Abstract
Background: The patterns of inbreeding coefficients (FIS) and fine spatial genetic structure (FSGS) were evaluated regarding the mating system and inbreeding depression of food-deceptive orchids, Dactylorhiza majalis, Dactylorhiza incarnata var. incarnata, and Dactylorhiza fuchsii, from NE Poland. Methods: We used 455 individuals, representing nine populations of three taxa and AFLPs, to estimate percent polymorphic loci and Nei’s gene diversity, which are calculated using the Bayesian method; FIS; FST; FSGS with the pairwise kinship coefficient (Fij); and AMOVA in populations. Results: We detected a relatively high proportion of polymorphic fragments (40.4–68.4%) and Nei’s gene diversity indices (0.140–0.234). The overall FIS was relatively low to moderate (0.071–0.312). The average Fij for the populations of three Dactylorhiza showed significantly positive values, which were observed between plants at distances of 1–10 m (20 m). FST was significant in each Dactylorhiza taxon, ranging from the lowest values in D. fuchsii and D. majalis (0.080–0.086, p < 0.05) to a higher value (0.163, p < 0.05) in D. incarnata var. incarnata. Molecular variance was the highest within populations (76.5–86.6%; p < 0.001). Conclusions: We observed concordant genetic diversity patterns in three food-deceptive, allogamous, pollinator-dependent, and self-compatible Dactylorhiza. FIS is often substantially higher than Fij with respect to the first class of FSGSs, suggesting that selfing (meaning of geitonogamy) is at least responsible for homozygosity. A strong FSGS may have evolutionary consequences in Dactylorhiza, and combined with low inbreeding depression, it may impact the establishment of inbred lines of D. majalis and D. incarnata var. incarnata.
1. Introduction
The mating system influences the genetic structure of plant populations by altering the drift/migration equilibrium, which is defined by the effective population size [1,2]. Theoretical and experimental studies have frequently shown that pollen transfer in outcrossing species results in lower genetic structure and higher genetic diversity than in self-pollinating species. In the latter, it is often attributed to a significant founder effect, which tends to increase inbreeding in selfers [2,3,4]. However, pollen flow may act in concert with life-history traits, such as dispersal mechanisms (by wind or animals), and combined, they exhibit high statistical power for predicting the scale of the fine spatial genetic structure (FSGS) within populations [5,6]. When pollen and seed dispersal are restricted, resulting in significant intra-population structure, biparental inbreeding can also influence the inbreeding coefficient. Therefore, following an isolation-by-distance model, a strong FSGS is frequent even within allogamous or potentially allogamous plants [6]. Higher levels of FSGS have also been highlighted for selfing and clonal species in low-density populations [6,7,8]. Variations in mating systems and different soil and climatic conditions may additionally contribute to different FSGS patterns [9]. FIS also reflects inbreeding in previous generations of perennials, resulting in the Wahlund effect on the population [10]. Mating systems and seed dispersal also influence FST via its impact on pollen-mediated and short and/or leptokurtic gene flow and the effective population size, especially during selfing and mating between relatives, by increasing inbreeding, which enhances genetic drift. A summary report by Duminil et al. [11], which analyzed data from 263 plant species, indicated that the inbreeding coefficient (FIS) observed at the adult plant stage enables the assessment of the effects of both biparental inbreeding and inbreeding depression on population genetic structure. In the early stages of the plant life cycle, inbreeding depression primarily impacts inbred progeny. As a result, the FIS of adult plants reflects information about both the selfing rate and inbreeding depression.
The mating system and gene flow within and among populations in Orchidaceae can generate common genetic diversity patterns and FSGSs [12]. Pollinator-mediated gene flow among populations, e.g., was higher in deceptive than in rewarding orchids [12,13]. The deceived pollinators generally visit only a limited number of flowers among plants within populations, facilitating cross-pollination and decreasing the chances of inbreeding [14,15,16,17,18,19]. Therefore, it can be hypothesized that the FSGS of these orchids is weak. In contrast, within-population genetic structure could be stronger in rewarding ones due to geitonogamy and mating among close relatives. However, based on earlier experimental orchid studies, dusty-like seed dispersal was usually limited, e.g., refs. [20,21,22,23,24,25]. These results agree with studies that have investigated the FSGSs of both deceptive orchids—e.g., Caladenia tentaculata [26], Cephalanthera longibracteata [22], Orchis cyclochila [23], Orchis purpurea [27], Orchis mascula [28,29], and Cymbidium goeringii [30]—and rewarding ones—e.g., Gymnadenia conopsea [31], Pogonia ophioglossoides [32], and Epipactis thunbergii [33]. Moreover, orchid germination success has been reported to be higher in the vicinity of mother plants because a mycorrhiza could favour the establishment of seedlings [34,35].
In this study, we focused on Dactylorhiza taxa, which are food-deceptive orchids that do not provide any rewards for their pollinators [36]. This genus can be considered a model due to its plant–pollinator interactions, natural selection, and consequent female reproductive success and its impact on genetic structure in food-deceptive plant groups [37,38,39]. In this context, the mating system and ID in food-deceptive D. majalis, D. incarnata var. incarnata, and D. fuchsii populations from NE Poland were documented in detail [40,41,42,43]. A mixed mating system was observed in all three studied Dactylorhiza taxa, similarly to Hedrén and Nordström’s study [44]. Ostrowiecka et al. [40] reported that pollinator behaviour in D. majalis likely encourages geitonogamy, which accounts for the formation of selfed seeds in fruits at various inflorescence levels, exhibiting germination potential comparable to that of outcrossed seeds within populations. Vallius et al. [45] and Hedrén and Nordstöm [44] proved that different D. incarnata varieties were characterized by a high level of inbreeding, and populations might consist of several inbred lines that were fixed for characters, especially with respect to flower colour. Wróblewska et al.’s [42] results corroborate with previous studies on Dactylorhiza concerning the low or medium level of fruit sets ranging from 7.4% to 77.5% [36,45,46]. In vitro experiments revealed that the seed germination of three Dactylorhiza taxa from both natural pollination and hand treatments (selfing and outcrossing) occurred at a relatively low level, up to 35% (with the exception of D. fuchsii and outcrossing experiments) [42]. In vitro asymbiotic seed germination was similar or slightly higher in selfing than crossing experiments in D. incarnata var. incarnata and D. majalis, while it was reversed in D. fuchsii [42]. Spontaneous autogamy in three Dactylorhiza taxa existed in <1% of pollination in the studied populations and most likely did not affect reproductive success [47,48]. The taxa are characterized as terrestrial, long-lived, self-compatible, tuberous perennial orchids that reproduce primarily via seeds, with vegetative reproduction occurring rarely [40,49]. Pollination is carried out by various insect groups, including Hymenoptera, Diptera, and Coleoptera and predominantly bees and bumblebees [40,41]. Molecular markers such as cpDNA (trnL, trnF, and psbC–trnK), internal transcribed spacer (ITS) sequences, and flow cytometry data have confirmed the taxonomic status of the three orchids studied [41].
Based on the estimates of an earlier ecological survey, e.g., natural fruit sets, a mixed mating system, and inbreeding depression from a controlled crosses treatment, orchid taxa from NE Poland were studied [40,42,43], in addition to the genetic reports of Hedrén and Nordstöm [44] and Naczk et al. [49]. We tested the following hypothesis: inbreeding coefficients are shaped at a high level in food-deceptive orchids D. majalis, D. incarnata var. incarnata, and D. fuchsii. We also assumed that seed dispersal mainly occurs over a short distance in orchids that are close to the mother plant, as was observed by many authors who experimentally researched seed dispersal; therefore, fine-scale genetic structure is stronger due to the effect of inbreeding and short-distance dispersal. Finally, the purpose of this study is to (1) estimate the inbreeding coefficient and the intensity of the FSGS using AFLP markers and (2) discuss how similar mating systems and different inbreeding depression shape the genetic diversity patterns of three food-deceptive Dactylorhiza taxa.
2. Materials and Methods
2.1. Study Sites
The present study was conducted from May to July between 2014 and 2017 across three populations of D. majalis (KA, SKI, and SKII), three populations of D. incarnata var. incarnata (ZB, RO, and MR), and three populations of D. fuchsii (BR, CM, and GR) in northeastern Poland (Figure 1). D. majalis grows in wet meadows filled with abundant, entomophilous, and rewarding plants. The study sites varied in the number of D. majalis individuals, with approximately 120–200 flowering individuals in SKI and SKII and ca. 1000 in KA. All meadows were managed extensively and mowed annually in late July or early August, and they were not subjected to artificial fertilization. The three populations of D. incarnata var. incarnata were of similar size, with MA having ca. 68–100 flowering plants, ZB with approximately 30–100, and RO with 35–200 (Figure 1). These populations were located in the Biebrza Valley and Rospuda Valley, occupying sedge communities with a low cover of rewarding plant species (ca. 10%). Dactylorhiza fuchsii was found in open hornbeam forests with a limited number of rewarding plants, specifically in the Białowieża Primeval Forest and nearby areas (CM and BR, with population sizes of approximately 84–133 flowering plants). One D. fuchsii population (GR, with ca. 140–193 flowering plants) was situated in the Biebrza Valley [42].

Figure 1.
Localities of nine Dactylorhiza populations in northeastern Poland. D. majalis (DM), KA, SKI, and SKII; D. incarnata var. incarnata (DI), ZB, MR, and RO; D. fuchsii (DF) CM, BR, and GR (Wróblewska et al. 2024a [24]).
The study involved samples from 455 individuals across nine populations of three Dactylorhiza taxa, including 162 individuals of D. majalis (DM), 129 of D. incarnata var. incarnata (DI), and 164 of D. fuchsii (DF) (Table 1; Figure 1). Although Dactylorhiza rarely regenerates clonally, one leaf sample was collected from individual shoots at least 1 m apart within D. majalis populations to minimize the effects of population substructure. For D. incarnata var. incarnata and D. fuchsii, samples were collected based on the positions of individuals within their respective populations, which were characterized by different flowering individual densities. Each sample from all populations was mapped using a grid coordinate system with a handheld GPS (Garmin GPSMAP 65s) to facilitate distance calculations between samples.

Table 1.
Locations of D. majalis (DM), D. incarnata var. incarnata (DI), and D. fuchsii (DF) populations in NE Poland and summary statistics of the genetic diversity and spatial genetic structure estimated using SPAGeDi 1.4 [6]. N—number of AFLP samples; PL%—frequency of polymorphic loci; H—Nei’s gene diversity; FIS—inbreeding coefficient; CI—the upper and lower 99% confidence interval values; Fij(1)—mean pairwise kinship coefficient among individuals at the first distance class; b1—regression slope of pairwise kinship at the first distance; Sp—the intensity of the FSGS according Veckemans and Hardy [6]. Voucher specimens were collected by Ada Wróblewska and deposited in the herbarium of the Faculty of Biology, University of Bialystok, Poland. * p < 0.05.
2.2. AFLP Analysis
Genomic DNA was extracted from dried leaf tissues using the Genomic Mini AX Plant kit (A & A Biotechnology, Gdansk, Poland), and the samples were genotyped for AFLP markers. The AFLP procedure, as outlined by Vos et al. [50], was adapted following the Applied Biosystems protocol (AFLPTM Plant Mapping). Initially, 12 primer pair combinations were tested on four selected samples from each Dactylorhiza taxon. The GeneScan 500 Liz-labelled size standard (Applied Biosystems, Waltham, MA, USA) was employed for DNA analysis on an ABI 3130. Subsequently, seven primer combinations were selected that yielded polymorphic, clear, and reproducible fragments of consistent intensities across the three Dactylorhiza taxa (D. majalis EcoR1-ACC/MseI-CAG, EcoR1-AGG/MseI-CAC; D. incarnata var. incarnata EcoR1-ACA/MseI-CAG, EcoR1-ACA/MseI-CTA; D. fuchsii EcoR1-AGG/MseI-CAG, EcoR1-ACC/MseI-CAT, EcoR1-ACC/MseI-CTA). Variable fragments in the 70–500 bp size range were recorded as present (1) or absent (0) using GeneMapper 4.0 (Applied Biosystems). To assess the repeatability of the AFLP results, three individuals from each population were fully replicated, starting from the restriction/ligation step of the AFLP process. The potential resampling of clones was evaluated using the AFLPdat R-script but was determined to be insignificant and therefore not corrected for.
To evaluate genetic diversity, the proportion of polymorphic fragments (PL%) and Nei’s gene diversity (H) were calculated using the Bayesian method with a nonuniform prior distribution of allele frequencies as proposed by Zhivotovsky [51] and implemented in AFLP-Surv version 1.0 [52]. The F statistic was determined through analysis of molecular variance (AMOVA) using Arlequin 3.11 [53], with the significance of variance components assessed using 1000 independent permutation runs.
The fine-scale genetic structure (FSGS) was analyzed through spatial autocorrelation using the pairwise kinship coefficient Fij for dominant markers [54]. Mean Fij estimates for pairs of individuals across specified distance classes were calculated and plotted against distance on a logarithmic scale with SPAGeDi 1.4 [6,54]. Distinct distance classes were created for each population of D. majalis, D. incarnata var. incarnata, and D. fuchsii due to varying spatial distribution/density patterns. To evaluate the significance of the FSGS, the regression slopes (b) of kinship coefficients against the natural logarithm of distance were compared to slopes obtained from the permutations of individual genotypes (10,000 random permutations). The extent of the FSGS was quantified using the Sp statistic as proposed by Vekemans and Hardy [6] and calculated as Sp = −b/(1 − F1), where b is the regression slope and F1 represents the average Fij between individuals. For each spatial distance class, the 99% confidence interval was determined using 10,000 permutations (with SPAGeDi) [55]. The probability value (p) was computed for each spatial distance class and coefficient.
To investigate the FIS, the Metropolis–Gibbs algorithm was applied in the I4A software based on dominant markers [56]. The data were run using prior values of beat distribution equal to α = β = 1.0 (corresponding to an “uninformative” flat distribution) and 60,000 repetitions, including a 10,000-step burn-in.
3. Results
Overall, 193, 215, and 263 polymorphic bands were scored in D. majalis, D. incarnata var. incarnata, and D. fuchsii, respectively. Considering the error rates (2%, 1.3%, and 1.5%, respectively), none of the samples may have represented clones.
Relatively high proportions of polymorphic fragments (PL% = 40.4–68.4%) and Nei’s gene diversity indices (H = 0.140–0.234) were detected among the three orchid species (Table 1). The overall FIS was relatively low to moderate, and it equaled 0.071–0.224 in D. incarnata var. incarnata and 0.079–0.134 in D. fuchsii; it reached the highest values of 0.192–0.312 in D. majalis.
The correlograms of the average Fij values for the populations of three Dactylorhiza taxa exhibited significantly positive values, which were observed in the short-distance classes. In D. majalis, the relatives were noted at a distance from 1 m to 10 m (Figure 2), and the values were significantly negative with respect to the longer distance classes (52–72 m) in the two other populations. Similar observations were made in two of the three populations of D. incarnata var. incarnata and D. fuchsii. Significant positive values were observed at a distance from 2 m to 20 m in D. incarnata var. incarnata (Figure 2) and from 2 m to 10 m in D. fuchsii (Figure 2). The bF values for D. majalis (−0.051–0.009), D. incarnata var. incarnata (−0.055–0.002), and D. fuchsii (−0.026–0.002) were almost all significant (permutation test, p < 0.05) (Table 1). The highest Sp values were observed for D. incarnata var. incarnata (0.063) and D. majalis (0.056) (Table 1).
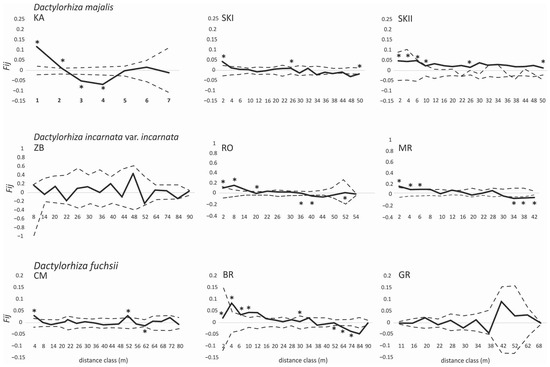
Figure 2.
Spatial correlograms for D. majalis, D. incarnata var. incarnata, and D. fuchsii populations with the mean pairwise kinship coefficients (Fij) of distance classes for AFLPs with respect to the hypothesis of random genetic structure obtained by permuting individual spatial locations, as implemented in SPAGeDi 1.4 [6]. The dotted lines indicate the 99% confidence intervals obtained from 10,000 permutations of genotypes. Codes of populations (KA, SKI, SKII, ZA, MR, RO, CM, BR, and GR; see Table 1); * p < 0.05.
Almost all FST values were significant in each Dactylorhiza taxon, ranging from the lowest values in D. fuchsii and D. majalis (0.080 and 0.086, p < 0.05, permutation test) to the highest value (0.163, p < 0.05, permutation test) in D. incarnata var. incarnata. The amount of molecular variance was highest within populations, and it was maintained at relatively higher and similar levels in D. majalis, D. incarnata var. incarnata, and D. fuchsii (AMOVA: 76.5%, 85.5%, and 86.6%; p < 0.001, respectively).
4. Discussion
The relationships among mating systems, inbreeding depression, biparental inbreeding, and their effects on FIS and FST have been infrequently documented in plant surveys [11]. Baskin and Baskin [57] summarized the impact of inbreeding depression on seed germination across 743 instances involving 233 species from 64 families. They found that in 50.1% of the cases, inbred and outcrossed seeds germinated at comparable frequencies, while 8.1% of inbred ones exhibited better germination rates than outcrossed seeds. Interestingly, the authors observed no strong correlation between decreased germination rates and increased FIS nor between increased germination rates and heightened levels of population genetic diversity. However, we observed concordant genetic diversity patterns in food-deceptive, allogamous, and pollinator-dependent populations, although these were also self-compatible with the mixed mating systems of D. majalis, D. incarnata var. incarnata, and D. fuchsii. Genetic diversity within studied Dactylorhiza populations was shaped at a relatively high level comparable to the data reported by Naczk et al. [49] and Hedrén and Nordström [44,58], suggesting that studied Dactylorhiza populations can be found via genetically different individuals and/or gene flow via leptokurtic dispersal. The genetic differentiation among them was low and significant (0.080–0.163), showing that gene flow (historical) in northeastern Poland was relatively high or populations were established from one source. However, the isolation processes of these Dactylorhiza populations were observed, resulting in the formation of a substructure.
Furthermore, the inbred population was shaped from moderate to high levels in three Dactylorhiza taxa, similarly to the studies of Hedrén and Nordström [44], Filippov et al. [59], and Naczk et al. [49]. Meanwhile, in D. majalis as the allotetraploid, FIS exhibited a wide range of values in the populations reported by Balao et al. [60], Hedrén and Nordström [44], and Naczk and Ziętara [61]. Let us assume the prediction that inbreeding is solely the result of mating among neighbouring plants. In this case, we expect FIS to be approximately equal to Fij at the smallest distance interval in the studied Dactylorhiza populations [6]. In our survey, FIS was substantially higher than Fij with respect to the first class of spatial distances in most Dactylorhiza populations, suggesting that selfing is at least partially responsible for homozygosity [56,62]. However, spontaneous autogamy in three Dactylorhiza taxa existed until 1% of pollination in the studied populations [47,48]. Hence, the only explanation of selfing in three Dactylorhiza taxa is the pollinator behaviour of bumblebees and other pollinators, which are known to promote geitonogamy and/or autogamy, explaining the development of selfed seeds [40,41]. The important factor shaping FIS was the slightly higher selfing frequency compared to outcross seeds germinated in in vitro treatments in D. majalis and D. incarnata var. incarnata, while in D. fuchsii, the germination pattern was reversed [42]. This phenomenon suggested that inbred and outbred D. majalis and D. incarnata var. incarnata seeds germinated at a similar or even slightly higher frequency. We stress the careful interpretation of the relationship between seed germination and FIS. This needs to be confirmed in further studies, including the growth and mortality observations of plants germinated from selfed and outcrossed seeds in the following stages. However, our data are related to a single studied Dactylorhiza species, and we can suppose that in D. fuchsii, a similar pattern exists in two out of three populations, such as D. majalis and D. incarnata var. incarnata. In the CM and BR populations, high inbreeding and slightly lower kinship coefficients supported the possibility of selfing (geitonogamy). The interesting question is whether biparental inbreeding can exist in food-deceptive Dactylorhiza taxa, even though pollinators spend a short time period on flowers and inflorescence and learn to avoid deceptive flowers. They typically only visit fewer flowers per plant and/or a few flowers between inflorescences within populations, promoting cross-pollination and skipping more plants between plant visits. In light of this outcrossing hypothesis [15], biparental inbreeding is rather unlikely. Using videotaping, Ostrowiecka et al. [40] observed that Apis mellifera visited three to five flowers on the same inflorescence within 11 to 40 s, contributing to geitonogamy. Our observations noted that A. mellifera pollinators did not return to the same flowers and avoided visiting all flowers on the inflorescences. Conversely, the bending of pollinaria serves as a mechanism to prevent geitonogamy and biparental inbreeding among closely related individuals. In Dactylorhiza, the average bending time is 39–54 s, which is considered relatively long for deceptive plants and similar to other deceptive Dactylorhiza taxa [63]. The bending time observed in each studied D. majalis population ranged from 8 s to 2 min and 5 s [40]. This relatively short bending time may be an opportunity for geitonogamy. This observation and the bending times in the studied Dactylorhiza populations support our hypothesis that geitonogamy cannot be completely ruled out in deceptive orchids compared to biparental inbreeding. Dactylorhiza seems to possess a more generalized pollination system, and numerous pollinators have been described and studied in detail. These pollinators can spend different amounts of time on the flowers, promoting geitonogamy.
Hand pollination using emasculated flowers was employed to assess the extent of apparent geitonogamy occurring via pollinators [64,65]. A previous fruit set observation from controlled pollination in three Dactylorhiza documented a moderate level of fruit set (35.4–40.5%). Simultaneously, emasculation experiments in their populations showed a significant decrease in fruiting between these treatments (D. majalis, 28.2% fruit set from emasculated flower, paired t = 2.68, df = 8, p < 0.002; D. incarnata var. incarnata, 14.6% fruit set from emasculated flower, paired t = 3.46, df = 10, p < 0.006; D. fuchsii, 28.2% fruit set from emasculated flower, paired t = 4.83, df = 10, p < 0.0007; Wróblewska et al. unpublished data [66]). This study concludes that selfing in three Dactylorhiza occurs mainly through geitonogamy. Kropf and Renner [17] have also pointed out the high levels of geitonogamous pollination in Dactylorhiza; measuring biparental inbreeding can be challenging in deceptive plants.
The FIS observed at the adult stage enabled the assessment of inbreeding depression impacts on the population’s genetic structure. In long-lived plants, FIS reflects inbreeding not only in the current generation but also in previous overlapping generations. However, other factors, such as the long lifespan of plants, can affect inbreeding depression [11]. In D. majalis, selfing (e.g., geitonogamy) and/or progeny and likely seed dispersal in the vicinity of the mother plant can manifest most strongly in spatial genetic structures. In the case of D. majalis, the results of the present study are inconsistent with those of Husband and Schemske [67], who concluded that purging is a significant evolutionary force in natural populations. Without reducing the genetic load, such fixation could reduce inbreeding depression [67,68]. However, inbreeding depression may be lower in long-standing populations with inbreeding than in populations with outcrossing, where selection may have purged the genome of its genetic load [67,68,69,70]. These two alternative approaches in a laboratory should be tested at a later stage of the life cycle of D. majalis, such as in seedlings and adult reproductive individuals.
5. Conclusions
Selfing (meaning of geitonogamy) and a strong fine-scale genetic structure may have additional and unexplored evolutionary consequences in Dactylorhiza, and combined with low inbreeding depression, they may strongly influence the establishment of inbred lines in the cases of D. majalis and D. incarnata var. incarnata. Currently, we cannot state that inbreeding depression may be widely viewed as the primary selective factor allowing transitions to complete selfing in Dactylorhiza. On the other hand, there are still limited studies on breeding systems and pollinator behaviour in deceptive multi-flowered orchids, which could shed light on geitonogamy and biparental inbreeding. Our study stressed that different Dactylorhiza food-deceptive taxa with varying levels of inbreeding depression can characterize similar FSGSs and inbreeding coefficients. Despite these distinct patterns of inbreeding depression, FSGSs comprise the formation of local family structures in Dactylorhiza taxa due to limited gene dispersal (e.g., seeds) and geitonogamy.
Author Contributions
Conceptualization, A.W.; methodology, A.W. and B.O.; software, A.W.; validation, A.W.; formal analysis, A.W.; investigation, A.W., B.O., E.J. and I.T.; writing—original draft preparation, A.W.; writing—review and editing, A.W.; visualization, A.W.; supervision, A.W.; project administration, A.W.; funding acquisition, A.W. and I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center in Poland (no. 2013/09/B/NZ8/03350).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data cited in the study are publicly available.
Acknowledgments
We thank Emilia Brzosko and Paweł Mirski for their help and support during fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hamrick, J.L.; Godt, M.J.W. Effects of life history traits on genetic diversity in plant species. Philos. Trans. Biol. Sci. 1996, 51, 1291–1298. [Google Scholar]
- Hamrick, J.L.; Linhart, V.B.; Mitfon, J. Relationships between life history characteristics and electrophoretically detectable genetic variation in plants. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 173–200. [Google Scholar] [CrossRef]
- Loveless, M.D.; Hamrick, J.L. Ecological determinants of genetic structure in plant populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Nybom, H.; Bartish, I.V. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 93–114. [Google Scholar] [CrossRef]
- Petit, R.J.; Duminil, J.; Fineschi, S.; Hampe, A.; Savini, D.; Vendramin, G.G. Comparative Organization of chloroplast; mitochondrial and nuclear diversity in plant populations. Mol. Ecol. 2005, 14, 689–701. [Google Scholar] [CrossRef]
- Vekemans, X.; Hardy, O.J. New insights from fine-scale spatial genetic structure analyses in plant populations. Mol. Ecol. 2004, 13, 921–935. [Google Scholar] [CrossRef]
- Volis, S.; Zaretsky, M.; Shulgina, I. Fine-scale spatial genetic structure in a predominantly selfing plant, role of seed and pollen dispersal. Heredity 2010, 105, 384–393. [Google Scholar] [CrossRef]
- Binks, R.M.; Millar, M.A.; Byrne, M. Not All Rare Species Are the Same: Contrasting Patterns of Genetic Diversity and Population Structure in Two Narrow-Range Endemic Sedges. Biol. J. Linn. Soc. 2015, 114, 873–886. [Google Scholar] [CrossRef]
- Mosca, E.; Di Pierro, E.A.; Budde, K.B.; Neale, D.B.; Gonzalez-Martınez, S.C. Environmental effects on fine-scale spatial genetic structure in four Alpine keystone forest tree species. Mol. Ecol. 2018, 27, 647–658. [Google Scholar] [CrossRef]
- Wahlund, S. Zusammensetzung von Population und Korrelationserscheinung vom Standpunkt der Vererbungslehre aus betrachtet. Hereditas 1928, 11, 65–106. [Google Scholar] [CrossRef]
- Duminil, J.; Hardy, O.J.; Petit, R.J. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol. Biol. 2009, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences, a spasmodic journey to diversification. Biol. J. Linn. Soc. 2005, 84, 1–54. [Google Scholar] [CrossRef]
- Cozzolino, S.; Schiestl, F.P.; Muller, A.; De Castro, O.; Nardella, A.M.; Widmer, A. Evidence for pollinator sharing in Mediterranean nectar-mimic orchids, absence of premating barriers? Proc. R. Soc. B Biol. Sci. 2005, 272, 1271–1278. [Google Scholar] [CrossRef]
- Neiland, M.R.M.; Wilcock, C.C. Fruit set; nectar reward; and rarity in the Orchidaceae. Am. J. Bot. 1998, 85, 1657–1671. [Google Scholar] [CrossRef]
- Jersáková, J.; Johnson, S.D.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef]
- Juillet, N.; Dunand-Martin, S.; Gigord, L.D.B. Evidence for Inbreeding Depression in the Food-Deceptive Colour-Dimorphic Orchid Dactylorhiza sambucina (L.) Soò. Plant Biol. 2006, 9, 147–151. [Google Scholar] [CrossRef]
- Kropf, M.; Renner, S.S. Pollinator-mediated selfing in two deceptive orchids and a review of pollinium tracking studies addressing geitonogamy. Oecologia 2008, 155, 497–508. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R. Lack of strong selection pressures maintains wide variation in floral traits in a food-deceptive orchid. Ann. Bot. 2020, 126, 445–453. [Google Scholar] [CrossRef]
- Machon, N.; Bardin, P.; Mazer, S.J.; Moret, J.; Godelle, B.; Austerlitz, F. Relationship between genetic structure and seed and pollen dispersal in the endangered orchid Spiranthes spiralis. New Phytol. 2003, 157, 677–687. [Google Scholar] [CrossRef]
- Jersáková, J.; Malinová, T. Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytol. 2007, 176, 237–241. [Google Scholar] [CrossRef]
- Brzosko, E.; Ostrowiecka, B.; Kotowicz, J.; Bolesta, M.; Gromotowicz, A.; Gromotowicz, M.; Orzechowska, A. Seed dispersal in six species of terrestrial orchids in Biebrza National Park. Acta Soc. Bot. Pol. 2017, 86, 3557. [Google Scholar] [CrossRef]
- Chung, M.Y.; Nason, J.D.; Chung, M.G. Spatial genetic structure in populations of the terrestrial orchid Cephalanthera longibracteata (Orchidaceae). Am. J. Bot. 2004, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y.; Nason, J.D.; Chung, M.G. Spatial genetic structure in populations of the terrestrial orchid Orchis cyclochila (Orchidaceae). Plant Syst. Evol. 2005, 254, 209–219. [Google Scholar] [CrossRef]
- Trapnell, D.W.; Hamrick, J.L. Mating patterns and geneflow in the Neotropical epiphytic orchid; Laelia rubescens. Mol. Ecol. 2005, 14, 75–84. [Google Scholar] [CrossRef]
- Trapnell, D.W.; Hamrick, J.L.; Nason, J.D. Three-dimensional fine-scale genetic structure of the Neotropicalepiphytic orchid; Laelia rubescens. Mol. Ecol. 2004, 13, 1111–1118. [Google Scholar] [CrossRef]
- Peakall, R.; Beattie, A.J. Ecological and genetic consequences of pollination by sexual deception in the orchid Calladenia tentaculata. Evol. 1996, 50, 2207–2220. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Vandepitte, K.; Honnay, O.; Roldán-Ruiz, I. Fine-scale genetic structure of life history stages in the food-deceptive orchid Orchis purpurea. Mol. Ecol. 2006, 15, 2801–2808. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Wiegand, T.; Vandepitte, K.; Brys, R.; Roldán-Ruiz, I.; Honnay, O. Multigenerational analysis of spatial structure in the terrestrial; food deceptive orchid Orchis mascula. J. Ecol. 2009, 97, 206–216. [Google Scholar] [CrossRef]
- Helsen, K.; Meekers, T.; Vranckx, G.; Roldán-Ruiz, I.; Vandepitte, K.; Honnay, O. A direct assessment of realized seed and pollen flow within and between two isolated populations of the food-deceptive orchid Orchis mascula. Plant Biol. 2016, 18, 139–146. [Google Scholar] [CrossRef]
- Chung, M.Y.; Nason, J.D.; Chung, M.G. Significant fine-demographic and scale genetic structure in expanding and senescing populations of the terrestrial orchid Cymbidium goeringii (Orchidaceae). Am. J. Bot. 2011, 98, 2027–2039. [Google Scholar] [CrossRef]
- Sletvold, N.; Grindeland, J.M.; Zu, P.; Ågren, J. Fine-scale genetic structure in the orchid Gymnadenia conopsea is not associated with local density of flowering plants. Am. J. Bot. 2024, 111, e16273. [Google Scholar] [CrossRef]
- Pandey, M.; Sharma, J. Efficiency of Microsatellite Isolation from Orchids via Next Generation Sequencing. Open J. Genet. 2012, 2, 167–172. [Google Scholar] [CrossRef]
- Chung, M.Y.; Chung, M.G. Extremely low levels of genetic diversity in the terrestrial orchid Epipactis thunbergii (Orchidaceae) in South Korea, implications for conservation. Bot. J. Linn. Soc. 2007, 155, 161–169. [Google Scholar] [CrossRef][Green Version]
- Diez, J.M. Hierarchical patterns of symbiotic orchid germination linked to adult proximity and environmental gradients. J. Ecol. 2007, 95, 159–170. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Wiegand, T.; Vandepitte, K.; Brys, R.; Roldán-Ruiz, I.; Honnay, O. Spatial variation in below-ground seed germination and divergent mycorrhizal associations correlate with spatial segregation of three co-occurring orchid species. J. Ecol. 2012, 100, 1328–1337. [Google Scholar] [CrossRef]
- Claessens, J.; Kleynen, J. The Flower of the European Orchid, Form and Function; Jean Claessens & Jacques Kleynen: Voerendaal, The Netherlands, 2011. [Google Scholar]
- Mattila, E.; Kuitunen, T.M. Nutrient versus pollination limitation in Platanthera bifolia and Dactylorhiza incarnata (Orchidaceae). Oikos 2000, 89, 360–366. [Google Scholar] [CrossRef]
- Sletvold, N.; Grindeland, J.M.; Ågren, J. Pollinator-mediated selection on floral display; spur length and flowering phenology in the deceptive orchid Dactylorhiza lapponica. New Phytol. 2010, 188, 385–392. [Google Scholar] [CrossRef]
- Trunschke, J.; Sletvold, N.; Ågren, J. Interaction intensity and pollinator-mediated selection. New Phytol. 2017, 214, 1381–1389. [Google Scholar] [CrossRef]
- Ostrowiecka, B.; Tałałaj, I.; Brzosko, E.; Jermakowicz, E.; Mirski, P.; Kostro-Ambroziak, A.; Mielczarek, Ł.; Lasoń, A.; Kupryjanowicz, J.; Kotowicz, J.; et al. Pollinators and visitors of the generalized food-deceptive orchid Dactylorhiza majalis in North-Eastern Poland. Biologia 2019, 74, 1247–1257. [Google Scholar] [CrossRef]
- Wróblewska, A.; Szczepaniak, L.; Bajguz, A.; Jędrzejczyk, I.; Tałałaj, I.; Ostrowiecka, B.; Brzosko, E.; Jermakowicz, E.; Mirski, P. Deceptive strategy in Dactylorhiza orchids, multidirectional evolution of floral chemistry. Ann. Bot. 2019, 123, 1005–1016. [Google Scholar] [CrossRef]
- Wróblewska, A.; Ostrowiecka, B.; Brzosko, E.; Jermakowicz, E.; Tałałaj, I.; Mirski, P. The patterns of inbreeding depression in food-deceptive Dactylorhiza orchids. Front. Plant Sci. 2024, 15, 1244393. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, A.; Ostrowiecka, B.; Kotowicz, J.; Jermakowicz, E.; Tałałaj, I.; Szefer, P. What are the drivers of female success in food-deceptive orchids? Ecol. Evol. 2024, 14, e11233. [Google Scholar] [CrossRef]
- Hedrén, M.; Nordström, S. Polymorphic populations of Dactylorhiza incarnata s.l. (Orchidaceae) on the Baltic island of Gotland, Morphology; habitat preference and genetic differentiation. Ann. Bot. 2009, 104, 527–542. [Google Scholar] [CrossRef]
- Vallius, E.; Salonen, V.; Kul, T. Factors of divergence in co-occurring varieties of Dactylorhiza incarnata (Orchidaceae). Plant Syst. Evol. 2004, 248, 177–189. [Google Scholar] [CrossRef]
- Kindlmann, P.; Jersáková, J. Effect of floral display on reproductive success in terrestrial orchids. Folia Geobot. 2006, 41, 47–60. [Google Scholar] [CrossRef]
- Tałałaj, I.; Kotowicz, J.; Brzosko, E.; Ostrowiecka, B.; Aleksandrowicz, O.; Wróblewska, A. Spontaneous caudicle reconfiguration in Dactylorhiza fuchsii, A new self-pollination mechanism for Orchideae. Plant Syst. Evol. 2019, 305, 269–280. [Google Scholar] [CrossRef]
- Siudek, K. The role of pollinarium reconfiguration as the mechanism of selfing in Dactylorhiza majalis and Dactylorhiza incarnata. Ph.D. Thesis, University of Bialystok, Faculty of Biology, Białystok, Poland, 2020; p. 68. [Google Scholar]
- Naczk, A.M.; Chybicki, I.J.; Ziętara, M.S. Genetic diversity of Dactylorhiza incarnata (Orchidaceae) in northern Poland. Acta Soc. Bot. Pol. 2016, 85, 1–14. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP, A New Technique for DNA Fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef]
- Zhivotovsky, L.A. Estimating population structure in diploids with multilocus dominant DNA markers. Mol. Ecol. 1999, 8, 907–913. [Google Scholar] [CrossRef]
- Vekemans, X.; Beauwens, T.; Lemaire, M.; Roldan-Ruiz, I. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol. 2002, 11, 139–151. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. ARLEQUIN (version 3.0), an integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar]
- Hardy, O.J. Estimation of pairwise relatedness between individuals and characterization of isolation-by-distance processes using dominant genetic markers. Mol. Ecol. 2003, 12, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Manly, B. Randomization. Bootstrap and Monte Carlo Methods in Biology, 3rd ed.; Chapman and Hall/CRC: London, UK, 2007. [Google Scholar]
- Chybicki, I.J.; Oleksa, A.; Burczyk, J. Increased inbreeding and strong kinship structure in Taxus baccata estimated from both AFLP and SSR data. Heredity 2011, 107, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Inbreeding depression and the cost of inbreeding on seed germination. Seed Sci. Res. 2015, 25, 355–385. [Google Scholar] [CrossRef]
- Hedrén, M.; Nordström, S. High levels of genetic diversity in marginal populations of the marsh orchid Dactylorhiza majalis subsp. majalis. Nord. J. Bot. 2018, 36, e01747. [Google Scholar] [CrossRef]
- Filippov, E.G.; Andronova, E.V.; Kazlova, V.M. Genetic structure of the populations of Dactylorhiza ochroleuca and D. incarnata (Orchidaceae) in the area of their joint growth in Russia and Belarus. Russ. J. Genet. 2017, 53, 661–671. [Google Scholar] [CrossRef]
- Balao, F.; Tannhäuser, M.; Lorenzo, M.T.; Hedrén, M.; Paun, O. Genetic differentiation and admixture between sibling allopolyploids in the Dactylorhiza majalis complex. Heredity 2016, 116, 351–361. [Google Scholar] [CrossRef]
- Naczk, A.M.; Ziętara, M.S. Genetic diversity in Dactylorhiza majalis subsp. majalis populations (Orchidaceae) of northern Poland. Nord. J. Bot. 2019, 37, e01989. [Google Scholar] [CrossRef]
- Geremew, A.; Stiers, I.; Sierens, T.; Kefalew, A.; Triest, L. Clonal growth strategy; diversity and structure, A spatiotemporal response to sedimentation in tropical Cyperus papirus swamps. PLoS ONE 2018, 13, e0190810. [Google Scholar] [CrossRef]
- Niiniaho, J. The Role of Geitonogamy in the Reproduction Success of a Nectarless Dactylorhiza maculata (Orchidaceae). Master’s Thesis, University of Jyväskylä, Jyväskylä, Finland, 2011. [Google Scholar]
- Eckert, C.G. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering; clonal plant. Ecology 2000, 81, 532–542. [Google Scholar] [CrossRef]
- Hayashi, T.; Ayre, B.M.; Bohman, B.; Brown, G.R.; Reiter, N.; Phillips, R.D. Pollination by multiple species of nectar foraging Hymenoptera in Prasophyllum innubum; a critically endangered orchid of the Australian Alps. Aust. J. Bot. 2024, 72, BT23110. [Google Scholar] [CrossRef]
- Wróblewska, A.; Ostrowiecka, B.; Tałałaj, I.; Jermakowicz, E.; Brzosko, E.; Mirski, P. (University of Bialystok, Faculty of Biology, Białystok, Poland). Emasculation of Dacylorhiza Flowers, Hand-Pollination Treatment. Unpublished work, 2024.
- Husband, B.C.; Schemske, D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Schoen, D. Correlated evolution of self fertilization and inbreeding depression, An experimental study of nine populations of Amsinckia (Boraginaceae). Evolution 1996, 50, 1478–1491. [Google Scholar] [CrossRef]
- Angeloni, F.; Ouborg, N.J.; Leimu, R. Meta-analysis on the association of population size and life history with inbreeding depression in plants. Biol. Conserv. 2011, 144, 35–43. [Google Scholar] [CrossRef]
- Keller, L.F.; Waller, D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).