Cryptic KMT2A::AFDN Fusion Due to AFDN Insertion into KMT2A in a Patient with Acute Monoblastic Leukemia
Abstract
1. Introduction
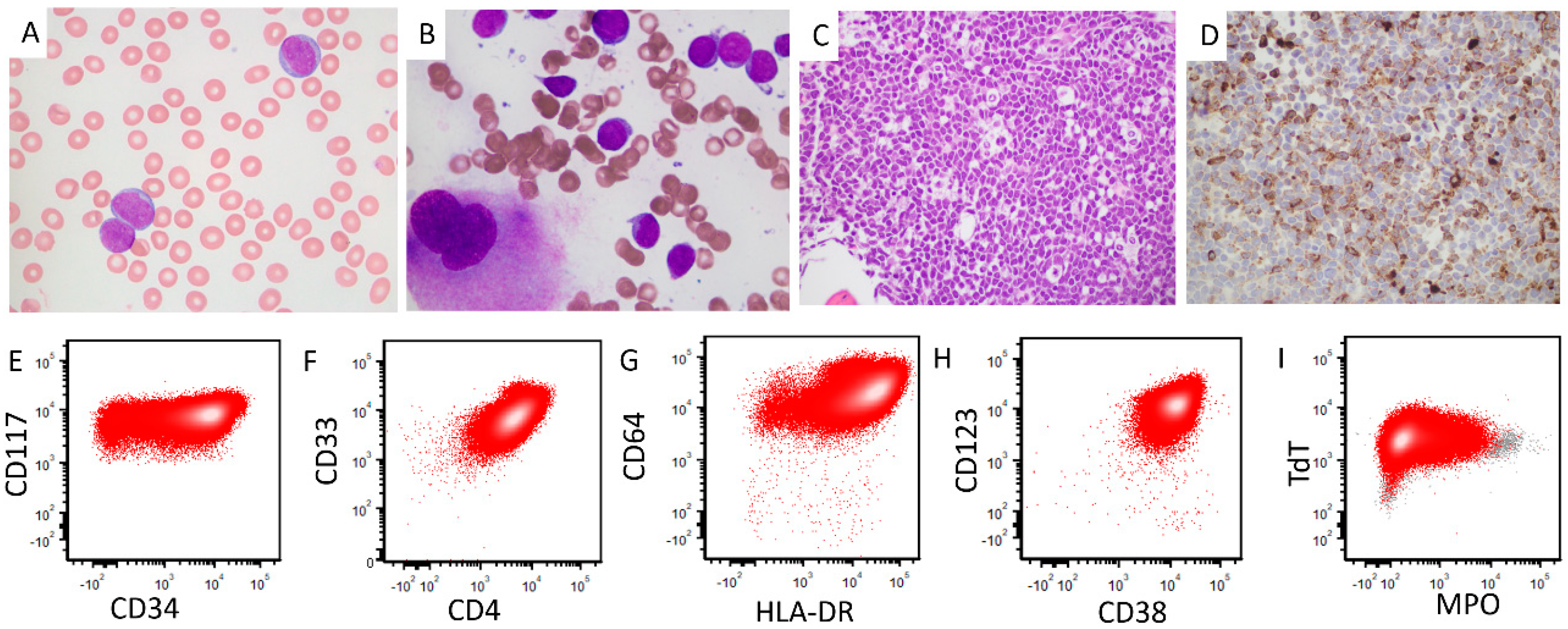
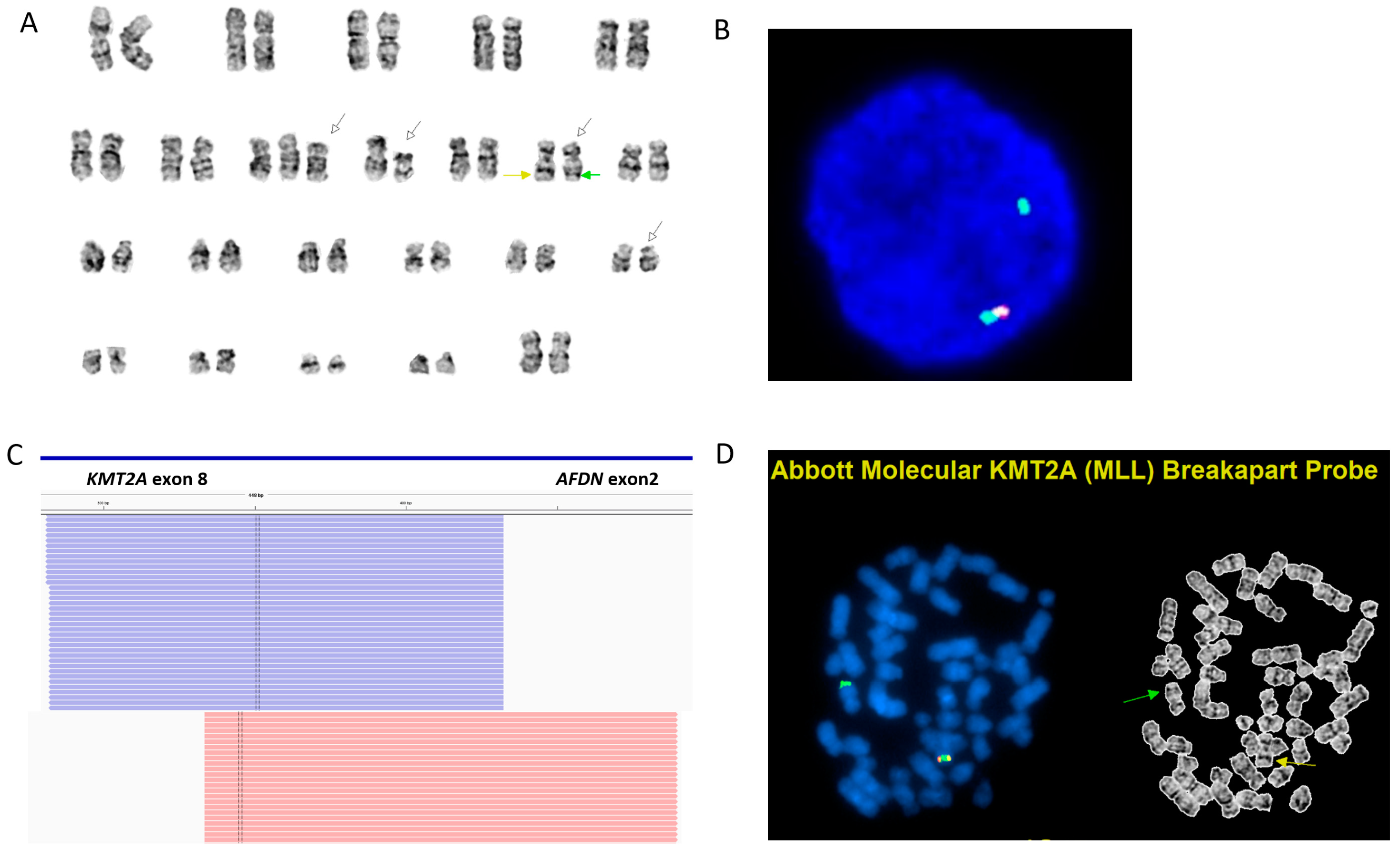
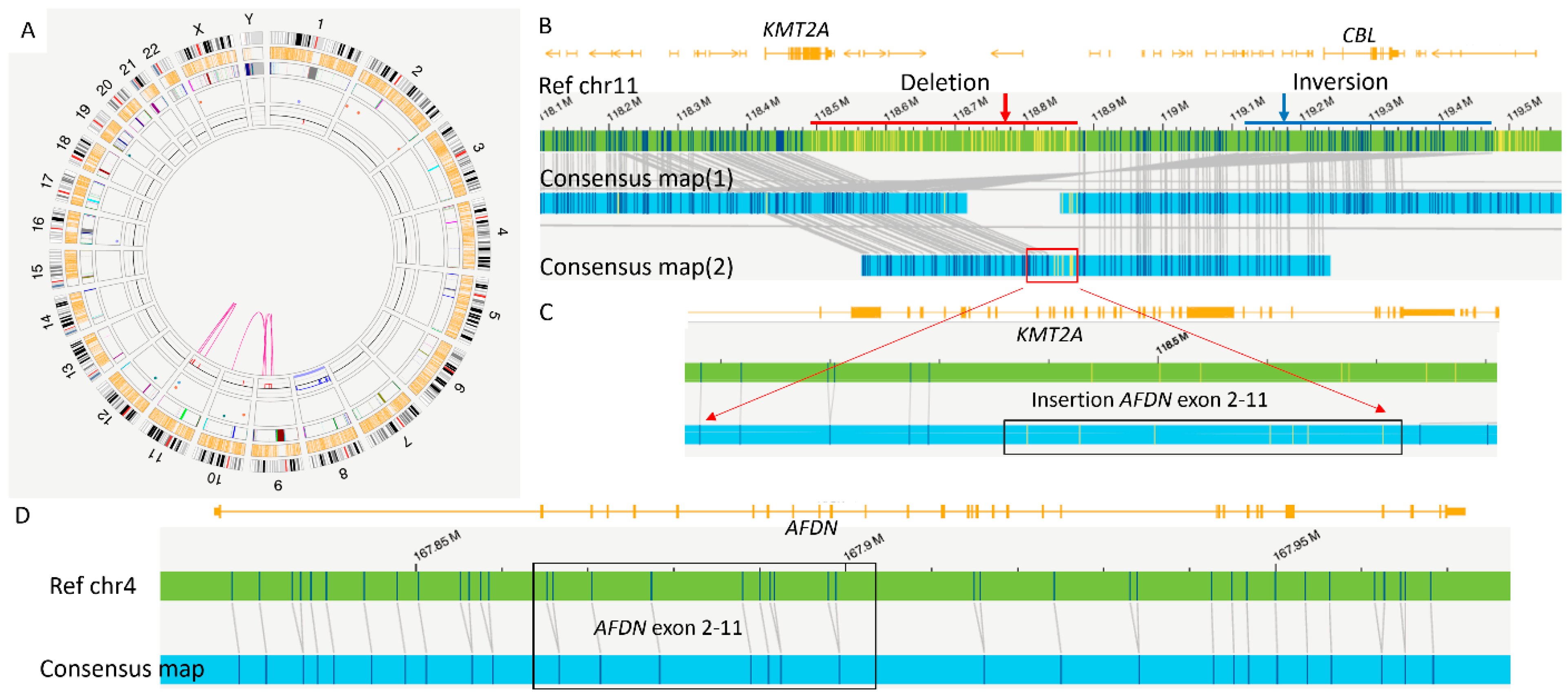
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, C.; Larghero, P.; Lopes, B.A.; Burmeister, T.; Gröger, D.; Sutton, R.; Venn, N.C.; Cazzaniga, G.; Abascal, L.C.; Tsaur, G.; et al. The KMT2A recombinome of acute leukemias in 2023. Leukemia 2023, 37, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Mrózek, K.; Heinonen, K.; Lawrence, D.; Carroll, A.J.; Koduru, P.R.; Rao, K.W.; Strout, M.P.; Hutchison, R.E.; Moore, J.O.; Mayer, R.J.; et al. Adult patients with de novo acute myeloid leukemia and t(9; 11)(p22; q23) have a superior outcome to patients with other translocations involving band 11q23: A cancer and leukemia group B study. Blood 1997, 90, 4532–4538. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.T.; Hess, J.L. Deregulation of the HOXA9/MEIS1 axis in acute leukemia. Curr. Opin. Hematol. 2016, 23, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Balgobind, B.V.; Raimondi, S.C.; Harbott, J.; Zimmermann, M.; Alonzo, T.A.; Auvrignon, A.; Beverloo, H.B.; Chang, M.; Creutzig, U.; Dworzak, M.N.; et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: Results of an international retrospective study. Blood 2009, 114, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Aldoss, I.; DiPersio, J.; Cuglievan, B.; Stone, R.; Arellano, M.; Thirman, M.J.; Patel, M.R.; Dickens, D.S.; Shenoy, S.; et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 2023, 615, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Martineau, M.; Berger, R.; Lillington, D.M.; Moorman, A.V.; Secker-Walker, L.M. The t(6;11)(q27;q23) translocation in acute leukemia: A laboratory and clinical study of 30 cases. Leukemia 1998, 12, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Lopes, B.A.; Caye-Eude, A.; Cavé, H.; Arfeuille, C.; Cuccuini, W.; Sutton, R.; Venn, N.C.; Oh, S.H.; Tsaur, G.; et al. Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL-USP2 fusions. Leukemia 2019, 33, 2306–2340. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, B.; Seong, M.-W.; Lee, D.S.; Hong, K.T.; Kang, H.J.; Yun, J.; Chang, Y.H. Cryptic KMT2A/MLLT10 fusion detected by next-generation sequencing in a case of pediatric acute megakaryoblastic leukemia. Cancer Genet. 2023, 276–277, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.E.; Greipp, P.T.; Baughn, L.B.; Falcon, C.P.; Jackson, C.C.; Peterson, J.F. Detection of a Cryptic KMT2A/AFDN Gene Fusion [ins(6;11)(q27;q23q23)] in a Pediatric Patient with Newly Diagnosed Acute Myeloid Leukemia. Lab Med. 2022, 53, e95–e99. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Hu, S.; Xu, J.; Loghavi, S.; Daver, N.; Toruner, G.A.; Wang, W.; Medeiros, L.J.; Tang, G. Detection of KMT2A Partial Tandem Duplication by Optical Genome Mapping in Myeloid Neoplasms: Associated Cytogenetics, Gene Mutations, Treatment Responses, and Patient Outcomes. Cancers 2024, 16, 4193. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.J.; Talley, P.J.; Reilly, J.T.; Stevenson, D.; Parsons, E.; Tighe, J. Detection of cryptic MLL insertions using a commercial dual-color fluorescence in situ hybridization probe. Cancer Genet. Cytogenet. 2003, 147, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Poirel, H.; Rack, K.; Delabesse, E.; Radford-Weiss, I.; Troussard, X.; Debert, C.; Leboeuf, D.; Bastard, C.; Picard, F.; Veil-Buzyn, A.; et al. Incidence and characterization of MLL gene (11q23) rearrangements in acute myeloid leukemia M1 and M5. Blood 1996, 87, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Moorman, A.; Haas, O.; Watmore, A.; Cheung, K.; Swanton, S.; Secker-Walker, L. Hematologic malignancies with t(4;11)(q21;q23)—A cytogenetic, morphologic, immunophenotypic and clinical study of 183 cases. Leukemia 1998, 12, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Soler, G.; Radford, I.; Meyer, C.; Marschalek, R.; Brouzes, C.; Ghez, D.; Romana, S.; Berger, R. MLL insertion with MLL-MLLT3 gene fusion in acute leukemia: Case report and review of the literature. Cancer Genet. Cytogenet. 2008, 183, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, L.M.; Suttorp, J.; Walter, C.; Antoniou, E.; Behrens, Y.L.; Göhring, G.; Awada, A.; von Neuhoff, N.; Reinhardt, D.; Schneider, M. Panel-based RNA fusion sequencing improves diagnostics of pediatric acute myeloid leukemia. Leukemia 2024, 38, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, S.; Wang, X.; Tang, G.; Li, S.; Wang, W.; Xu, J.; Pierce, S.A.; Jia, F.; Jorgensen, J.L.; Ravandi, F.; et al. Comprehensive immunophenotypic study of acute myeloid leukemia with KMT2A (MLL) rearrangement in adults: A single-institution experience. Cytom. B Clin. Cytom. 2022, 102, 123–133. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Toruner, G.A.; Thakral, B.; Patel, K.P.; Pemmaraju, N.; Wang, S.A.; Kanagal-Shamanna, R.; Tang, G.; Issa, G.C.; Loghavi, S.; et al. Cryptic KMT2A::AFDN Fusion Due to AFDN Insertion into KMT2A in a Patient with Acute Monoblastic Leukemia. Genes 2025, 16, 317. https://doi.org/10.3390/genes16030317
Wei Q, Toruner GA, Thakral B, Patel KP, Pemmaraju N, Wang SA, Kanagal-Shamanna R, Tang G, Issa GC, Loghavi S, et al. Cryptic KMT2A::AFDN Fusion Due to AFDN Insertion into KMT2A in a Patient with Acute Monoblastic Leukemia. Genes. 2025; 16(3):317. https://doi.org/10.3390/genes16030317
Chicago/Turabian StyleWei, Qing, Gokce A. Toruner, Beenu Thakral, Keyur P. Patel, Naveen Pemmaraju, Sa A. Wang, Rashmi Kanagal-Shamanna, Guilin Tang, Ghayas C. Issa, Sanam Loghavi, and et al. 2025. "Cryptic KMT2A::AFDN Fusion Due to AFDN Insertion into KMT2A in a Patient with Acute Monoblastic Leukemia" Genes 16, no. 3: 317. https://doi.org/10.3390/genes16030317
APA StyleWei, Q., Toruner, G. A., Thakral, B., Patel, K. P., Pemmaraju, N., Wang, S. A., Kanagal-Shamanna, R., Tang, G., Issa, G. C., Loghavi, S., Medeiros, L. J., & DiNardo, C. (2025). Cryptic KMT2A::AFDN Fusion Due to AFDN Insertion into KMT2A in a Patient with Acute Monoblastic Leukemia. Genes, 16(3), 317. https://doi.org/10.3390/genes16030317







