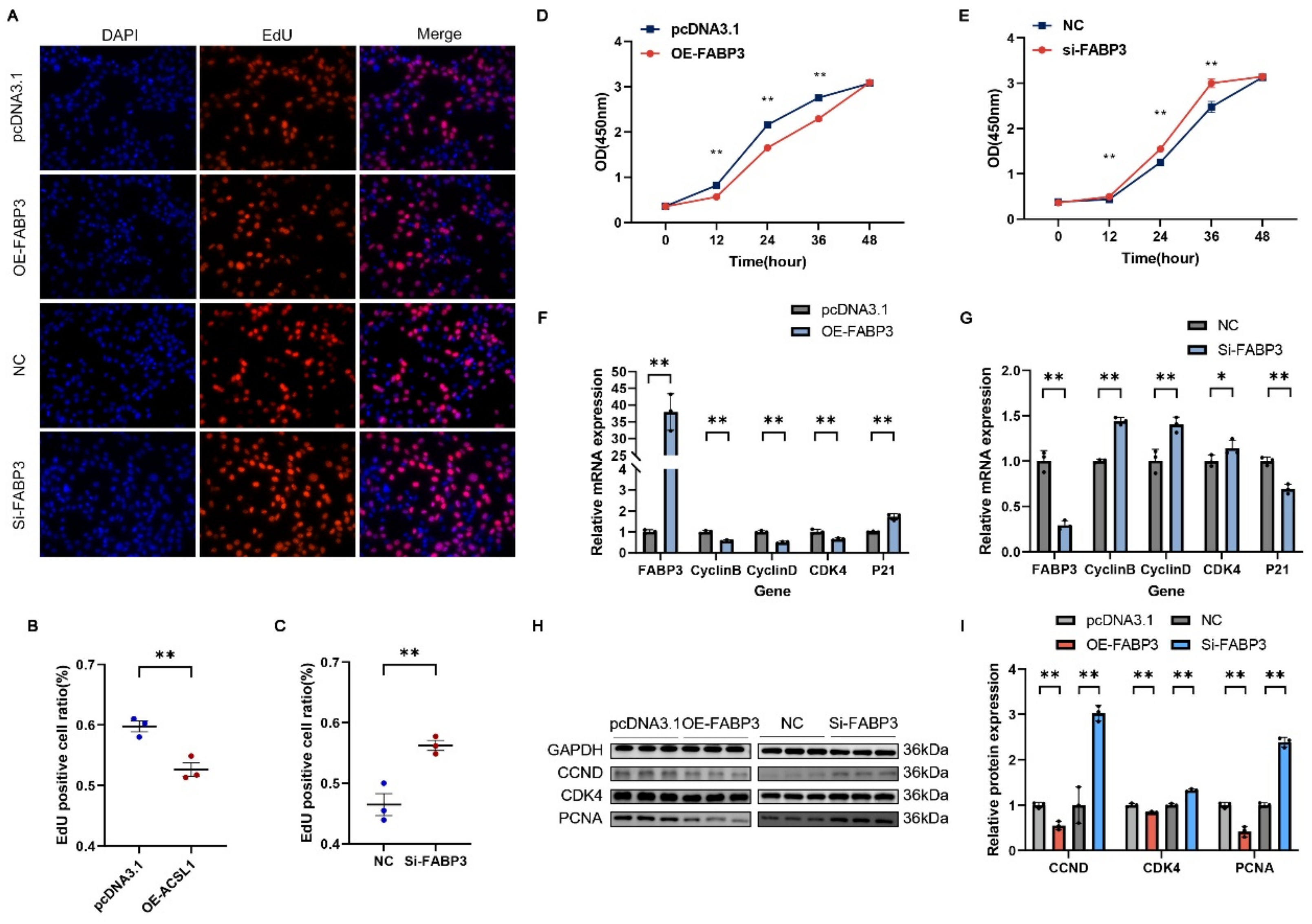
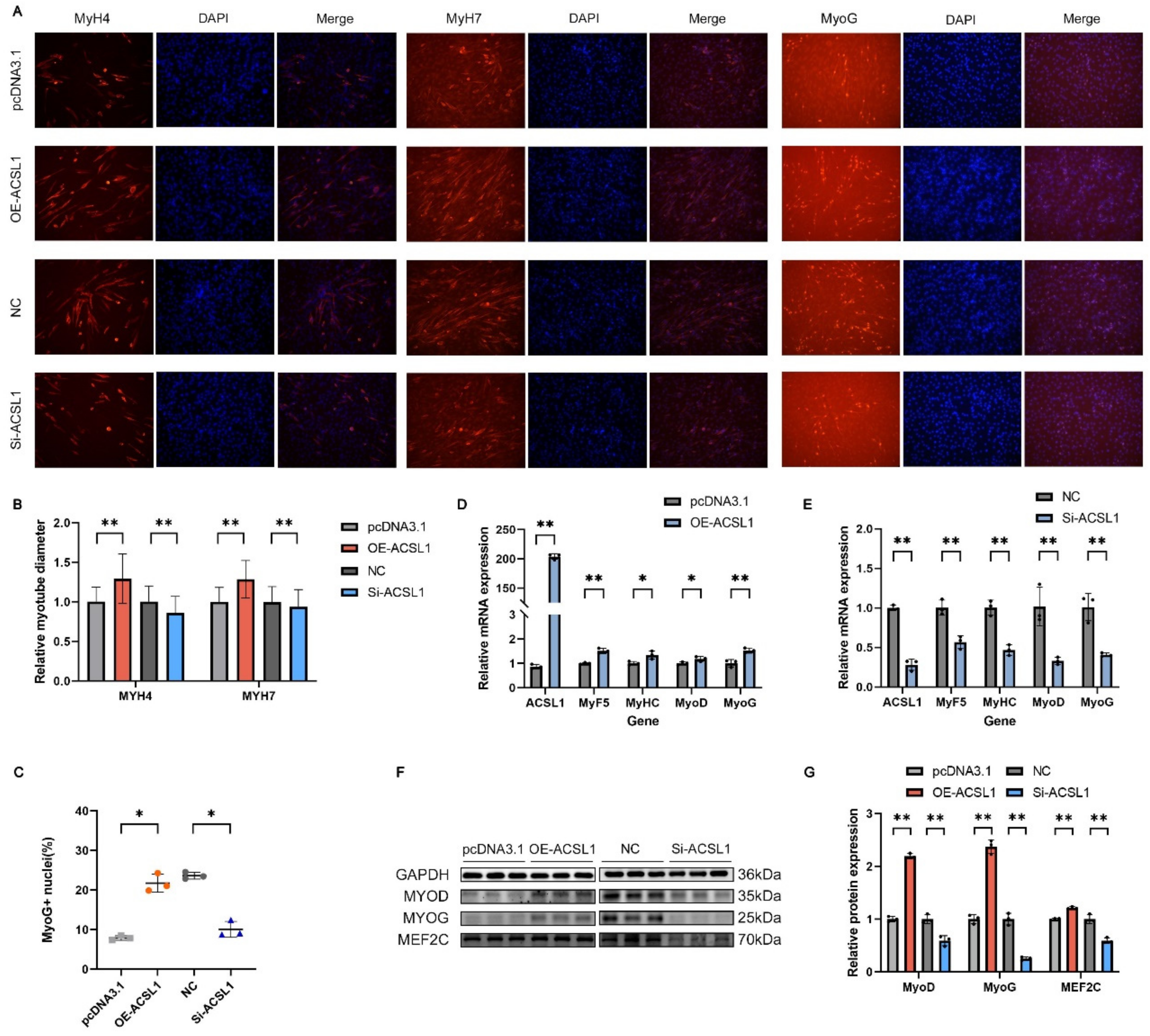
2.5. RNA Extraction and Quantitative PCR Analysis
2.6. Cell Proliferation Analysis via CCK-8 Kit
2.7. EdU Analysis
2.8. Fluorescent Immunolabeling
2.9. Western Blot Assay
3. Results
3.1. Morphological Variations and ELISA Results
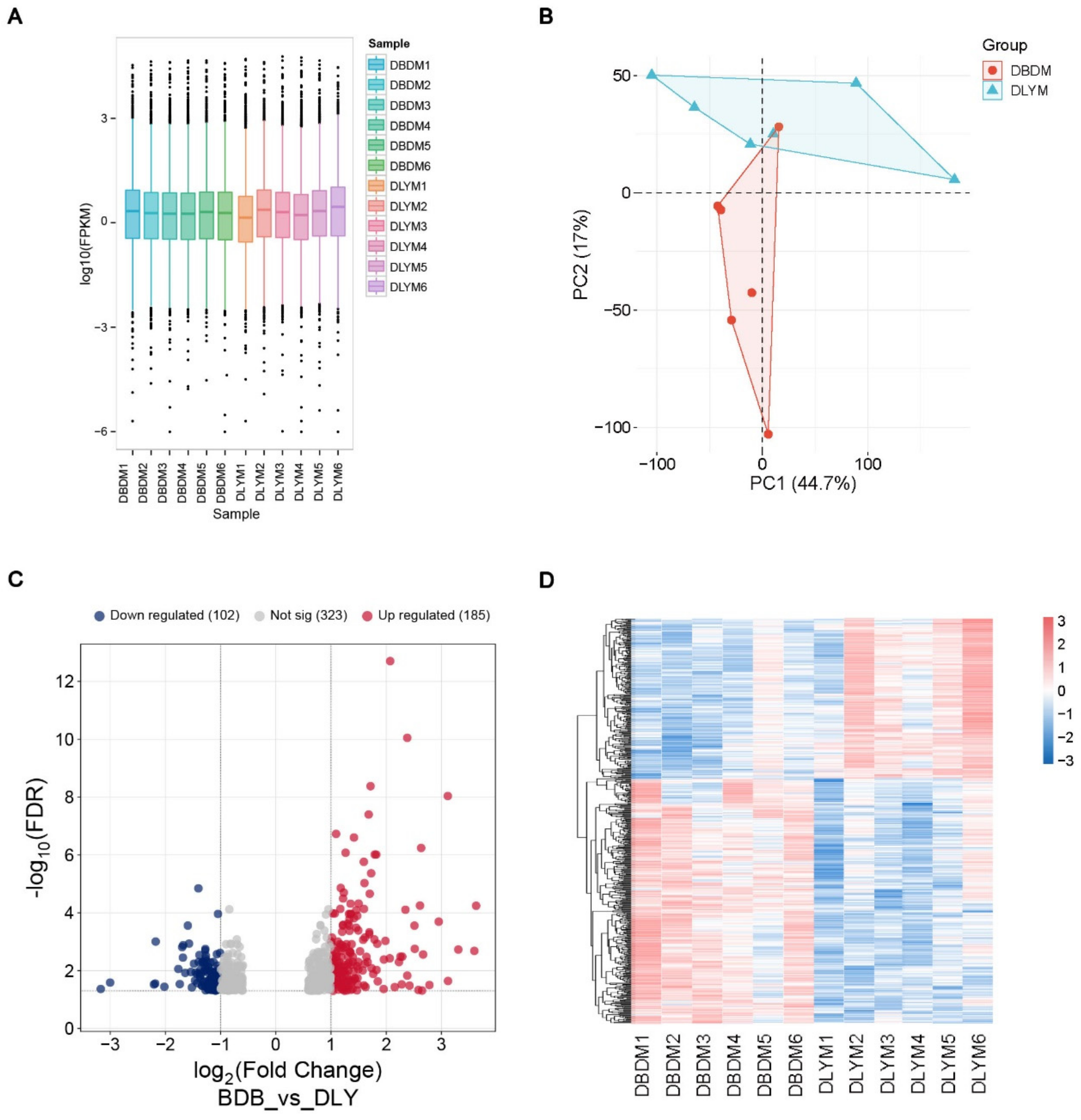
3.2. DEGs in the Longissimus Dorsi Muscle of DLY and DBD
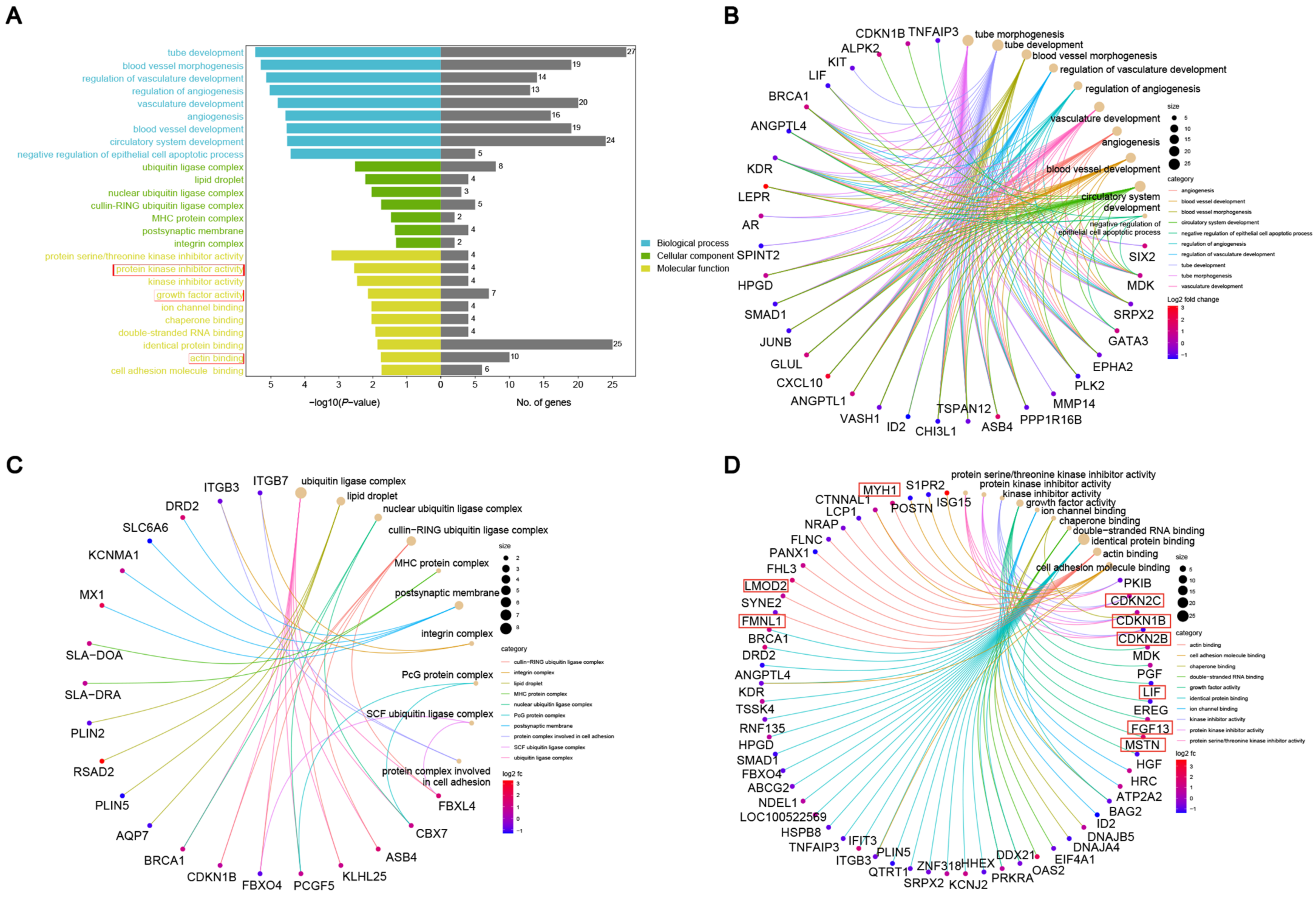
3.3. GO Enrichment
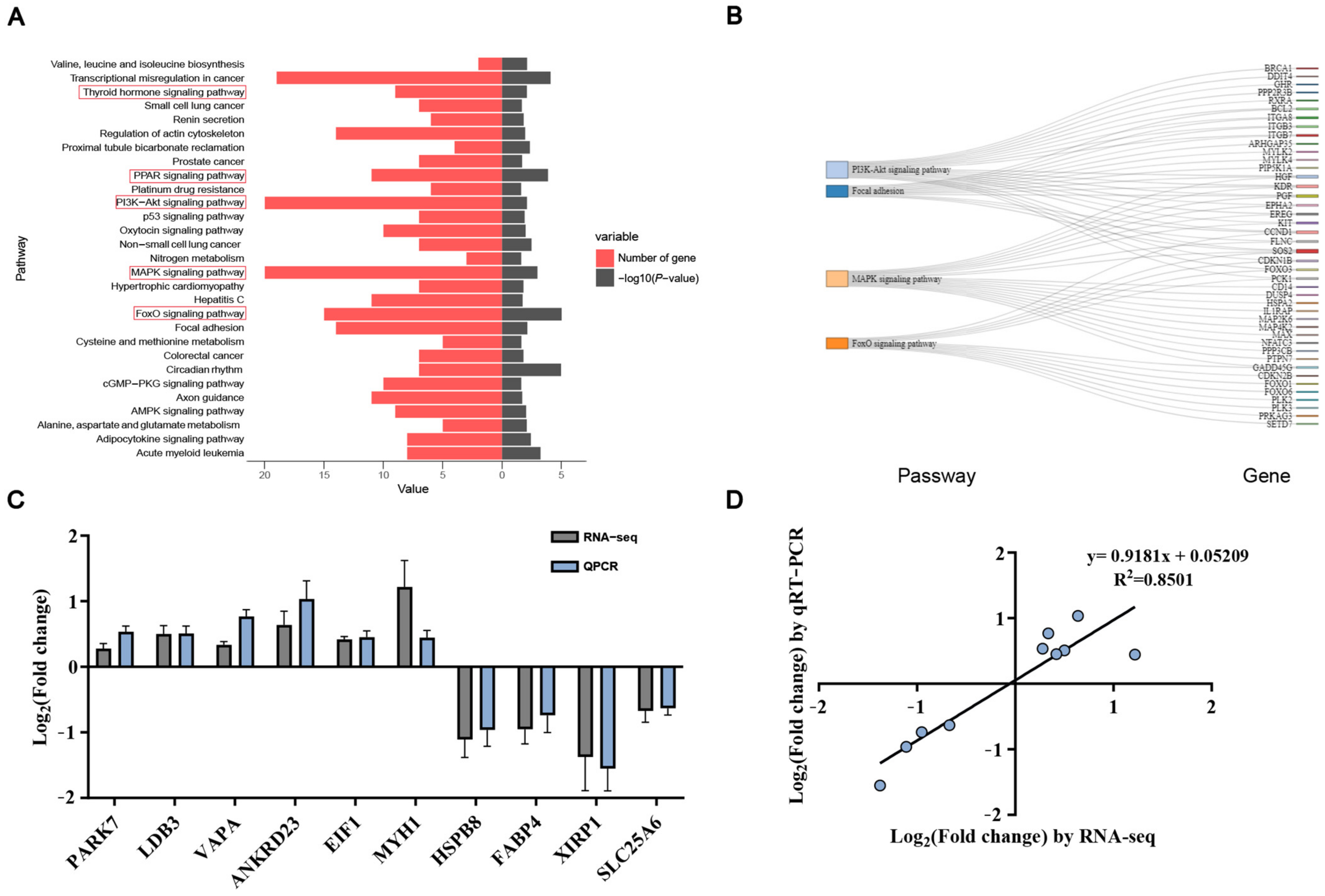
3.4. KEGG Enrichment Analysis and qRT-PCR Validation
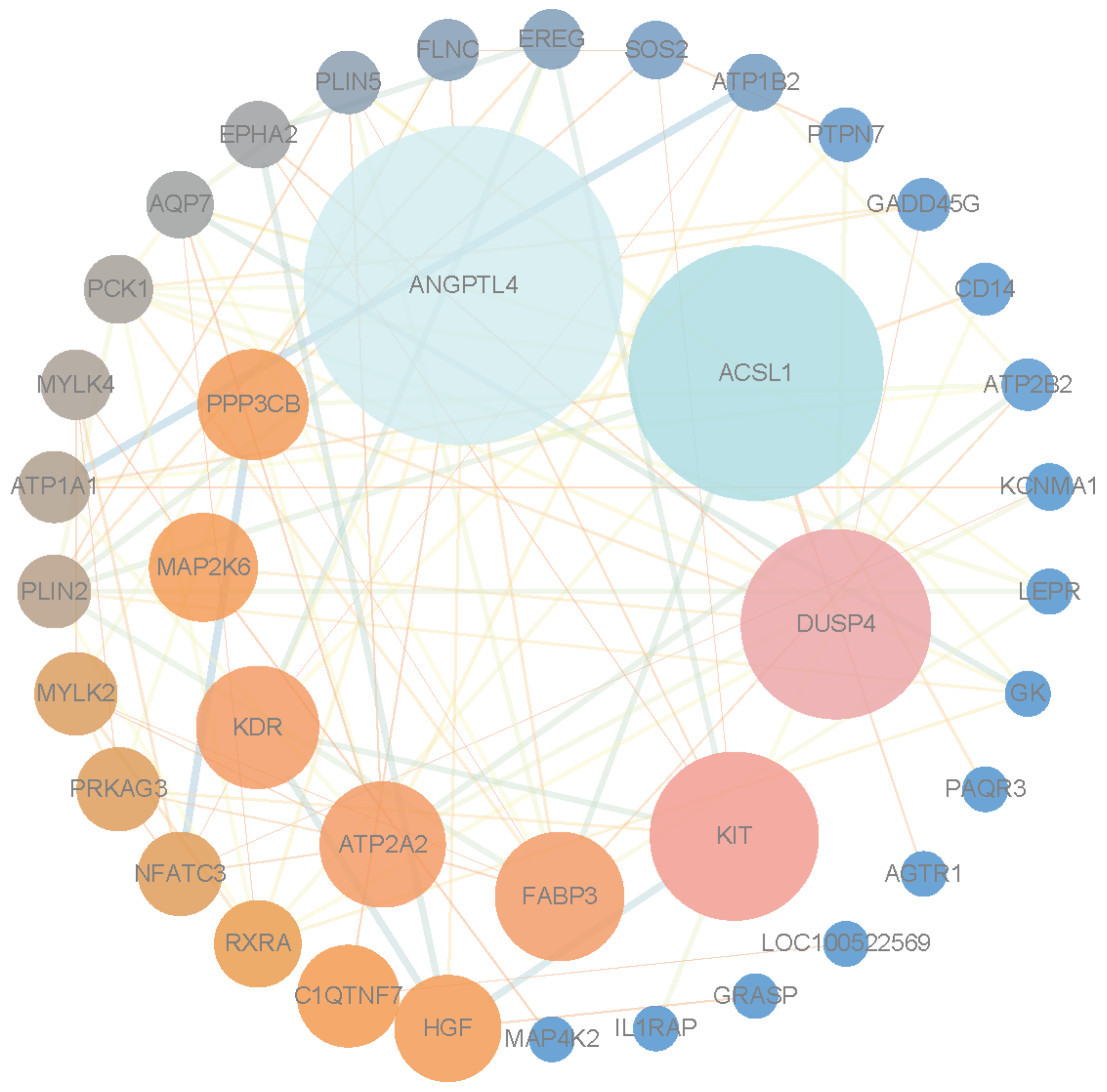
3.5. Protein–Protein Interaction Network Analysis and Identification of Hub Genes
3.6. ACSL1 Inhibits the Proliferation of C2C12
3.7. FABP3 Inhibits the Proliferation of C2C12
3.8. ACSL1 Facilitates the Differentiation of C2C12
3.9. FABP3 Promotes the Differentiation of C2C12
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. China Statistical Yearbook 2024; National Bureau of Statistics of China: Beijing, China, 2024.
- Gao, J.; Sun, L.; Tu, W.; Cao, M.; Zhang, S.; Xu, J.; He, M.; Zhang, D.; Dai, J.; Wu, X.; et al. Characterization of Meat Metabolites and Lipids in Shanghai Local Pig Breeds Revealed by LC–MS-Based Method. Foods 2024, 13, 2327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, Y.; Huang, L.; An, S.; Liu, D.; Zhang, J.; Miao, Z. Effects of Acorns on Meat Quality and Lipid Metabolism-Related Gene Expression in Muscle Tissues of Yuxi Black Pigs. Metabolites 2024, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, F.; Liufu, S.; Li, Z.; Gong, Y.; Ma, H. Integrated analysis of muscle lncRNA and mRNA of Chinese indigenous breed Ningxiang pig in four developmental stages. Front. Vet.-Sci. 2024, 11, 1465389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, R.; Yu, Y.; Wang, Y.; Zheng, M.; Zhao, G.; Wen, J.; Wang, S.; Cui, H. Integrative multiomics analysis identifies key genes regulating intramuscular fat deposition during development. Poult. Sci. 2024, 103, 104404. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology that Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef]
- Da Paixão, A.O.; Bolin, A.P.; Silvestre, J.G.; Rodrigues, A.C. Palmitic Acid Impairs Myogenesis and Alters Temporal Expression of miR-133a and miR-206 in C2C12 Myoblasts. Int. J. Mol. Sci. 2021, 22, 2748. [Google Scholar] [CrossRef]
- Cai, L.; Huang, Y.; Zhong, L.; Zou, X.; Ji, J.; Liu, X.; Huang, C.; Zeng, Q.; Yang, B.; Xiao, S.; et al. Using phenotypic and genotypic big data to investigate the effect of muscle fiber characteristics on meat quality and eating quality traits in pigs. Meat Sci. 2023, 198, 109122. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Zheng, P. Dihydromyricetin improves meat quality and promotes skeletal muscle fiber type transformations via AMPK signaling in growing–finishing pigs. Food Funct. 2022, 13, 3649–3659. [Google Scholar] [CrossRef]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]
- Tan, X.; He, Y.; He, Y.; Yan, Z.; Chen, J.; Zhao, R.; Sui, X.; Zhang, L.; Du, X.; Irwin, D.M.; et al. Comparative Proteomic Analysis of Glycolytic and Oxidative Muscle in Pigs. Genes 2023, 14, 361. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, S.; Kim, J.-M. Genetic correlation between biopsied and post-mortem muscle fibre characteristics and meat quality traits in swine. Meat Sci. 2022, 186, 108735. [Google Scholar] [CrossRef]
- Cho, I.C.; Park, H.B.; Ahn, J.S.; Han, S.-H.; Lee, J.-B.; Lim, H.-T.; Yoo, C.-K.; Jung, E.-J.; Kim, D.-H.; Sun, W.-S.; et al. A functional regulatory variant of MYH3 influences muscle fiber-type composition and intramuscular fat content in pigs. PLoS Genet. 2019, 15, e1008279. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhou, F.; Yao, Z.; Zhan, Y.; Fan, Z.; Meng, X.; Zhang, Z.; Liu, L.; Yang, J.; et al. Increased Accuracy of Genomic Prediction Using Preselected SNPs from GWAS with Imputed Whole-Genome Sequence Data in Pigs. Animals 2023, 13, 3871. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, G.; Cidan, Y.; Shi, B.; Tan, Z.; Zhang, J.; Basang, W. Comprehensive Multi-Omic Evaluation of the Microbiota and Metabolites in the Colons of Diverse Swine Breeds. Animals 2024, 14, 1221. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lai, T.; Fan, Y.; Yu, C.; Li, W.; Li, M.; Lei, S.; Zhang, J.; Xu, W.; Wang, Z.; et al. Transcriptome and targeted metabolome analysis of lipid profiles, nutrients compositions and volatile compounds in longissimus dorsi of different pig breeds. Anim. Biosci. 2024, in press. [CrossRef] [PubMed]
- Xu, S.; Zheng, W.; Zeng, Y.; Tian, X.; Xu, X. Analysis of volatile flavor and lipids in different breeds of pork using electronic noses, GC-MS and UPLC-MS/MS. J. Chromatogr. A 2025, 1746, 465783. [Google Scholar] [CrossRef]
- Yang, L.; Lin, X.; Chen, Y.; Peng, P.; Lan, Q.; Zhao, H.; Wei, H.; Yin, Y.; Liu, M. Association analysis of the sorting nexin 29 (SNX29) gene copy number variations with growth traits in Diannan small-ear (DSE) pigs. Anim. Biotechnol. 2024, 35, 2309956. [Google Scholar] [CrossRef]
- Liu, M.; Lan, Q.; Yang, L.; Deng, Q.; Wei, T.; Zhao, H.; Peng, P.; Lin, X.; Chen, Y.; Ma, H.; et al. Genome-Wide Association Analysis Identifies Genomic Regions and Candidate Genes for Growth and Fatness Traits in Diannan Small-Ear (DSE) Pigs. Animals 2023, 13, 1571. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Cai, J.; Ni, Y.; Xiao, L.; Zhang, J. Transcriptome analysis in comparing carcass and meat quality traits of Jiaxing Black Pig and Duroc x Duroc x Berkshire x Jiaxing Black Pig crosses. Gene 2022, 808, 145978. [Google Scholar] [CrossRef]
- Pruitt, S.C.; Freeland, A.; Rusiniak, M.E.; Kunnev, D.; Cady, G.K. Cdkn1b overexpression in adult mice alters the balance between genome and tissue ageing. Nat. Commun. 2013, 4, 2626. [Google Scholar] [CrossRef]
- Nanda, V.; Downing, K.P.; Ye, J.; Xiao, S.; Kojima, Y.; Spin, J.M.; DiRenzo, D.; Nead, K.T.; Connolly, A.J.; Dandona, S.; et al. CDKN2B Regulates TGFβ Signaling and Smooth Muscle Cell Investment of Hypoxic Neovessels. Circ. Res. 2016, 118, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Xiao, D.; Fang, X.; Wang, J. Oxidative modification of miR-30c promotes cardiac fibroblast proliferation via CDKN2C mismatch. Sci. Rep. 2024, 14, 13085. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Robinson, K.; Sundquist, T.; Chan, S.S.K. In vivo PSC differentiation as a platform to identify factors for improving the engraftability of cultured muscle stem cells. Front. Cell Dev. Biol. 2024, 12, 1362671. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shi, X.; Wu, G.; Zhu, J.; Song, C.; Zhang, Q.; Yang, G. FGF13 regulates proliferation and differentiation of skeletal muscle by down-regulating Spry. Cell Prolif. 2015, 48, 550–560. [Google Scholar] [CrossRef]
- Coscia, S.M.; Moore, A.S.; Thompson, C.P.; Tirrito, C.F.; Ostap, E.M.; Holzbaur, E.L.F. An interphase actin wave promotes mitochondrial content mixing and organelle homeostasis. Nat. Commun. 2024, 15, 3793. [Google Scholar] [CrossRef]
- Larrinaga, T.M.; Farman, G.P.; Mayfield, R.M.; Yuen, M.; Ahrens-Nicklas, R.C.; Cooper, S.T.; Pappas, C.T.; Gregorio, C.C. Lmod2 is necessary for effective skeletal muscle contraction. Sci. Adv. 2024, 10, eadk1890. [Google Scholar] [CrossRef]
- Dreher, S.I.; Grubba, P.; von Toerne, C.; Moruzzi, A.; Maurer, J.; Goj, T.; Birkenfeld, A.L.; Peter, A.; Loskill, P.; Hauck, S.M.; et al. IGF1 promotes human myotube differentiation toward a mature metabolic and contractile phenotype. Am. J. Physiol. Physiol. 2024, 326, C1462–C1481. [Google Scholar] [CrossRef]
- Lv, C.; Niu, S.; Yan, S.; Bai, C.; Yu, X.; Hou, J.; Gao, W.; Zhang, J.; Zhao, Z.; Yang, C.; et al. Low-density lipoprotein receptor-related protein 1 regulates muscle fiber development in cooperation with related genes to affect meat quality. Poult. Sci. 2019, 98, 3418–3425. [Google Scholar] [CrossRef]
- Li, W.; Kai, L.; Wei, W.; Fan, Y.; Wang, Y.; Lu, Z. Dietary metabolizable energy and crude protein levels affect Taihe silky fowl growth performance, meat quality, and cecal microbiota during fattening. Poult. Sci. 2024, 103, 104363. [Google Scholar] [CrossRef]
- Park, J.; Moon, S.S.; Song, S.; Cheng, H.; Im, C.; Du, L.; Kim, G.-D. Comparative review of muscle fiber characteristics between porcine skeletal muscles. J. Anim. Sci. Technol. 2024, 66, 251–265. [Google Scholar] [CrossRef]
- Al-Moadhen, H.; Lees, J.C.; van der Werf, J.H.J.; McGilchrist, P. The Impact of Genetic and Non-Genetic Factors on Lamb Loin Shear Force. Animals 2024, 14, 2628. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, Y.; Dou, Y.; Li, C.; Song, C.; Zhang, Z.; Qi, K.; Li, X.; Qiao, R.; Wang, K.; et al. Effect of miR-149-5p on intramuscular fat deposition in pigs based on metabolomics and transcriptomics. BMC Genom. 2023, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Li, L.; Ren, M.; Zhou, M.; Li, S. Transcriptome Profiling of Different Developmental Stages on Longissimus Dorsi to Identify Genes Underlying Intramuscular Fat Content in Wannanhua Pigs. Genes 2023, 14, 903. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Zhao, W.; Li, M.; Li, C. Transcriptome Analysis Reveals Long Intergenic Non-Coding RNAs Contributed to Intramuscular Fat Content Differences between Yorkshire and Wei Pigs. Int. J. Mol. Sci. 2020, 21, 1732. [Google Scholar] [CrossRef]
- Lozier, N.R.; Kopchick, J.J.; de Lacalle, S. Relative Contributions of Myostatin and the GH/IGF-1 Axis in Body Composition and Muscle Strength. Front. Physiol. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Hai, C.; Hao, Z.; Bu, L.; Lei, J.; Liu, X.; Zhao, Y.; Bai, C.; Su, G.; Yang, L.; Li, G. Increased rumen Prevotella enhances BCAA synthesis, leading to synergistically increased skeletal muscle in myostatin-knockout cattle. Commun. Biol. 2024, 7, 1575. [Google Scholar] [CrossRef]
- Shan, B.; Yan, M.; Yang, K.; Lin, W.; Yan, J.; Wei, S.; Wei, W.; Chen, J.; Zhang, L. MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition. Genes 2022, 13, 260. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, Z.; Zhang, Q.; Hao, W.; Pang, Y.; Zhang, D.; Liu, D. Transcriptional Regulation Associated with Subcutaneous Adipogenesis in Porcine ACSL1 Gene. Biomolecules 2023, 13, 1057. [Google Scholar] [CrossRef]
- Li, T.; Wei, H.; Zhang, S.; Liu, X.; Xing, L.; Liu, Y.; Gong, R.; Li, J. Intermittent cold stimulation affects energy metabolism and improves stress resistance in broiler heart. Poult. Sci. 2024, 103, 103190. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Tan, J.; Shen, C.; Deng, S.; Fu, X.; Gao, S.; Li, H.; Zhang, X.; Cai, W. Targeting ACSL1 promotes cardiomyocyte proliferation and cardiac regeneration. Life Sci. 2022, 294, 120371. [Google Scholar] [CrossRef]
- Nan, J.; Lee, J.S.; Lee, S.A.; Lee, D.-S.; Park, K.S.; Chung, S.S. An Essential Role of the N-terminal Region of ACSL1 in Linking Free Fatty Acids to Mito-chondrial β-Oxidation in C2C12 Myotubes. Mol. Cells 2021, 44, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Khamoui, A.V.; Tokmina-Roszyk, D.; Rossiter, H.B.; Fields, G.B.; Visavadiya, N.P. Hepatic proteome analysis reveals altered mitochondrial metabolism and suppressed acyl-CoA synthetase-1 in colon-26 tumor-induced cachexia. Physiol. Genom. 2020, 52, 203–216. [Google Scholar] [CrossRef]
- Li, Q.; Tao, Z.; Shi, L.; Ban, D.; Zhang, B.; Yang, Y.; Zhang, H.; Wu, C. Expression and genome polymorphism of ACSL1 gene in different pig breeds. Mol. Biol. Rep. 2012, 39, 8787–8792. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Bliss, E.; Whiteside, E.; Hoffman, B.; Mills, D.E. Biomarkers to measure respiratory muscle damage following inspiratory pressure threshold loading in healthy young men. J. Appl. Physiol. 2023, 134, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.T.; Jayanetti, K.; Oubraim, S.; Hillowe, A.; Frank, E.; Jong, J.; Wang, L.; Wang, H.; Ojima, I.; Haj-Dahmane, S.; et al. Fatty acid binding proteins are novel modulators of synaptic epoxyeicosatrienoic acid signaling in the brain. Sci. Rep. 2023, 13, 15234. [Google Scholar] [CrossRef]
- Peng, H.; Hu, M.; Liu, Z.; Lai, W.; Shi, L.; Zhao, Z.; Ma, H.; Li, Y.; Yan, S. Transcriptome Analysis of the Liver and Muscle Tissues of Dorper and Small-Tailed Han Sheep. Front. Genet. 2022, 13, 868717. [Google Scholar] [CrossRef]
- Xiong, L.; Yao, X.; Pei, J.; Wang, X.; Guo, S.; Cao, M.; Bao, P.; Wang, H.; Yan, P.; Guo, X. Do microbial-gut-muscle mediated by SCFAs, microbial-gut-brain axis mediated by insulin simul-taneously regulate yak IMF deposition? Int. J. Biol. Macromol. 2024, 257 Pt 1, 128632. [Google Scholar] [CrossRef]
- Zelencova-Gopejenko, D.; Videja, M.; Grandane, A.; Pudnika-Okinčica, L.; Sipola, A.; Vilks, K.; Dambrova, M.; Jaudzems, K.; Liepinsh, E. Heart-Type Fatty Acid Binding Protein Binds Long-Chain Acylcarnitines and Protects against Lipotoxicity. Int. J. Mol. Sci. 2023, 24, 5528. [Google Scholar] [CrossRef]
- Zhuang, L.; Mao, Y.; Liu, Z.; Li, C.; Jin, Q.; Lu, L.; Tao, R.; Yan, X.; Chen, K. FABP3 Deficiency Exacerbates Metabolic Derangement in Cardiac Hypertrophy and Heart Failure via PPARα Pathway. Front. Cardiovasc. Med. 2021, 8, 722908. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Granado-Casas, M.; de Oca, A.P.-M.; Julian, M.T.; Domingo, M.; Codina, P.; Santiago-Vacas, E.; Cediel, G.; Julve, J.; Rossell, J.; et al. Fatty Acid Binding Proteins 3 and 4 Predict Both All-Cause and Cardiovascular Mortality in Subjects with Chronic Heart Failure and Type 2 Diabetes Mellitus. Antioxidants 2023, 12, 645. [Google Scholar] [CrossRef]
- Keller, F.; Denicolò, S.; Leierer, J.; Kruus, M.; Heinzel, A.; Kammer, M.; Ju, W.; Nair, V.; Burdet, F.; Ibberson, M.; et al. Association of Urinary Epidermal Growth Factor, Fatty Acid-Binding Protein 3, and Vascular Cell Adhesion Molecule 1 Levels with the Progression of Early Diabetic Kidney Disease. Kidney Blood Press. Res. 2024, 49, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Sierzega, M.; Drabik, A.; Sanak, M.; Chrzan, R.; Richter, P. Dissecting the importance and origin of circulating myokines in gastric cancer cachexia. Front. Endocrinol. 2024, 15, 1437197. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, S.; Wojdała, A.L.; Bellomo, G.; Toja, A.; Chipi, E.; Piersma, S.R.; Pham, T.V.; Gaetani, L.; Jimenez, C.R.; Parnetti, L.; et al. Potential diagnostic value of CSF metabolism-related proteins across the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2023, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Pesämaa, I.; Müller, S.A.; Robinson, S.; Darcher, A.; Paquet, D.; Zetterberg, H.; Lichtenthaler, S.F.; Haass, C. A microglial activity state biomarker panel differentiates FTD-granulin and Alzheimer’s disease patients from controls. Mol. Neurodegener. 2023, 18, 70. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, L.; Lv, Y.; Liao, R.; Zhang, K.; Zhou, J.; Zhang, S.; Xu, J.; He, M.; Wu, C.; et al. Transcriptomic and metabolomic dissection of skeletal muscle of crossbred Chongming white goats with different meat production performance. BMC Genom. 2024, 25, 443. [Google Scholar] [CrossRef]
- Abouleisa, R.R.E.; Salama, A.B.M.; Ou, Q.; Tang, X.-L.; Solanki, M.; Guo, Y.; Nong, Y.; McNally, L.; Lorkiewicz, P.K.; Kassem, K.M.; et al. Transient Cell Cycle Induction in Cardiomyocytes to Treat Subacute Ischemic Heart Failure. Circulation 2022, 145, 1339–1355. [Google Scholar] [CrossRef]
- Kumar, S.; Maurya, V.K.; Chitti, S.V.; Kabir, R.; Shanker, K.; Nayak, D.; Khurana, A.; Manchanda, R.K.; Gadugu, S.; Kumar, V.; et al. Wound Healing Activity of a Novel Formulation SKRIN via Induction of Cell Cycle Progression and Inhibition of PCNA–p21 Complex Interaction Leading to Cell Survival and Proliferation. ACS Pharmacol. Transl. Sci. 2021, 4, 352–364. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, B.H.; Wang, X.; Huang, C.-P.; Xu, S.-M.; Wang, J.-L.; Huang, T.-E.; Xiao, W.-L.; Tian, X.-L.; Lan, X.-Q.; et al. Handelin alleviates cachexia- and aging-induced skeletal muscle atrophy by improving protein homeostasis and inhibiting inflammation. J. Cachexia Sarcopenia Muscle 2024, 15, 173–188. [Google Scholar] [CrossRef]
- Sosa, P.; Alcalde-Estévez, E.; Asenjo-Bueno, A.; Plaza, P.; Carrillo-López, N.; Olmos, G.; López-Ongil, S.; Ruiz-Torres, M.P. Aging-related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle. J. Cachex-Sarcopenia Muscle 2021, 12, 1266–1279. [Google Scholar] [CrossRef]


| Name | Sense | Antisense |
|---|---|---|
| Si-FABP3 | GGA AGC UAG UGG ACA GCA ATT | UUG CUG UCC ACU AGC UUC CTT |
| Si-ACSL1 | GUG UCA UGG AGC UAA GAU ATT | UAU CUU AGC UCC AUG ACA CTT |
| Si-NC | UUC UCC GAA CGU GUC ACG UTT | ACG UGA CAC GUU CGG AGA ATT |
| Target Gene | Oligonucleotide Sequence | Annealing Temp (°C) | Product Size (bp) | GeneBank No. |
|---|---|---|---|---|
| EIF1 | F: AATTTGCCTGCAATGGCACT | 59 | 130 | XM_003482982. 4 |
| R: TTCAGCTGGTCGTCCTTAGC | ||||
| HSPB8 | F: TCCTGCTGCTTCTCCTCGTGTT | 63 | 79 | XM_001929585. 5 |
| R: TTAAGCCGGAGGAGCTGATGGT | ||||
| MYH1 | F: AACCTCTCCAAGTTCCGCAAGC | 64 | 68 | NM_001104951. 2 |
| R: TCAGCAATGTCAGCCCGTTCCT | ||||
| VAPA | F: TGGTGAAGCTGTCGGAGGAGAA | 63 | 136 | XM_021096055. 1 |
| R: AGTGAAGGAAGAGGGCTGGTGA | ||||
| ANKRD23 | F: TGCCTGTGGAGCCCATATTGAC | 63 | 108 | NM_001315720. 1 |
| R: CCATAGAGCAGCAGCACCTTCA | ||||
| PARK7 | F: ATCGGAGTCTGCTGCTGTGAAG | 63 | 138 | NM_001078663. 1 |
| R: AGTGGGTGCGTCGTAACTTTGC | ||||
| LDB3 | F: AGCCCTTCGGGAATAGCCTCTT | 64 | 163 | XM_005657425. 3 |
| R: AAGCAGGTGTCGTGCCAGGTAT | ||||
| FABP4 | F: TGCCTTCAAATTGGGCCAGGAA | 64 | 112 | NM_001002817. 1 |
| R: TCCATCCCACTTCTGCACCTGT | ||||
| SLC25A6 | F: ATCACCAAGTCTGACGGCATCC | 63 | 161 | NM_214418. 2 |
| R: GCGATCATCCAGCTCACCACAA | ||||
| XIRP1 | F: CTGCTGTGACGAGACCTGACTT | 63 | 61 | NM_001143928. 1 |
| R: TGCTGGCTGGAGTCCTCATCAT | ||||
| CyclinB | F: AACTTCAGCCTGGGTCG | 57 | 249 | NM_172301. 3 |
| R: CAGGGAGTCTTCACTGTAGGA | ||||
| CyclinD | F: TAGGCCCTCAGCCTCACTC | 60 | 80 | NM_001379248. 1 |
| R: CCACCCCTGGGATAAAGCAC | ||||
| CDK4 | F: CGAGCGTAAGGCTGATGGAT | 60 | 177 | NM_001355005. 1 |
| R: CCAGGCCGCTTAGAAACTGA | ||||
| p21 | F: GAGCAAAGTGTGCCGTTGTC | 60 | 112 | NM_001111099. 2 |
| R: AAAGTTCCACCGTTCTCGGG | ||||
| MyoD | F: AAGACGACTCTCACGGCTTG | 60 | 169 | NM_010866. 2 |
| R: GCAGGTCTGGTGAGTCGAAA | ||||
| MyoG | F: CAATGCACTGGAGTTCGGT | 58 | 134 | NM_031189. 2 |
| R: CTGGGAAGGCAACAGACAT | ||||
| MyHC | F: CAGAAGCAACGAGAGGAG | 55 | 88 | XM_017314318. 3 |
| R: GGTCAGCAGAGTTCAGATT | ||||
| MyF5 | F: CAGGAATGCCATCCGCTACA | 60 | 78 | XM_006513319. 3 |
| R: CCCGGCAGGCTGTAATAGTT | ||||
| ACSL1 | F: CCTGGACGACTTGTTGAA | 54 | 170 | NM_001302163. 2 |
| R: CATCTATCTGCGACCTGAA | ||||
| FABP3 | F: ACCTGGAAGCTAGTGGACAG | 59 | 106 | NM_010174. 2 |
| R: TGATGGTAGTAGGCTTGGTCAT | ||||
| GAPDH | F: AGGTGGTGAAGCAGGCATCTGA | 64 | 114 | NM_001289726. 2 |
| R: CGGCATCGAAGGTGGAAGAGTG |
| Sample ID | Clean Reads | GC (%) | Q30 (%) | Mapped Reads | Mapping Rate |
|---|---|---|---|---|---|
| DLYM1 | 41,023,660 | 53.46 | 95.62 | 39,655,191 | 96.66% |
| DLYM2 | 43,610,182 | 52.12 | 95.61 | 42,249,383 | 96.88% |
| DLYM3 | 39,986,234 | 52.84 | 95.08 | 38,650,277 | 96.66% |
| DLYM4 | 40,895,390 | 53.38 | 95.53 | 39,504,038 | 96.60% |
| DLYM5 | 40,335,290 | 52.31 | 94.58 | 38,875,712 | 96.38% |
| DLYM6 | 41,858,308 | 51.59 | 95.43 | 40,451,926 | 96.64% |
| DBDM1 | 42,224,020 | 51.61 | 95.59 | 40,808,828 | 96.65% |
| DBDM2 | 41,301,168 | 51.55 | 95.55 | 39,924,198 | 96.67% |
| DBDM3 | 42,115,656 | 53.14 | 95.64 | 40,779,108 | 96.83% |
| DBDM4 | 40,474,448 | 52.71 | 95.53 | 39,095,163 | 96.59% |
| DBDM5 | 43,631,020 | 52.36 | 95.74 | 42,239,474 | 96.81% |
| DBDM6 | 43,820,494 | 52.07 | 95.78 | 42,400,827 | 96.76% |
| Gene | Passway | Function | Reference |
|---|---|---|---|
| CDKN1B | protein kinase inhibitor activity | Reduced muscle fiber diameter. | [21] |
| CDKN2B | protein kinase inhibitor activity | Involved in TGFβ signaling regulation, impacting smooth muscle cell proliferation and apoptosis. | [22] |
| CDKN2C | protein kinase inhibitor activity | Reduces the expression of cyclin-dependent kinase CDK4, thereby inhibiting the proliferation of muscle cells. | [23] |
| LIF | growth factor activity | Facilitates both the self-renewal and differentiation processes of muscle stem cells. | [24] |
| FGF13 | growth factor activity | Upregulates the p27 mRNA level, downregulates the expression of Cyclin E and Spry1 proteins, activates the ERK1/2 signaling pathway, and inhibits myoblast differentiation. | [25] |
| FMNL1 | actin binding | Regulates the content of actin. | [26] |
| LMOD2 | actin binding | Regulates the sarcomere length in skeletal muscle, and its deficiency results in reduced muscle contractility. | [27] |
| MYH1 | actin binding | The myosin heavy chain encoded by MYH1 is a major component of fast-twitch muscle fibers (Type II fibers). | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Liufu, S.; Wen, S.; Wang, K.; Chen, W.; Xiao, L.; Liu, X.; Yi, L.; Liu, J.; Xu, X.; et al. Identification and Functional Validation of ACSL1 and FABP3 as Muscle-Related Genes Screened by Transcriptomics in Crossbred Duroc × Berkshire × Diannan Small-Eared Pigs. Genes 2025, 16, 520. https://doi.org/10.3390/genes16050520
Chen B, Liufu S, Wen S, Wang K, Chen W, Xiao L, Liu X, Yi L, Liu J, Xu X, et al. Identification and Functional Validation of ACSL1 and FABP3 as Muscle-Related Genes Screened by Transcriptomics in Crossbred Duroc × Berkshire × Diannan Small-Eared Pigs. Genes. 2025; 16(5):520. https://doi.org/10.3390/genes16050520
Chicago/Turabian StyleChen, Bohe, Sui Liufu, Sheng Wen, Kaiming Wang, Wenwu Chen, Lanlin Xiao, Xiaolin Liu, Lei Yi, Jingwen Liu, Xin Xu, and et al. 2025. "Identification and Functional Validation of ACSL1 and FABP3 as Muscle-Related Genes Screened by Transcriptomics in Crossbred Duroc × Berkshire × Diannan Small-Eared Pigs" Genes 16, no. 5: 520. https://doi.org/10.3390/genes16050520
APA StyleChen, B., Liufu, S., Wen, S., Wang, K., Chen, W., Xiao, L., Liu, X., Yi, L., Liu, J., Xu, X., Liu, C., Wen, W., Ma, H., & Deng, Q. (2025). Identification and Functional Validation of ACSL1 and FABP3 as Muscle-Related Genes Screened by Transcriptomics in Crossbred Duroc × Berkshire × Diannan Small-Eared Pigs. Genes, 16(5), 520. https://doi.org/10.3390/genes16050520




