The E3 Ligase UBR5/Hyd Ensures Meiotic Fidelity Through Catalysis-Independent Regulation of β2-Tubulin in Drosophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Husbandry and Stocks
2.2. Immunofluorescence Staining and Microscopy
2.3. Mosaic Clonal Analysis
2.4. Genomic Editing by CRISPR-Cas9
2.5. Statistical Analyses
3. Results
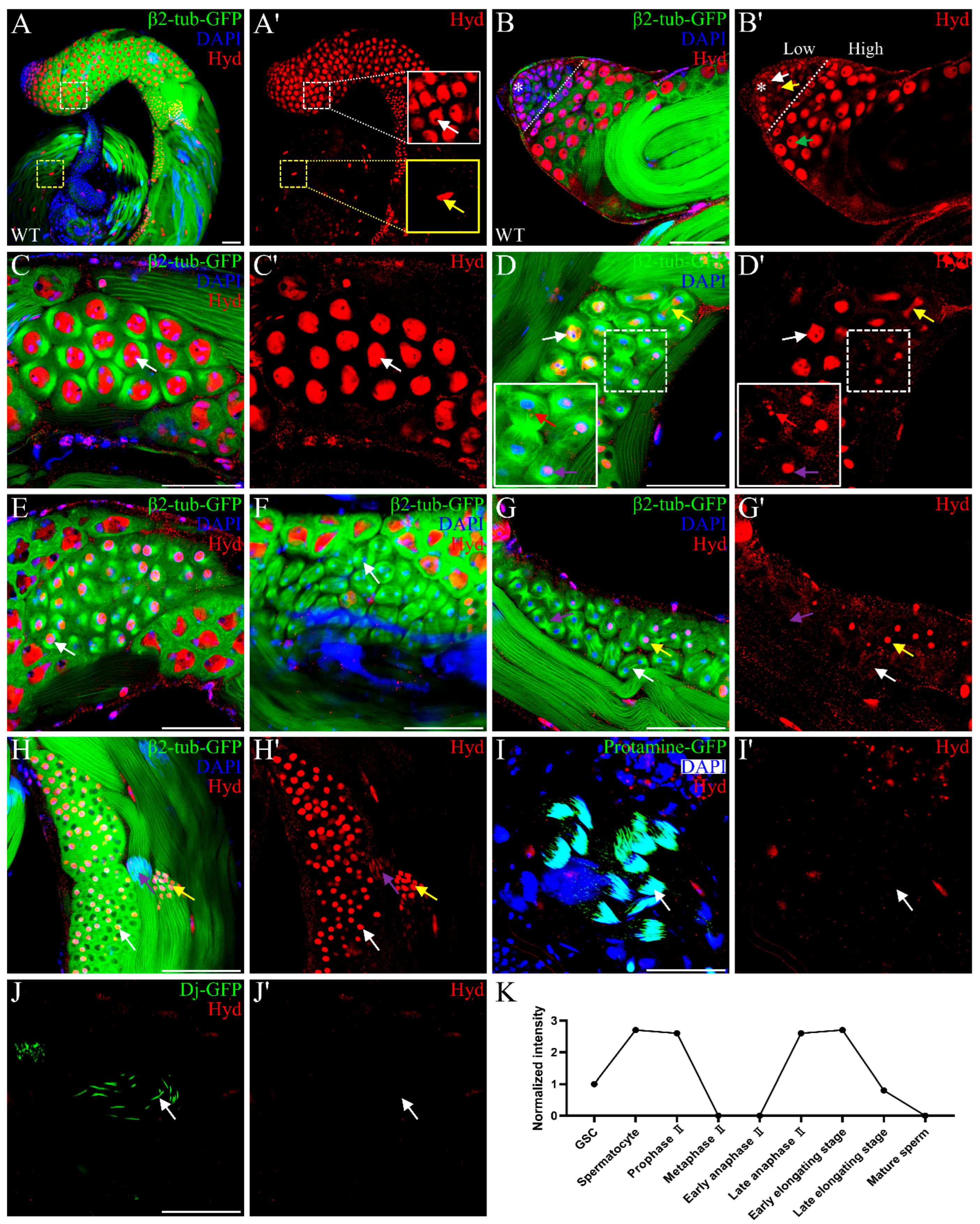
3.1. Hyd Displays a Dynamic, Stage-Specific Expression Pattern During Spermatogenesis
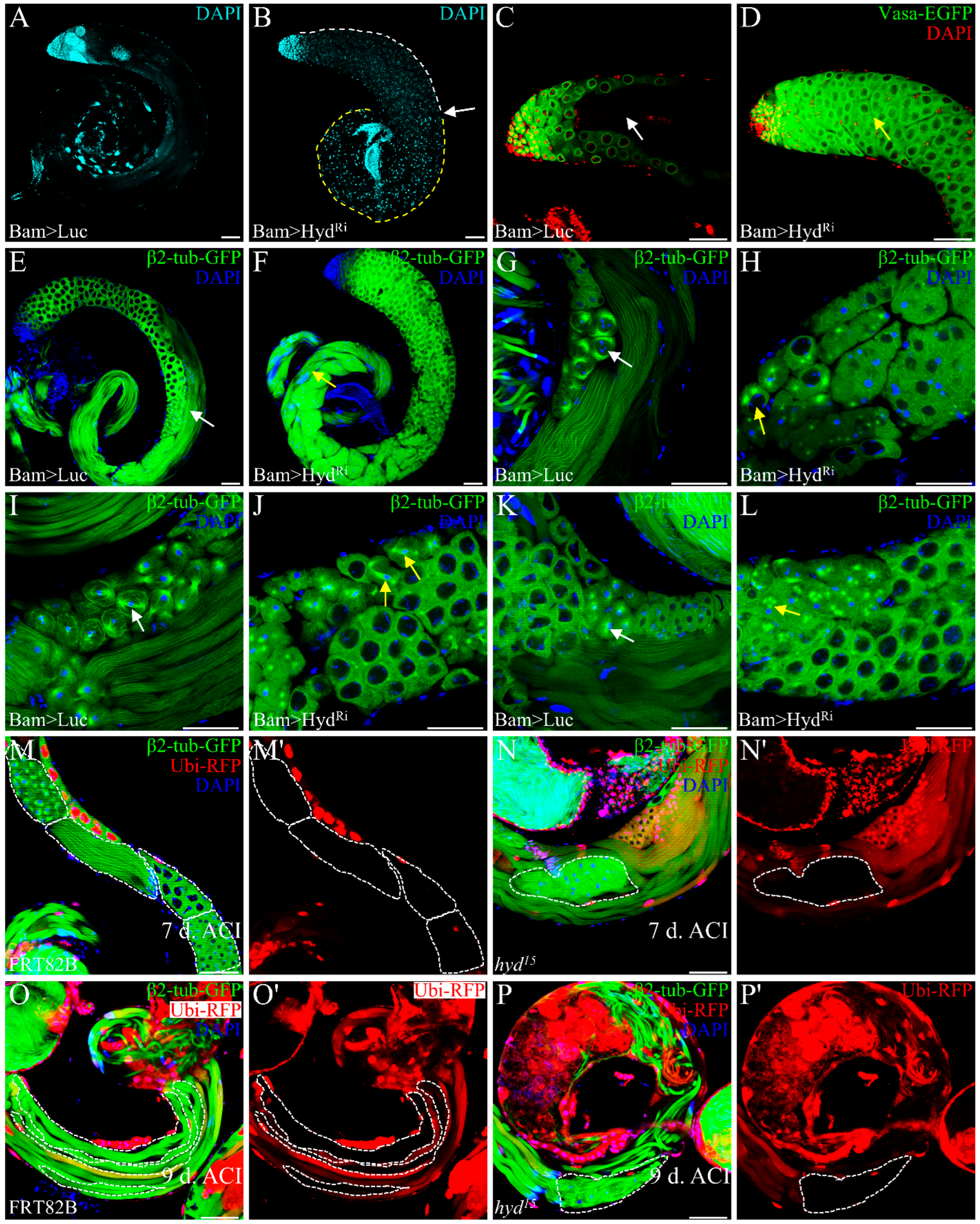
3.2. Hyd Depletion Disrupts Meiotic Progression and Spermatid Differentiation
3.3. Hyd Regulates Germ Cell Differentiation Independently of Its E3 Ubiquitin Ligase Activity
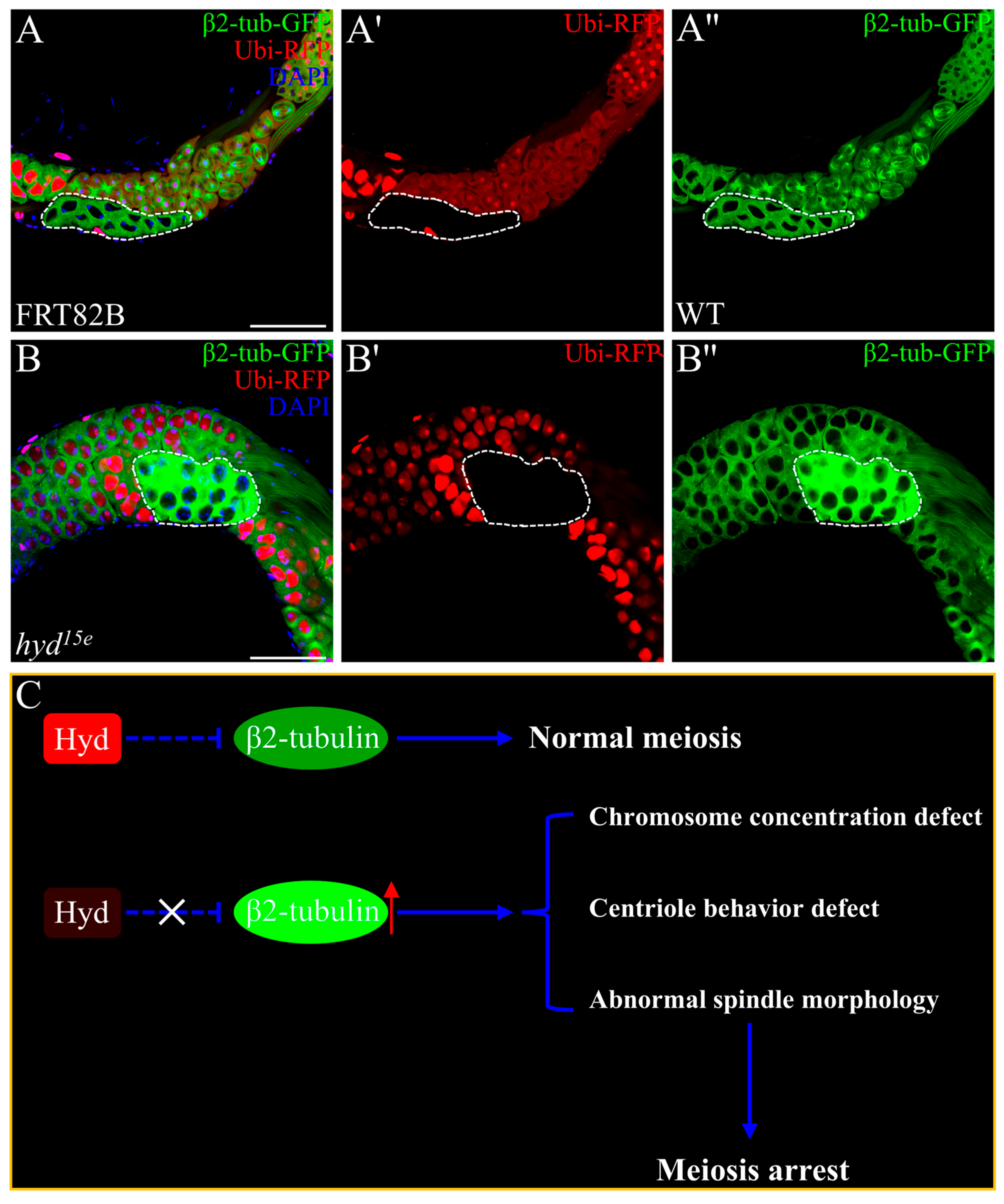
3.4. Hyd Deletion Leads to Aberrant Accumulation of β2-Tubulin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R.J.T.L. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar]
- Kanippayoor, R.L.; Alpern, J.H.; Moehring, A.J. Protamines and spermatogenesis in Drosophila and Homo sapiens: A comparative analysis. Spermatogenesis 2013, 3, e24376. [Google Scholar] [CrossRef]
- Kemphues, K.J.; Kaufman, T.C.; Raff, R.A.; Raff, E.C. The Testis-Specific Beta-Tubulin Subunit in Drosophila-Melanogaster Has Multiple Functions in Spermatogenesis. Cell 1982, 31, 655–670. [Google Scholar] [CrossRef]
- Frappaolo, A.; Piergentili, R.; Giansanti, M.G. Microtubule and Actin Cytoskeletal Dynamics in Male Meiotic Cells of Drosophila melanogaster. Cells 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.J.; Cauchi, L.M.; Dunleavy, J.E.; Burke, R.; Hime, G.R.; O’Bryan, M.K.J.H.R.U. A comparison of spermatogenesis between flies and men—Conserved processes of male gamete production. Hum. Reprod. Update 2025, dmaf018. [Google Scholar] [CrossRef]
- Yu, J.; Lan, X.; Chen, X.; Yu, C.; Xu, Y.; Liu, Y.; Xu, L.; Fan, H.Y.; Tong, C. Protein synthesis and degradation are essential to regulate germline stem cell homeostasis in Drosophila testes. Development 2016, 143, 2930–2945. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.; Wen, Y.; Liu, Y.J.; Zhou, T.; Ni, B.X.; Lin, Y.; Dong, J.; Zhou, Z.M.; Hu, Z.B.; et al. Identification of seven genes essential for male fertility through a genome-wide association study of non-obstructive azoospermia and RNA interference-mediated large-scale functional screening in Drosophila. Hum. Mol. Genet. 2015, 24, 1493–1503. [Google Scholar] [CrossRef]
- EMatunis, L.; Stine, R.R.; de Cuevas, M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis 2012, 2, 137–144. [Google Scholar] [CrossRef] [PubMed]
- White-Cooper, H. Tissue, cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis 2012, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, L.J.; de Cuevas, M.; Matunis, E. Genetics of Gonadal Stem Cell Renewal. Annu. Rev. Cell Dev. Bi. 2015, 31, 291–315. [Google Scholar] [CrossRef]
- White-Cooper, H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010, 139, 11–21. [Google Scholar] [CrossRef]
- Demarco, R.S.; Eikenes, A.H.; Haglund, K.; Jones, D.L. Investigating spermatogenesis in Drosophila melanogaster. Methods 2014, 68, 218–227. [Google Scholar] [CrossRef]
- de Cuevas, M.; Matunis, E.L. The stem cell niche: Lessons from the Drosophila testis. Development 2011, 138, 2861–2869. [Google Scholar] [CrossRef]
- Tasaki, T.; Zakrzewska, A.; Dudgeon, D.D.; Jiang, Y.; Lazo, J.S.; Kwon, Y.T. The substrate recognition domains of the N-end rule pathway. J. Biol. Chem. 2009, 284, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Liu, Y.; Prada, M.L.; Mohan, A.K.; Gupta, A.; Jaiswal, A.; Sharma, M.; Merisaari, J.; Haikala, H.M.; Talvinen, K.; et al. UBR5 Is Coamplified with MYC in Breast Tumors and Encodes an Ubiquitin Ligase That Limits MYC-Dependent Apoptosis. Cancer Res. 2020, 80, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Shearer, R.F.; Iconomou, M.; Watts, C.K.W.; Saunders, D.N. Functional Roles of the E3 Ubiquitin Ligase UBR5 in Cancer. Mol. Cancer Res. 2015, 13, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Lei, H.; Deng, H.; Deng, S.; Tirado, C.R.; Wang, M.; Mu, P.; Zheng, Y.; Pan, D. Hyd/UBR5 defines a tumor suppressor pathway that links Polycomb repressive complex to regulated protein degradation in tissue growth control and tumorigenesis. Genes Dev. 2024, 38, 675–691. [Google Scholar] [CrossRef]
- Mansfield, E.; Hersperger, E.; Biggs, J.; Shearn, A. Genetic and molecular analysis of hyperplastic discs, a gene whose product is required for regulation of cell proliferation in Drosophila melanogaster imaginal discs and germ cells. Dev. Biol. 1994, 165, 507–526. [Google Scholar] [CrossRef]
- Martin, P.; Martin, A.; Shearn, A. Studies of l(3)c43hs1 a polyphasic, temperature-sensitive mutant of Drosophila melanogaster with a variety of imaginal disc defects. Dev. Biol. 1977, 55, 213–232. [Google Scholar] [CrossRef]
- Pertceva, J.A.; Dorogova, N.V.; Bolobolova, E.U.; Nerusheva, O.O.; Fedorova, S.A.; Omelyanchuk, L.V. The role of Drosophila hyperplastic discs gene in spermatogenesis. Cell Biol. Int. 2010, 34, 991–996. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Q.; Chan, B.; Liu, H.; Singh, S.R.; Manley, J.; Lee, J.; Weideman, A.M.; Hou, G.; Hou, S.X. Whole-animal genome-wide RNAi screen identifies networks regulating male germline stem cells in Drosophila. Nat. Commun. 2016, 7, 12149. [Google Scholar] [CrossRef]
- Chen, D.; McKearin, D.M. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 2003, 130, 1159–1170. [Google Scholar] [CrossRef]
- Kelso, R.J.; Buszczak, M.; Quinones, A.T.; Castiblanco, C.; Mazzalupo, S.; Cooley, L. Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 2004, 32, D418–D420. [Google Scholar] [CrossRef]
- Alzyoud, E.; Nemeth, D.; Vedelek, V.; Szogi, T.; Toth, V.P.; Krecsmarik, M.; Abraham, E.; Lipinszki, Z.; Sinka, R. Versatile gamma-tubulin complexes contribute to the dynamic organization of MTOCs during Drosophila spermatogenesis. Commun. Biol. 2024, 7, 1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Tang, X.F.; Chen, Y.J.; Cao, J.; Huang, Q.Z.; Ling, X.M.; Ren, W.Y.; Liu, S.Q.; Wu, Y.H.; Ray, L.; et al. Hyperplastic discs differentially regulates the transcriptional outputs of hedgehog signaling. Mech Develop. 2014, 133, 117–125. [Google Scholar] [CrossRef]
- Ligoxygakis, P.; Cammarata-Mouchtouris, A.; Nguyen, X.-H.; Acker, A.; Bonnay, F.; Goto, A.; Orian, A.; Fauvarque, M.-O.; Boutros, M.; Reichhart, J.-M.; et al. Hyd ubiquitinates the NF-κB co-factor Akirin to operate an effective immune response in Drosophila. PLoS Pathog. 2020, 16, e1008458. [Google Scholar]
- Moncrieff, S.; Moncan, M.; Scialpi, F.; Ditzel, M. Regulation of hedgehog Ligand Expression by the N-End Rule Ubiquitin-Protein Ligase Hyperplastic Discs and the Drosophila GSK3beta Homologue, Shaggy. PLoS ONE 2015, 10, e0136760. [Google Scholar] [CrossRef]
- Xu, T.; Rubin, G.M. Analysis of Genetic Mosaics in Developing and Adult Drosophila Tissues. Development 1993, 117, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.T. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Fabian, L.; Brill, J.A. Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Ye, X.; Lin, Y.; Zeng, L.; Luo, Y.; Zhou, L.; Shen, C.; Hu, W.; Yan, D.; et al. The Drosophila Dpp/BMP signaling directly regulates dpp transcription for optimal ligand dosage. Development 2025, dev.204488. [Google Scholar]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef]
- Gratz, S.J.; Rubinstein, C.D.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. CRISPR-Cas9 Genome Editing in Drosophila. Curr. Protoc. Mol. Biol. 2015, 111, 31.2.1–31.2.20. [Google Scholar] [CrossRef]
- Jianbo, W.; Xin, L.; Zhiyang, G.; Lin, P.; Xian, L.; Xiahe, H.; Yingchun, W.; Zhaohui, W. RNA Kinase CLP1/Cbc Regulates Meiosis Initiation in Spermatogenesis. Hum. Mol. Genet. 2021, 30, 1569–1578. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Cenci, G.; Bonaccorsi, S.; Pisano, C.; Verni, F.; Gatti, M. Chromatin and Microtubule Organization during Premeiotic, Meiotic and Early Postmeiotic Stages of Drosophila-Melanogaster Spermatogenesis. J. Cell Sci. 1994, 107, 3521–3534. [Google Scholar] [CrossRef]
- Baker, C.C.; Gim, B.S.; Fuller, M.T. Cell type-specific translational repression of Cyclin B during meiosis in males. Development 2015, 142, 3394–3402. [Google Scholar] [CrossRef]
- Fari, K.; Takacs, S.; Ungar, D.; Sinka, R. The role of acroblast formation during Drosophila spermatogenesis. Biol. Open 2016, 5, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Blumer, N.; Kampfer, M.; Renkawitz-Pohl, R. Flagellar mitochondrial association of the male-specific Don Juan protein in Drosophila spermatozoa. J. Cell Sci. 1998, 111, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Meng, S.; Lu, Y.; Trombly, M.I.; Chen, J.; Lin, C.; Turk, A.; Wang, X. Mammalian hyperplastic discs homolog EDD regulates miRNA-mediated gene silencing. Mol. Cell 2011, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kemphues, K.J.; Raff, E.C.; Raff, R.A.; Kaufman, T.C. Mutation in a Testis-Specific Beta-Tubulin in Drosophila—Analysis of Its Effects on Meiosis and Map Location of the Gene. Cell 1980, 21, 445–451. [Google Scholar] [CrossRef]
- Wu, B.; Song, M.; Dong, Q.; Xiang, G.; Li, J.; Ma, X.; Wei, F. UBR5 promotes tumor immune evasion through enhancing IFN-gamma-induced PDL1 transcription in triple negative breast cancer. Theranostics 2022, 12, 5086–5102. [Google Scholar] [CrossRef]
- Kemphues, K.J.; Raff, R.A.; Kaufman, T.C.; Raff, E.C. Mutation in a Structural Gene for a Beta-Tubulin Specific to Testis in Drosophila-Melanogaster. Proc. Natl. Acad. Sci. USA 1979, 76, 3991–3995. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Khaleghpour, K.; Kahvejian, A.; Gehring, K. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. USA 2001, 98, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C.; Sonenberg, N.; Burley, S.K. X-ray structure of the human hyperplastic discs protein: An ortholog of the C-terminal domain of poly(A)-binding protein. Proc. Natl. Acad. Sci. USA 2001, 98, 4414–4419. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Lin, L.; Zhang, Y.; Shen, C.; Qi, Y.; Lin, X. The E3 Ligase UBR5/Hyd Ensures Meiotic Fidelity Through Catalysis-Independent Regulation of β2-Tubulin in Drosophila. Genes 2025, 16, 1245. https://doi.org/10.3390/genes16111245
Zhou L, Lin L, Zhang Y, Shen C, Qi Y, Lin X. The E3 Ligase UBR5/Hyd Ensures Meiotic Fidelity Through Catalysis-Independent Regulation of β2-Tubulin in Drosophila. Genes. 2025; 16(11):1245. https://doi.org/10.3390/genes16111245
Chicago/Turabian StyleZhou, Lin, Lang Lin, Yan Zhang, Chenghao Shen, Yun Qi, and Xinhua Lin. 2025. "The E3 Ligase UBR5/Hyd Ensures Meiotic Fidelity Through Catalysis-Independent Regulation of β2-Tubulin in Drosophila" Genes 16, no. 11: 1245. https://doi.org/10.3390/genes16111245
APA StyleZhou, L., Lin, L., Zhang, Y., Shen, C., Qi, Y., & Lin, X. (2025). The E3 Ligase UBR5/Hyd Ensures Meiotic Fidelity Through Catalysis-Independent Regulation of β2-Tubulin in Drosophila. Genes, 16(11), 1245. https://doi.org/10.3390/genes16111245





