Molecular Cargo of Exosomes in Prostate Cancer: A Multi-Omics Perspective on Liquid Biopsies
Abstract
1. Introduction
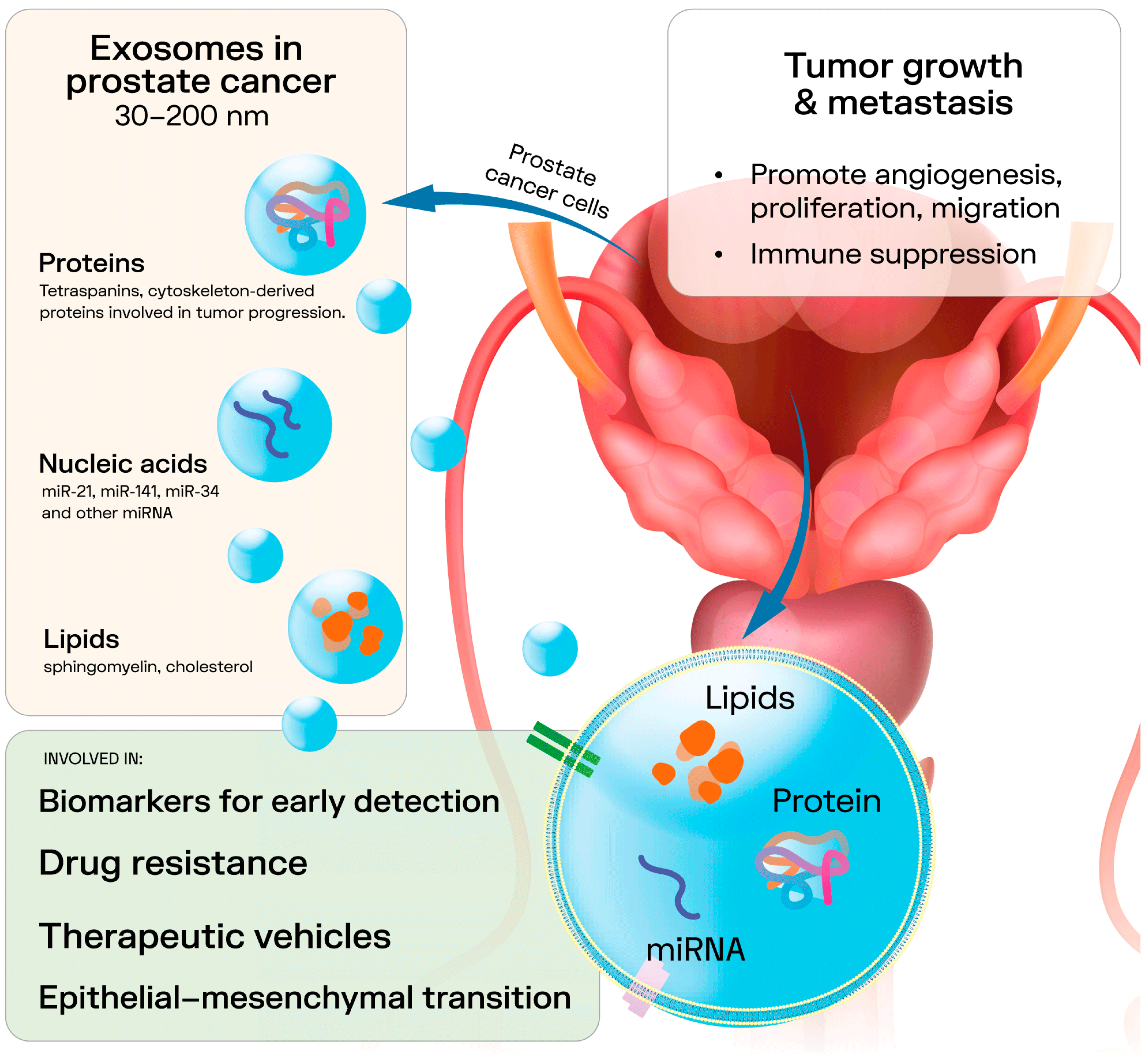
2. Exosomes
3. Exosome Isolation and Identification
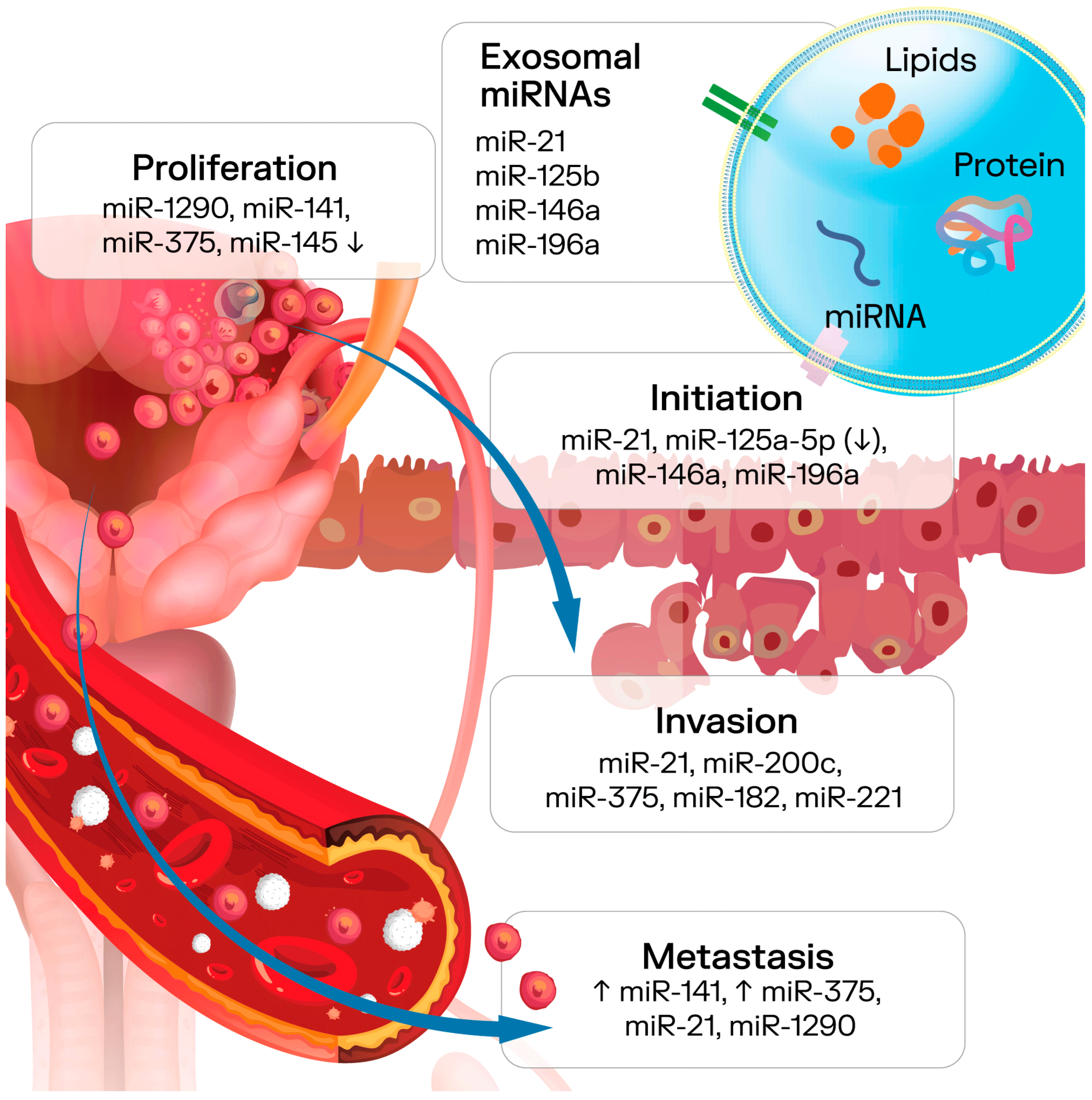
4. Tumor Microenvironment
5. Exosomes’ Potential for PCa Diagnostics
6. Exosomes’ Potential as a PCa Therapy
7. Exosomes’ Potential as a PCa Prognostic
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Commission. Prostate Cancer Burden in EU-27. 2021. Available online: https://ecis.jrc.ec.europa.eu (accessed on 20 August 2025).
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef] [PubMed]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Clinical practice. Screening for prostate cancer. N. Engl. J. Med. 2011, 365, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Van der Kwast, T.H.; Roobol, M.J. Defining the threshold for significant versus insignificant prostate cancer. Nat. Rev. Urol. 2013, 10, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Rajvansh, R.; Drake, J.M. Emerging Role of Extracellular Vesicles in Prostate Cancer. Endocrinology 2021, 162, bqab139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Gobbi, L.; Bucca, B.; Moriconi, M.; Viscuso, P.; Gentilucci, A.; Mariotti, G.; Cattarino, S.; et al. Exosome Analysis in Prostate Cancer: How They Can Improve Biomarkers’ Performance. Curr. Issues Mol. Biol. 2023, 45, 6085–6096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Semjonow, A.; Brandt, B.; Oberpenning, F.; Roth, S.; Hertle, L. Discordance of assay methods creates pitfalls for the interpretation of prostate-specific antigen values. Prostate Suppl. 1996, 7, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D.; et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤ 4.0 ng per milliliter. N. Engl. J. Med. 2004, 350, 2239–2246, Erratum in N. Engl. J. Med. 2004, 351, 1470. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, T.; Shim, S.R.; Basile, G.; Baboudjian, M.; Kói, T.; Przydacz, M.; Abufaraj, M.; Ploussard, G.; Kasivisvanathan, V.; Rivas, J.G.; et al. Magnetic Resonance Imaging in Prostate Cancer Screening: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 745–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wegelin, O.; Exterkate, L.; van der Leest, M.; Kelder, J.C.; Bosch, J.L.H.R.; Barentsz, J.O.; Somford, D.M.; van Melick, H.H.E. Complications and Adverse Events of Three Magnetic Resonance Imaging-based Target Biopsy Techniques in the Diagnosis of Prostate Cancer Among Men with Prior Negative Biopsies: Results from the FUTURE Trial, a Multicentre Randomised Controlled Trial. Eur. Urol. Oncol. 2019, 2, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Tilki, D. Biomarkers in prostate cancer—Current clinical utility and future perspectives. Crit. Rev. Oncol. Hematol. 2017, 120, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome:exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrasini, N.; Lionetti, V. Exosomes in Critical Illness. Crit. Care. Med. 2017, 45, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in Cancer Diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2021, 10, 281–294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vlaeminck-Guillem, V. Extracellular Vesicles in Prostate Cancer Carcinogenesis, Diagnosis, and Management. Front. Oncol. 2018, 8, 222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brzozowski, J.S.; Bond, D.R.; Jankowski, H.; Goldie, B.J.; Burchell, R.; Naudin, C.; Smith, N.D.; Scarlett, C.J.; Larsen, M.R.; Dun, M.D.; et al. Extracellular vesicles with altered tetraspanin CD9 and CD151 levels confer increased prostate cell motility and invasion. Sci. Rep. 2018, 8, 8822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, L.; Zhang, K.; Qing, Y.; Li, D.; Cui, M.; Jin, P.; Xu, T. Proteomic and lipidomic analysis of exosomes derived from ovarian cancer cells and ovarian surface epithelial cells. J. Ovarian Res. 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, J.; Ding, M.; Xu, K.; Yang, C.; Mao, L.J. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget 2017, 8, 97693–97700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corcoran, C.; Rani, S.; O’Driscoll, L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate 2014, 74, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Shi, T.; Srivastava, S.; Kagan, J.; Liu, T.; Rodland, K.D. Proteomic Analysis of Exosomes for Discovery of Protein Biomarkers for Prostate and Bladder Cancer. Cancers 2020, 12, 2335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 131, 56482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chuo, S.T.; Chien, J.C.; Lai, C.P. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Libregts, S.F.W.M.; Arkesteijn, G.J.A.; Németh, A.; Nolte-‘t Hoen, E.N.M.; Wauben, M.H.M. Flow cytometric analysis of extracellular vesicle subsets in plasma: Impact of swarm by particles of non-interest. J. Thromb. Haemost. 2018, 16, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles. 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paluskievicz, C.M.; Cao, X.; Abdi, R.; Zheng, P.; Liu, Y.; Bromberg, J.S. T Regulatory Cells and Priming the Suppressive Tumor Microenvironment. Front. Immunol. 2019, 10, 2453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.S.; Chen, Q.C.; Zong, H.T.; Xia, Q. Exosome miRNA-203 promotes M1 macrophage polarization and inhibits prostate cancer tumor progression. Mol. Cell. Biochem. 2024, 479, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 are associated with treatment outcome in metastatic castration resistant prostate cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanshen, Z.; Lifen, Y.; Xilian, W.; Zhong, D.; Huihong, M. miR-92a promotes proliferation and inhibits apoptosis of prostate cancer cells through the PTEN/Akt signaling pathway. Libyan J. Med. 2021, 16, 1971837, Erratum in Libyan J. Med. 2025, 20, 2475687. https://doi.org/10.1080/19932820.2025.2475687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wan, X.; Huang, W.; Yang, S.; Zhang, Y.; Zhang, P.; Kong, Z.; Li, T.; Wu, H.; Jing, F.; Li, Y. Androgen-induced miR-27A acted as a tumor suppressor by targeting MAP2K4 and mediated prostate cancer progression. Int. J. Biochem. Cell Biol. 2016, 79, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Gu, J.; Zhou, D.; Li, L.; Cheng, W.; Wang, Y.; Tang, T.; Wang, X. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp. Mol. Med. 2020, 52, 1809–1822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zennami, K.; Graham, M.; Chikara, S.; Sysa-Shah, P.; Rafiqi, F.H.; Wang, R.; Abel, B.; Zeng, Q.; Krueger, T.E.G.; Brennen, W.N.; et al. Cell Type-Specific Effects of miR-21 Loss Attenuate Tumor Progression in MYC-Driven Prostate Cancer. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Liu, Q.; Liao, Q. Long noncoding RNA: A dazzling dancer in tumor immune microenvironment. J. Exp. Clin. Cancer Res. 2020, 39, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Chen, J.Q.; Liu, T.J.; Chen, Y.L.; Ma, Z.K.; Fan, Y.Z.; Wang, Z.X.; Xu, S.; Wang, K.; Wang, X.Y.; et al. Knocking down AR promotes osteoblasts to recruit prostate cancer cells by altering exosomal circ-DHPS/miR-214-3p/CCL5 pathway. Asian J. Androl. 2024, 26, 195–204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, Y.; Gao, X. Inhibition of cancer cell-derived exosomal microRNA-183 suppresses cell growth and metastasis in prostate cancer by upregulating TPM1. Cancer Cell Int. 2021, 21, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, T.; Glavich, G.J.; Pahuski, M.; Short, A.; Semmes, O.J.; Yang, L.; Galkin, V.; Drake, R.; Esquela-Kerscher, A. Characterization and Evidence of the miR-888 Cluster as a Novel Cancer Network in Prostate. Mol. Cancer Res. 2018, 16, 669–681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Sui, B.; Fan, W.; Lei, L.; Zhou, L.; Yang, L.; Diao, Y.; Zhang, Y.; Li, Z.; Liu, J.; et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J. Extracell Vesicles 2021, 10, e12056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furesi, G.; de Jesus Domingues, A.M.; Alexopoulou, D.; Dahl, A.; Hackl, M.; Schmidt, J.R.; Kalkhof, S.; Kurth, T.; Taipaleenmäki, H.; Conrad, S.; et al. Exosomal miRNAs from Prostate Cancer Impair Osteoblast Function in Mice. Int. J. Mol. Sci. 2022, 23, 1285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamid, Y.; Rabbani, R.D.; Afsara, R.; Nowrin, S.; Ghose, A.; Papadopoulos, V.; Sirlantzis, K.; Ovsepian, S.V.; Boussios, S. Exosomal Liquid Biopsy in Prostate Cancer: A Systematic Review of Biomarkers for Diagnosis, Prognosis, and Treatment Response. Int. J. Mol. Sci. 2025, 26, 802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Dong, Y.; Wang, K.J.; Deng, Z.; Zhang, W.; Shen, H.F. Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. Neoplasma 2020, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- de Nóbrega, M.; Dos Reis, M.B.; Pereira, É.R.; de Souza, M.F.; de Syllos Cólus, I.M. The potential of cell-free and exosomal microRNAs as biomarkers in liquid biopsy in patients with prostate cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 2893–2910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, J.; Zeng, X.; Wang, X.; Wu, Y.; Lei, P.; Wang, Z.; Yang, C.; Hu, Z. Effective Diagnosis of Prostate Cancer Based on mRNAs From Urinary Exosomes. Front. Med. 2022, 9, 736110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Yu, C.; Jiang, K.; Yang, G.; Yang, S.; Tan, S.; Li, T.; Liang, H.; He, Q.; Wei, F.; et al. Unveiling potential: Urinary exosomal mRNAs as non-invasive biomarkers for early prostate cancer diagnosis. BMC. Urol. 2024, 24, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Q.; Chiu, P.K.; Wong, C.Y.; Cheng, C.K.; Teoh, J.Y.; Ng, C.F. Identification of piRNA Targets in Urinary Extracellular Vesicles for the Diagnosis of Prostate Cancer. Diagnostics 2021, 11, 1828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markert, L.; Holdmann, J.; Klinger, C.; Kaufmann, M.; Schork, K.; Turewicz, M.; Eisenacher, M.; Savelsbergh, A. Small RNAs as biomarkers to differentiate benign and malign prostate diseases: An alternative for transrectal punch biopsy of the prostate? PLoS ONE 2021, 16, e0247930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.H.; Ji, J.; Wang, B.C.; Chen, H.; Yang, Z.H.; Wang, K.; Luo, C.L.; Zhang, W.W.; Wang, F.B.; Zhang, X.L. Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell. Physiol. Biochem. 2018, 46, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Wang, B.Y.; Luo, L.; Li, Q.; Meng, Z.A.; Xia, T.L.; Deng, W.M.; Yang, M.; Zhou, J.; Zhang, X.; et al. A urine extracellular vesicle lncRNA classifier for high-grade prostate cancer and increased risk of progression: A multi-center study. Cell Rep. Med. 2023, 4, 101240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Ji, J.; Lyu, J.; Jin, X.; He, X.; Mo, S.; Xu, H.; He, J.; Cao, Z.; Chen, X.; et al. A Novel Urine Exosomal lncRNA Assay to Improve the Detection of Prostate Cancer at Initial Biopsy: A Retrospective Multicenter Diagnostic Feasibility Study. Cancers 2021, 13, 4075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soekmadji, C.; Corcoran, N.M.; Oleinikova, I.; Jovanovic, L.; Australian Prostate Cancer Collaboration BioResource; Ramm, G.A.; Nelson, C.C.; Jenster, G.; Russell, P.J. Extracellular vesicles for personalized therapy decision support in advanced metastatic cancers and its potential impact for prostate cancer. Prostate 2017, 77, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, X.; Huang, Y.; Zhang, X.; Sun, W.; Du, Y.; Xu, Z.; Kou, H.; Zhu, S.; Liu, C.; et al. Prostate cancer cell-derived exosomal IL-8 fosters immune evasion by disturbing glucolipid metabolism of CD8 + T cell. Cell Rep. 2023, 42, 113424. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; García-Cortés, Á.; Torres, M.; Ancizu-Marckert, J.; Ignacio Pascual, J.; Díez-Caballero, F.; Enrique Robles, J.; Rosell, D.; Miñana, B.; Mateos, B.; et al. Characterisation of the perioperative changes of exosomal immune-related cytokines induced by prostatectomy in early-stage prostate cancer patients. Cytokine 2021, 141, 155471. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Capasso, C.; Del Prete, S.; Di Raimo, R.; Falchi, M.; Angelini, D.F.; Sciarra, A.; Maggi, M.; Supuran, C.T.; et al. Plasmatic exosomes from prostate cancer patients show increased carbonic anhydrase IX expression and activity and low pH. J. Enzyme. Inhib. Med. Chem. 2020, 35, 280–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huertas-Lárez, R.; Muñoz-Moreno, L.; Recio-Aldavero, J.; Román, I.D.; Arenas, M.I.; Blasco, A.; Sanchís-Bonet, Á.; Bajo, A.M. Induction of more aggressive tumoral phenotypes in LNCaP and PC3 cells by serum exosomes from prostate cancer patients. Int. J. Cancer 2023, 153, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiong, W.Y.; Hou, H.J.; Xu, Q.; Cai, X.L.; Zeng, T.X.; Ha, X.Q. Exosomal miRNAs as Biomarkers of Cancer: A Meta-Analysis. Clin. Lab. 2019, 65, e23956. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, C.; Zhang, Y.F.; He, H.; Wang, D.; Lv, H.X.; Yang, Z.J.; Wang, J.; Ren, Y.Q.; Zhang, W.B.; et al. Integrating plasma exosomal miRNAs, ultrasound radiomics and tPSA for the diagnosis and prediction of early prostate cancer: A multi-center study. Clin. Transl. Oncol. 2025, 27, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Sonar, S. Decoding the mysteries of prostate cancer via cutting-edge liquid biopsy-based urine exosomes profiling. J. Liq. Biopsy 2024, 6, 100162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, M.; Bajo-Santos, C.; Hessvik, N.P.; Lorenz, S.; Fromm, B.; Berge, V.; Sandvig, K.; Linē, A.; Llorente, A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer 2017, 16, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foj, L.; Ferrer, F.; Serra, M.; Arévalo, A.; Gavagnach, M.; Giménez, N.; Filella, X. Exosomal and Non-Exosomal Urinary miRNAs in Prostate Cancer Detection and Prognosis. Prostate 2017, 77, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Paskeh, M.D.A.; Okina, E.; Gholami, M.H.; Hushmandi, K.; Hashemi, M.; Kalu, A.; Zarrabi, A.; Nabavi, N.; Rabiee, N.; et al. Molecular Landscape of LncRNAs in Prostate Cancer: A focus on pathways and therapeutic targets for intervention. J. Exp. Clin. Cancer Res. 2022, 41, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Endzeliņš, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Soboļevska, K.; Ābols, A.; Rodriguez, M.; Šantare, D.; Rudņickiha, A.; Lietuvietis, V.; et al. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barceló, M.; Castells, M.; Bassas, L.; Vigués, F.; Larriba, S. Semen miRNAs Contained in Exosomes as Non-Invasive Biomarkers for Prostate Cancer Diagnosis. Sci. Rep. 2019, 9, 13772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, Y.L.; Chu, P.Y.; Lee, B.H.; Chen, K.C.; Yang, C.Y.; Kuo, W.H.; Shen, T.L. Basics and applications of tumor-derived extracellular vesicles. J. Biomed. Sci. 2019, 26, 35. [Google Scholar] [CrossRef]
- Wang, S.; Du, P.; Cao, Y.; Ma, J.; Yang, X.; Yu, Z.; Yang, Y. Cancer associated fibroblasts secreted exosomal miR-1290 contributes to prostate cancer cell growth and metastasis via targeting GSK3β. Cell Death Discov. 2022, 8, 371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vardaki, I.; Corn, P.; Gentile, E.; Song, J.H.; Madan, N.; Hoang, A.; Parikh, N.; Guerra, L.; Lee, Y.C.; Lin, S.C.; et al. Radium-223 Treatment Increases Immune Checkpoint Expression in Extracellular Vesicles from the Metastatic Prostate Cancer Bone Microenvironment. Clin. Cancer Res. 2021, 27, 3253–3264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aaltomaa, S.; Lipponen, P.; Ala-Opas, M.; Kosma, V.M. Expression and prognostic value of CD44 standard and variant v3 and v6 isoforms in prostate cancer. Eur. Urol. 2001, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, B.; Wu, A.; Wetterskog, D.; Attard, G. Blood-based liquid biopsies for prostate cancer: Clinical opportunities and challenges. Br. J. Cancer 2022, 127, 1394–1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coman, R.A.; Schitcu, V.H.; Budisan, L.; Raduly, L.; Braicu, C.; Petrut, B.; Coman, I.; Berindan-Neagoe, I.; Al Hajjar, N. Evaluation of miR-148a-3p and miR-106a-5p as Biomarkers for Prostate Cancer: Pilot Study. Genes 2024, 15, 584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, T.; Mizutani, K.; Kameyama, K.; Kawakami, K.; Fujita, Y.; Nakane, K.; Kanimoto, Y.; Ehara, H.; Ito, H.; Seishima, M.; et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol. Oncol. 2015, 33, 385.e15–385.e20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Guan, H.; Mizokami, A.; Keller, E.T.; Xu, X.; Liu, X.; Tan, J.; Hu, L.; Lu, Y.; et al. Exosome-derived microRNAs contribute to prostate cancer chemoresistance. Int. J. Oncol. 2016, 49, 838–846. [Google Scholar] [CrossRef]
- Kharaziha, P.; Chioureas, D.; Rutishauser, D.; Baltatzis, G.; Lennartsson, L.; Fonseca, P.; Azimi, A.; Hultenby, K.; Zubarev, R.; Ullén, A.; et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget 2015, 6, 21740–21754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; McDermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin β4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int. J. Oncol. 2015, 47, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, T.; Zhou, W.; Feng, C.; Zhou, Z.; Chen, Y.; Li, D.; Chen, L.; Zhao, J.; Zhang, Y.; et al. CAF-derived exosomal miR-196b-5p after androgen deprivation therapy promotes epithelial-mesenchymal transition in prostate cancer cells through HOXC8/NF-κB signaling pathway. Biol. Direct. 2025, 20, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Guo, H.; Liu, S.; Hu, Y.; Huang, Y.; Rong, J.; Yuan, F.; Wang, R.; Wang, Z. Blocking secretion of exosomes by GW4869 dampens CD8+ T cell exhaustion and prostate cancer progression. Hum. Cell 2025, 38, 131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pszczółkowska-Kępa, B.; Olejarz, W.; Głuszko, A.; Wałpuski, G.; Lorenc, T.; Brzozowska, B. Exosome-mediated modulation of radioresistance: The radiation-induced bystander effect in prostate cancer cells. PLoS ONE 2025, 20, e0330501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.Y.; Moon, H.W.; Jo, M.S.; Lee, J.Y. Exosomal miR-664a-5p as a therapeutic target biomarker for PARP inhibitor response in prostate cancer. Am. J. Cancer Res. 2024, 14, 3789–3799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puhka, M.; Takatalo, M.; Nordberg, M.E.; Valkonen, S.; Nandania, J.; Aatonen, M.; Yliperttula, M.; Laitinen, S.; Velagapudi, V.; Mirtti, T.; et al. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics 2017, 7, 3824–3841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Del Re, M.; Conteduca, V.; Crucitta, S.; Gurioli, G.; Casadei, C.; Restante, G.; Schepisi, G.; Lolli, C.; Cucchiara, F.; Danesi, R.; et al. Androgen receptor gain in circulating free DNA and splicing variant 7 in exosomes predict clinical outcome in CRPC patients treated with abiraterone and enzalutamide. Prostate. Cancer Prostatic. Dis. 2021, 24, 524–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malla, B.; Aebersold, D.M.; Dal Pra, A. Protocol for serum exosomal miRNAs analysis in prostate cancer patients treated with radiotherapy. J. Transl. Med. 2018, 16, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Chen, W.; Zhang, C.; He, Q.; Wang, X.; Han, J.; Gao, P.; Wang, K.; Xie, H.; Gao, F.; et al. Prostate Cancer Cells Secrete PD-1 in Exosomes to Enhance Myeloid-Derived Suppressor Cell Activity and Promote Tumor Immune Evasion. Cancer Res. 2025, 85, 3435–3453. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Pang, B.; Li, J.; Gao, N.; Fan, T.; Li, Y. Emerging Role of Exosomes in Liquid Biopsy for Monitoring Prostate Cancer Invasion and Metastasis. Front. Cell Dev. Biol. 2021, 9, 679527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Sorokin, I.; Aleksic, I.; Fisher, H.; Kaufman, R.P., Jr.; Winer, A.; McNeill, B.; Gupta, R.; Tilki, D.; Fleshner, N.; et al. Expression of Small Noncoding RNAs in Urinary Exosomes Classifies Prostate Cancer into Indolent and Aggressive Disease. J. Urol. 2020, 204, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Luo, Z.H.; He, Y.D.; Wang, B.Y.; Xia, T.L.; Deng, W.M.; Zhang, L.X.; Tang, X.M.; Meng, Z.A.; Gao, X.; et al. Plasma extracellular vesicle circRNA signature and resistance to abiraterone in metastatic castration-resistant prostate cancer. Br. J. Cancer 2023, 128, 1320–1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zavridou, M.; Strati, A.; Bournakis, E.; Smilkou, S.; Tserpeli, V.; Lianidou, E. Prognostic Significance of Gene Expression and DNA Methylation Markers in Circulating Tumor Cells and Paired Plasma Derived Exosomes in Metastatic Castration Resistant Prostate Cancer. Cancers 2021, 13, 780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kretschmer, A.; Tutrone, R.; Alter, J.; Berg, E.; Fischer, C.; Kumar, S.; Torkler, P.; Tadigotla, V.; Donovan, M.; Sant, G.; et al. Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome. World J. Urol. 2022, 40, 983–989. [Google Scholar] [CrossRef]
- Ruiz-Plazas, X.; Altuna-Coy, A.; Alves-Santiago, M.; Vila-Barja, J.; García-Fontgivell, J.F.; Martínez-González, S.; Segarra-Tomás, J.; Chacón, M.R. Liquid Biopsy-Based Exo-oncomiRNAs Can Predict Prostate Cancer Aggressiveness. Cancers 2021, 13, 250. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wu, L.; Zhai, Y.; Zhu, Y.; Wang, W.; Chen, D.; Xing, N.; Ye, X.; Yang, F. Identification and verification of exosome-related gene signature to predict the cancer status and prognosis of prostate cancer. Discov. Oncol. 2025, 16, 1680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, B.; Luo, T.; Wu, Y.; Hu, J.; Yang, C.; Wu, J.; Luo, Y.; Shangguan, W.; Li, W.; Yang, L.; et al. Urinary exosomal FAM153C-RPL19 chimeric RNA as a diagnostic and prognostic biomarker for prostate cancer in Chinese patients. Cancer Lett. 2025, 631, 217938. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to ad vanced approaches. J. Extracell. Vesicles 2024, 13, e12404, Erratum in J. Extracell. Vesicles 2024, 13, e12451. https://doi.org/10.1002/jev2.12451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Year | Exosomal Biomarkers | Sample Source | Exosomal Contents | Clinical Practice | Change in Expression | Reference |
|---|---|---|---|---|---|---|
| 2017 | miR-196a-5p, miR-34a-5p, miR-143-3p, miR-501-3p, miR-92a-1-5p | Urine | miRNAs | 20 PCa vs. 9 healthy people | Reduced | [76] |
| 2017 | miR-21, miR-141, miR-375 | Urine | miRNAs | 60 PCa vs. 10 healthy people | Increased | [77] |
| 2020 | (miR-125a-5p/miR-141-3p) ratio | Plasma | miRNAs | 31 PCa vs. 19 healthy people | [59] | |
| 2022 | lncRNA-p21 | Urine | lncRNAs | Identify PCa with benign disease | Increased | [78] |
| 2017 | miR-375 | Serum | miRNAs | 50 PCa vs. 22 BPH | Increased | [79] |
| 2019 | (PSA + miR-142-3p + miR-142-5p + miR-223-3p) panel | Semen | miRNAs | 31 PCa vs. 24 BPH | Increased | [80] |
| 2019 | Survivin | Plasma | miRNAs | Early detection of PCa | Increased | [81] |
| Cargo Type | Representative Molecules | Molecular/Functional Role | Disease Context in Prostate Cancer | Clinical Implication | Key References |
|---|---|---|---|---|---|
| miRNAs | miR-141, miR-21, miR-375, miR-1290, miR-423-5p | Regulate EMT, AR signaling, cell survival, immune modulation | Elevated in metastatic and CRPC patients; associated with treatment resistance and tumor aggressiveness | Diagnostic: Distinguish PCa vs. benign disease; Prognostic: Predict metastatic progression and biochemical recurrence | [48,77,82] |
| lncRNAs | PCA3, SChLAP1, MALAT1, PCAT-1 | Compete with miRNAs, alter chromatin remodeling, modulate AR co-regulators | Overexpressed in high-risk PCa and detectable in urinary exosomes | Non-invasive diagnosis (especially urine-based tests), risk stratification | [65,66,67] |
| Proteins | PSA, PSMA, PD-L1, ITGB4, CD44v6 | Promote immune evasion, metastatic adhesion, chemoresistance signaling | Elevated in exosomes from drug-resistant tumor cells; PD-L1+ exosomes correlate with immune suppression | Predictive biomarker for therapy response (ADT/AR-targeted therapy/docetaxel); potential therapeutic targets | [68,83,84] |
| Metabolites & Enzymes | Carbonic anhydrase IX, γ-glutamyltransferase (GGT), Lactate-associated metabolites | Regulate extracellular acidification, oxidative stress adaptation, metabolic reprogramming | Support tumor cell survival under hypoxia, promote bone niche formation in metastasis | Prognostic markers for aggressiveness; potential targets for metabolic intervention | [71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, R.A.; Nutu, A.; Strilciuc, S.; Budisan, L.; Berindan-Neagoe, I. Molecular Cargo of Exosomes in Prostate Cancer: A Multi-Omics Perspective on Liquid Biopsies. Genes 2025, 16, 1437. https://doi.org/10.3390/genes16121437
Coman RA, Nutu A, Strilciuc S, Budisan L, Berindan-Neagoe I. Molecular Cargo of Exosomes in Prostate Cancer: A Multi-Omics Perspective on Liquid Biopsies. Genes. 2025; 16(12):1437. https://doi.org/10.3390/genes16121437
Chicago/Turabian StyleComan, Roxana Andra, Andreea Nutu, Stefan Strilciuc, Liviuta Budisan, and Ioana Berindan-Neagoe. 2025. "Molecular Cargo of Exosomes in Prostate Cancer: A Multi-Omics Perspective on Liquid Biopsies" Genes 16, no. 12: 1437. https://doi.org/10.3390/genes16121437
APA StyleComan, R. A., Nutu, A., Strilciuc, S., Budisan, L., & Berindan-Neagoe, I. (2025). Molecular Cargo of Exosomes in Prostate Cancer: A Multi-Omics Perspective on Liquid Biopsies. Genes, 16(12), 1437. https://doi.org/10.3390/genes16121437









