CTLA4 Alteration and Neurologic Manifestations: A New Family with Large Phenotypic Variability and Literature Review
Abstract
1. Introduction
2. Subjects and Methods
2.1. Clinical Phenotype
2.2. Genetic Analysis and In Silico Predictions
3. Results
3.1. Genetic Findings
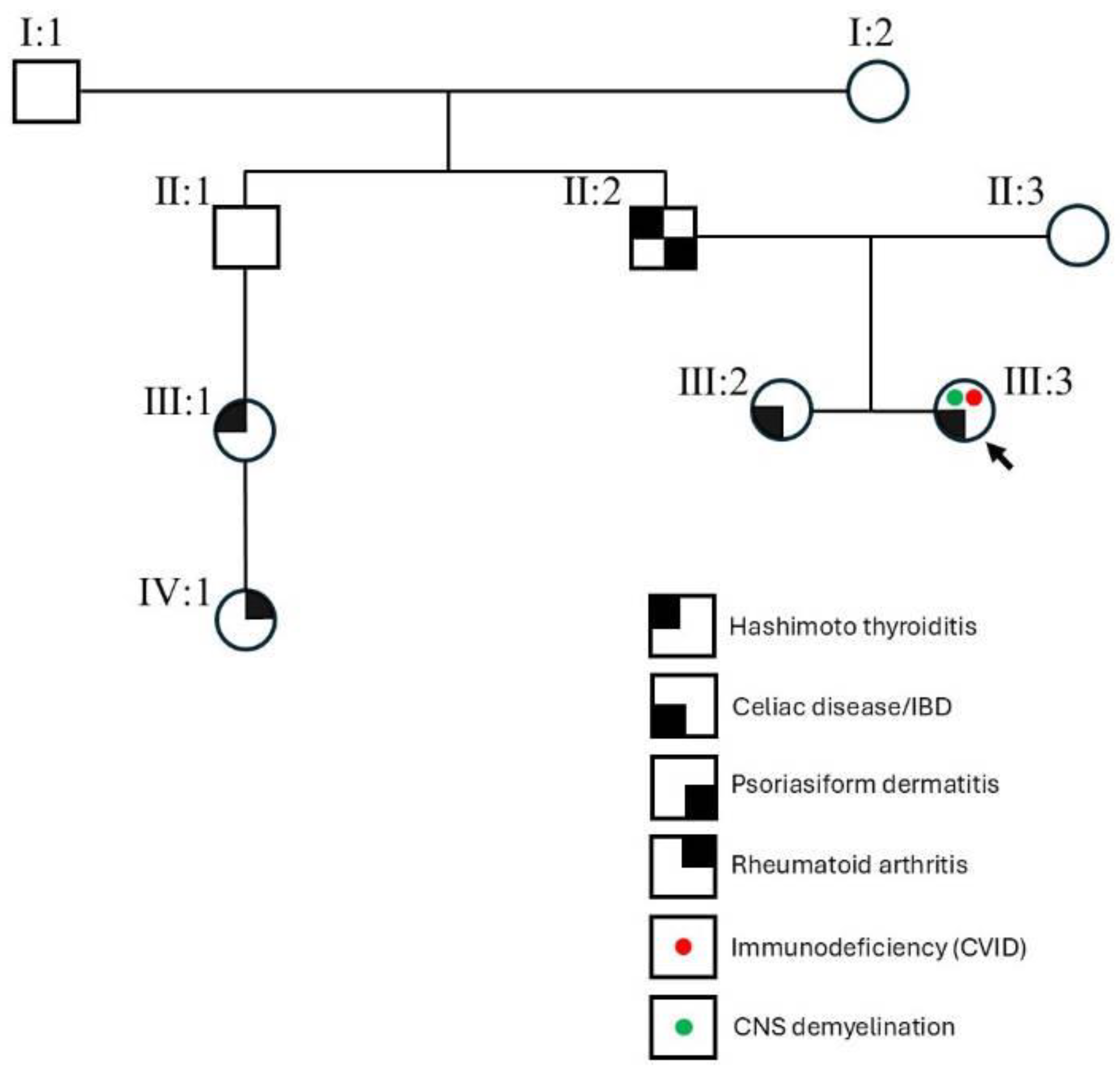
3.2. Phenotypic Constellation in CTLA4 c.436G>A Carriers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, G.R.; Kim, W.J.; Lim, S.; Lee, H.G.; Koo, J.H.; Nam, K.H.; Kim, S.M.; Park, S.D.; Choi, J.M. In Vivo Induction of Regulatory T Cells Via CTLA-4 Signaling Peptide to Control Autoimmune Encephalomyelitis and Prevent Disease Relapse. Adv. Sci. 2021, 8, 2004973. [Google Scholar] [CrossRef] [PubMed]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, H.S.; Ouyang, W.; Lo, B.; Deenick, E.K.; Niemela, J.E.; Avery, D.T.; Schickel, J.N.; Tran, D.Q.; Stoddard, J.; Zhang, Y.; et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014, 345, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef]
- Schindler, M.K.; Pittaluga, S.; Enose-Akahata, Y.; Su, H.C.; Rao, V.K.; Rump, A.; Jacobson, S.; Cortese, I.; Reich, D.S.; Uzel, G. Haploinsufficiency of immune checkpoint receptor CTLA4 induces a distinct neuroinflammatory disorder. J. Clin. Investig. 2020, 130, 5551–5561. [Google Scholar] [CrossRef]
- Schwab, C.; Gabrysch, A.; Olbrich, P.; Patiño, V.; Warnatz, K.; Wolff, D.; Hoshino, A.; Kobayashi, M.; Imai, K.; Takagi, M.; et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J. Allergy Clin. Immunol. 2018, 142, 1932–1946. [Google Scholar] [CrossRef]
- Lanz, A.L.; Riester, M.; Peters, P.; Schwerd, T.; Lurz, E.; Hajji, M.S.; Rohlfs, M.; Ley-Zaporozhan, J.; Walz, C.; Kotlarz, D.; et al. Abatacept for treatment-refractory pediatric CTLA4-haploinsufficiency. Clin. Immunol. 2021, 229, 108779. [Google Scholar] [CrossRef]
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.S.; Schäffer, A.A.; Grüning, B.A.; et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Boeva, V.; Popova, T.; Bleakley, K.; Chiche, P.; Cappo, J.; Schleiermacher, G.; Janoueix-Lerosey, I.; Delattre, O.; Barillot, E. Control-FREEC: A tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 2012, 28, 423–425. [Google Scholar] [CrossRef]
- Magi, A.; Tattini, L.; Cifola, I.; D’Aurizio, R.; Benelli, M.; Mangano, E.; Battaglia, C.; Bonora, E.; Kurg, A.; Seri, M.; et al. EXCAVATOR: Detecting copy number variants from whole-exome sequencing data. Genome Biol. 2013, 14, R120. [Google Scholar] [CrossRef] [PubMed]
- Slatter, M.A.; Engelhardt, K.R.; Burroughs, L.M.; Arkwright, P.D.; Nademi, Z.; Skoda-Smith, S.; Hagin, D.; Kennedy, A.; Barge, D.; Flood, T.; et al. Hematopoietic stem cell transplantation for CTLA4 deficiency. J. Allergy Clin. Immunol. 2016, 138, 615–619.e1. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Hoshino, A.; Yoshida, K.; Ueno, H.; Imai, K.; Piao, J.; Kanegane, H.; Yamashita, M.; Okano, T.; Muramatsu, H.; et al. Genetic heterogeneity of uncharacterized childhood autoimmune diseases with lymphoproliferation. Pediatr. Blood Cancer. 2018, 65, e26831. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, E.; Ciullini Mannurita, S.; Hagin, D.; Vignoli, M.; Anover-Sombke, S.; DeBoer, S.; Segundo, G.R.S.; Allenspach, E.J.; Favre, C.; Ochs, H.D.; et al. Clinical, Immunological, and Molecular Heterogeneity of 173 Patients With the Phenotype of Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX) Syndrome. Front. Immunol. 2018, 9, 2411. [Google Scholar] [CrossRef]
- Ureshino, H.; Koarada, S.; Kamachi, K.; Yoshimura, M.; Yokoo, M.; Kubota, Y.; Ando, T.; Ichinohe, T.; Morio, T.; Kimura, S. Immune dysregulation syndrome with de novo CTLA4 germline mutation responsive to abatacept therapy. Int. J. Hematol. 2020, 111, 897–902. [Google Scholar] [CrossRef]
- Siddiqi, A.E.; Liu, A.Y.; Charville, G.W.; Kunder, C.A.; Uzel, G.; Sadighi Akha, A.A.; Oak, J.; Martin, B.; Sacha, J.; Lewis, D.B.; et al. Disseminated Pneumocystis jirovecii Infection with Osteomyelitis in a Patient with CTLA-4 Haploinsufficiency. J. Clin. Immunol. 2020, 40, 412–414. [Google Scholar] [CrossRef]
- Kolcava, J.; Litzman, J.; Bednarik, J.; Stulik, J.; Stourac, P. Neurological manifestation of immune system dysregulation resulting from CTLA-4 receptor mutation: A case report. Mult. Scler. Relat. Disord. 2020, 45, 102313. [Google Scholar] [CrossRef]
- Zaremehrjardi, F.; Baniadam, L.; Seif, F.; Arshi, S.; Bemanian, M.H.; Shokri, S.; Rezaeifar, A.; Fallahpour, M.; Nabavi, M. A Patient with CTLA-4 Haploinsufficiency with Multiple Autoimmune Presentations: A Case Report. Iran. J. Immunol. 2020, 17, 244–249. [Google Scholar]
- Similuk, M.N.; Yan, J.; Ghosh, R.; Oler, A.J.; Franco, L.M.; Setzer, M.R.; Kamen, M.; Jodarski, C.; DiMaggio, T.; Davis, J.; et al. Clinical exome sequencing of 1000 families with complex immune phenotypes: Toward comprehensive genomic evaluations. J. Allergy Clin. Immunol. 2022, 150, 947–954. [Google Scholar] [CrossRef]
- Siggs, O.M.; Russell, A.; Singh-Grewal, D.; Wong, M.; Chan, P.; Craig, M.E.; O’Loughlin, T.; Stormon, M.; Goodnow, C.C. Preponderance of CTLA4 Variation Associated With Autosomal Dominant Immune Dysregulation in the MYPPPY Motif. Front. Immunol. 2019, 10, 1544. [Google Scholar] [CrossRef]
- Ueda, H.; Howson, J.M.; Esposito, L.; Heward, J.; Snook, H.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.; Smith, A.N.; Di Genova, G.; et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, T.; Ino, R.; Takahashi, N.; Gotoh, N.; Minato, Y.; Takizawa, M.; Yokohama, A.; Handa, H.; Saitoh, T.; Tsukamoto, N.; et al. PDCD1 and CTLA4 polymorphisms affect the susceptibility to, and clinical features of, chronic immune thrombocytopenia. Br. J. Haematol. 2018, 180, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Qiu, L.J.; Zhang, M.; Wen, P.F.; Ye, X.R.; Liang, Y.; Pan, H.F.; Ye, D.Q. CTLA-4 CT60 (rs3087243) polymorphism and autoimmune thyroid diseases susceptibility: A comprehensive meta-analysis. Endocr. Res. 2014, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, Z.; Zhang, J.; Cai, Z.; Zeng, H.; Chen, M.; Huang, J. Association of the CTLA4 gene CT60/rs3087243 single-nucleotide polymorphisms with Graves’ disease. Biomed. Rep. 2015, 3, 691–696. [Google Scholar] [CrossRef]
- Kamesh, L.; Heward, J.M.; Williams, J.M.; Gough, S.C.; Chavele, K.M.; Salama, A.; Pusey, C.; Savage, C.O.; Harper, L. CT60 and +49 polymorphisms of CTLA 4 are associated with ANCA-positive small vessel vasculitis. Rheumatology 2009, 48, 1502–1505. [Google Scholar] [CrossRef]
- Vandenborre, K.; Van Gool, S.W.; Kasran, A.; Ceuppens, J.L.; Boogaerts, M.A.; Vandenberghe, P. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 1999, 98, 413–421. [Google Scholar] [CrossRef]
- Zeissig, S.; Petersen, B.S.; Tomczak, M.; Melum, E.; Huc-Claustre, E.; Dougan, S.K.; Laerdahl, J.K.; Stade, B.; Forster, M.; Schreiber, S.; et al. Early-onset Crohn’s disease and autoimmunity associated with a variant in CTLA-4. Gut 2015, 64, 1889–1897. [Google Scholar] [CrossRef]
- Ayrignac, X.; Goulabchand, R.; Jeziorski, E.; Rullier, P.; Carra-Dallière, C.; Lozano, C.; Portales, P.; Vincent, T.; Viallard, J.F.; Menjot de Champfleur, N.; et al. Two neurologic facets of CTLA4-related haploinsufficiency. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e751. [Google Scholar] [CrossRef]
- Egg, D.; Rump, I.C.; Mitsuiki, N.; Rojas-Restrepo, J.; Maccari, M.E.; Schwab, C.; Gabrysch, A.; Warnatz, K.; Goldacker, S.; Patiño, V.; et al. Therapeutic options for CTLA-4 insufficiency. J. Allergy Clin. Immunol. 2022, 149, 736–746. [Google Scholar] [CrossRef]
- Azizi, G.; Hesari, M.F.; Sharifinejad, N.; Fayyaz, F.; Chavoshzadeh, Z.; Mahdaviani, S.A.; Alan, M.S.; Jamee, M.; Tavakol, M.; Sadri, H.; et al. The Autoimmune Manifestations in Patients with Genetic Defects in the B Cell Development and Differentiation Stages. J. Clin. Immunol. 2023, 43, 819–834. [Google Scholar] [CrossRef]
- Sellebjerg, F.; Krakauer, M.; Khademi, M.; Olsson, T.; Sørensen, P.S. FOXP3, CBLB and ITCH gene expression and cytotoxic T lymphocyte antigen 4 expression on CD4+CD25high T cells in multiple sclerosis. Clin. Exp. Immunol. 2012, 170, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Rad, I.A.; Ahmadi-Salmasi, B. CTLA-4, PD-1 and TIM-3 expression predominantly downregulated in MS patients. J. Neuroimmunol. 2018, 323, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kiapour, N.; Kapoor, S.; Khan, T.; Thamilarasan, M.; Tao, Y.; Cohen, S.; Miller, R.; Sobel, R.A.; Markovic-Plese, S. IL-11 Induces Encephalitogenic Th17 Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2019, 203, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Fransen, N.L.; Crusius, J.B.A.; Smolders, J.; Mizee, M.R.; van Eden, C.G.; Luchetti, S.; Remmerswaal, E.B.M.; Hamann, J.; Mason, M.R.J.; Huitinga, I. Post-mortem multiple sclerosis lesion pathology is influenced by single nucleotide polymorphisms. Brain Pathol. 2020, 30, 106–119. [Google Scholar] [CrossRef]
- Basile, M.S.; Bramanti, P.; Mazzon, E. The Role of Cytotoxic T-Lymphocyte Antigen 4 in the Pathogenesis of Multiple Sclerosis. Genes 2022, 13, 1319. [Google Scholar] [CrossRef]
- Seyedsadr, M.; Wang, Y.; Elzoheiry, M.; Shree Gopal, S.; Jang, S.; Duran, G.; Chervoneva, I.; Kasimoglou, E.; Wrobel, J.A.; Hwang, D.; et al. IL-11 induces NLRP3 inflammasome activation in monocytes and inflammatory cell migration to the central nervous system. Proc. Natl. Acad. Sci. USA 2023, 120, e2221007120. [Google Scholar] [CrossRef]
- Lin, T.W.; Hu, Y.C.; Yang, Y.H.; Chien, Y.H.; Lee, N.C.; Yu, H.H.; Chiang, B.L.; Wang, L.C. CTLA-4 gene mutation and multiple sclerosis: A case report and literature review. J. Microbiol. Immunol. Infect. 2022, 55, 545–548. [Google Scholar] [CrossRef]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef]
- Renton, A.E.; Pliner, H.A.; Provenzano, C.; Evoli, A.; Ricciardi, R.; Nalls, M.A.; Marangi, G.; Abramzon, Y.; Arepalli, S.; Chong, S.; et al. A genome-wide association study of myasthenia gravis. JAMA Neurol. 2015, 72, 396–404. [Google Scholar] [CrossRef]
- Coustal, C.; Goulabchand, R.; Labauge, P.; Guilpain, P.; Carra-Dallière, C.; Januel, E.; Jeziorski, E.; Salle, V.; Viallard, J.F.; Boutboul, D.; et al. Clinical, Radiologic, and Immunologic Features of Patients With CTLA4 Deficiency With Neurologic Involvement. Neurology 2023, 101, e1560–e1566. [Google Scholar] [CrossRef]
- Grammatikos, A.; Johnston, S.; Rice, C.M.; Gompels, M. A Family with a Novel CTLA4 Haploinsufficiency Mutation and Neurological Symptoms. J. Clin. Immunol. 2021, 41, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yin, H.; Wang, L.; Cui, L.; Wang, R. Systemic autoimmune diseases complicated with hydrocephalus: Pathogenesis and management. Neurosurg. Rev. 2019, 42, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lebbé, C.; Weber, J.S.; Maio, M.; Neyns, B.; Harmankaya, K.; Hamid, O.; O’Day, S.J.; Konto, C.; Cykowski, L.; McHenry, M.B.; et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann. Oncol. 2014, 25, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, L.A.; Held, K.; Beltrán, E.; Berking, C.; Prinz, J.C.; Junker, A.; Tietze, J.K.; Ertl-Wagner, B.; Straube, A.; Kümpfel, T.; et al. CTLA4 as Immunological Checkpoint in the Development of Multiple Sclerosis. Ann. Neurol. 2016, 80, 294–300. [Google Scholar] [CrossRef]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Galmiche, S.; Lheure, C.; Kramkimel, N.; Franck, N.; Boitier, F.; Dupin, N.; Turc, G.; Psimaras, D.; Aractingi, S.; Guégan, S. Encephalitis induced by immune checkpoint inhibitors in metastatic melanoma: A monocentric retrospective study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e440–e443. [Google Scholar] [CrossRef]
- Oliveira, M.C.B.; de Brito, M.H.; Simabukuro, M.M. Central Nervous System Demyelination Associated With Immune Checkpoint Inhibitors: Review of the Literature. Front. Neurol. 2020, 11, 538695. [Google Scholar] [CrossRef]
- Berti, A.; Bortolotti, R.; Dipasquale, M.; Kinspergher, S.; Prokop, L.; Grandi, G.; Inchiostro, S.; Paolazzi, G.; Caffo, O.; Veccia, A. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit. Rev. Oncol. Hematol. 2021, 162, 103351. [Google Scholar] [CrossRef]
- Casagrande, S.; Sopetto, G.B.; Bertalot, G.; Bortolotti, R.; Racanelli, V.; Caffo, O.; Giometto, B.; Berti, A.; Veccia, A. Immune-Related Adverse Events Due to Cancer Immunotherapy: Immune Mechanisms and Clinical Manifestations. Cancers 2024, 16, 1440. [Google Scholar] [CrossRef]
- Klein, O.R.; Bonfim, C.; Abraham, A.; Ruggeri, A.; Purtill, D.; Cohen, S.; Wynn, R.; Russell, A.; Sharma, A.; Ciccocioppo, R.; et al. Transplant for non-malignant disorders: An International Society for Cell & Gene Therapy Stem Cell Engineering Committee report on the role of alternative donors, stem cell sources and graft engineering. Cytotherapy 2023, 25, 463–471. [Google Scholar]
| Study (PMID) | Immunological | Gastrointestinal | Respiratory | Neurological | Hematological | Skin | Endocrine | Skeletal | Others |
|---|---|---|---|---|---|---|---|---|---|
| 29729943 (Individual #53, fam V.II.1) | Lymphadenopathy | Lymphocytic organ infiltration (retroperitoneum) | ITP; AIHA; autoimmune neutropenia; Evans syndrome | Renal involvement | |||||
| 29729943 (Individual #99, fam SS.II.1) | Hypogammaglobulinemia (low IgG, IgM, IgA); fungal infections (Candida) | Splenomegaly; diarrhea/enteropathy, atrophic gastritis | Respiratory involvement, (upper and lower respiratory tract); severe respiratory infections (pneumonia); GLILD; bronchiectasis; lymphadenopathy and lymphocytic organ infiltration (lungs) | Lymphadenopathy and lymphocytic organ infiltration (brain); retinal tear due to lymphocytic infiltrations into the retina | RA | ||||
| 28960754 | Autoimmune lymphoproliferative syndrome (ALPS) | Hepatomegaly; splenomegaly | Evans syndrome | ||||||
| 31993940 | Positivity for anti-parietal cell antibody; hypogammaglobulinemia (low IgG) | Recurrent diarrhea; atrophic gastritis; lymphoid hyperplasia at the ileal end | Recurrent respiratory infection | Dysphagia since age 3 years | IDA; ITP; leukopenia; | Bilateral axillary lymphadenopathy | Cervical lymphadenopathy; bilateral knee arthralgia; steroid refractory RA | ||
| 32996901 | Reduction of CD19+ B cells, low levels of total IgG and IgA, and a normal IgE level; CMV infection; reduced PBMC count | Chronic diarrhea; enteropathy; cholecystectomy (probably due to AIHA) | Chronic sinusitis | ITP; Evans syndrome | Alopecia areata | Hypothyroidism, diabetes mellitus type 1 | |||
| 35753512 (P0003698) | Chronic infections; fever | Abdominal pain; splenomegaly; diarrhea | Abnormal lung morphology; pulmonary obstruction | Abnormality of vision; nausea; vomiting; anxiety; seizures; abdominal pain; distal muscle weakness; headache | AIHA; IDA | Urticaria; blepharitis | Weight loss | ||
| 32623363 | Hypogammaglobulinemia; polyvalent allergy | Crohn’s disease; sphincter dysfunction (bowel) | Bilateral pulmonary interstitial infiltration; chronic pansinusitis | Progressive headache and focal right-side-sensitive epileptic paroxysm; presence of an isolated infiltrating mass (16 × 20 × 22 mm) and lesions in the white-matter (left parieto-occipital region); T2-hyperintense lesion in the right counterpart; positive sensory symptoms in both hands; static tremor and hypesthesia of the left upper extremity (suspected MS); idiopathic intracranial hypertension; moderate central paraparesis of the lower extremities; hypesthesia; disseminated intramedullary lesions; CSF oligoclonal bands | Autoimmune thyroiditis | Juvenile seronegative RA | Recurrent uveitis, with permanent moderate vision loss due to papilledema; sphincter dysfunction (bladder) | ||
| 31955317 | Immunodeficiency with low IgG and undetectable IgA and IgM levels; CD19+ B cells were absent; lower count of CD4+ T cells; elevated lactate dehydrogenase level | Cholestasis; mediastinal adenopathy; hepato-splenomegaly, multiple lytic lesions of the liver | Interstitial fibrosis of the left lung with focal honeycombing and lymphocytes infiltrates in the fibrotic areas; sinopulmonary infections; progressive respiratory distress with hypoxemia; dyspnea | ITP progressed to pancytopenia with lymphopenia by age 23 years; erythroid and megakaryocytic hyperplasia | Anasarca; axillary lymphadenopathy | Multiple lytic lesions in the skull, ribs, and spine; cervical lymphadenopathy | |||
| 36790564 | Unspecified primary B cell defects | ||||||||
| 30443250 | Immune dysregulation-IPEX | ||||||||
| Current study | Immunodeficiency; hypogammaglobulinemia (low IgG, IgA, IgM, IgE); CVID | Chronic diarrhea; enteropathy; gallstones; celiac disease/IBD; poliposis | Interstitial pneumopathy | Hypodensity in the pons region; vermis lesions; hydrocephalus; seizures; CNS demyelination | Autoimmune thrombocytopenia; hemolytic anemia | Psoriasiform dermatitis (father) | Hashimoto thyroiditis (father and cousin) | Osteoporosis; RA (niece) | Growth delay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genio, E.; Lecca, M.; Ciccocioppo, R.; Errichiello, E. CTLA4 Alteration and Neurologic Manifestations: A New Family with Large Phenotypic Variability and Literature Review. Genes 2025, 16, 306. https://doi.org/10.3390/genes16030306
Genio E, Lecca M, Ciccocioppo R, Errichiello E. CTLA4 Alteration and Neurologic Manifestations: A New Family with Large Phenotypic Variability and Literature Review. Genes. 2025; 16(3):306. https://doi.org/10.3390/genes16030306
Chicago/Turabian StyleGenio, Edoardo, Mauro Lecca, Rachele Ciccocioppo, and Edoardo Errichiello. 2025. "CTLA4 Alteration and Neurologic Manifestations: A New Family with Large Phenotypic Variability and Literature Review" Genes 16, no. 3: 306. https://doi.org/10.3390/genes16030306
APA StyleGenio, E., Lecca, M., Ciccocioppo, R., & Errichiello, E. (2025). CTLA4 Alteration and Neurologic Manifestations: A New Family with Large Phenotypic Variability and Literature Review. Genes, 16(3), 306. https://doi.org/10.3390/genes16030306






