Genome-Wide Insights into Internalizing Symptoms in Admixed Latin American Children
Abstract
1. Introduction
2. Methods
2.1. Recruitment of Study Participants and Ethical Considerations
2.2. Psychological Assessment Tool
2.3. Genotyping and Quality Control
2.4. Genotype Imputation
2.5. Population Genetic Structure and Linkage Disequilibrium
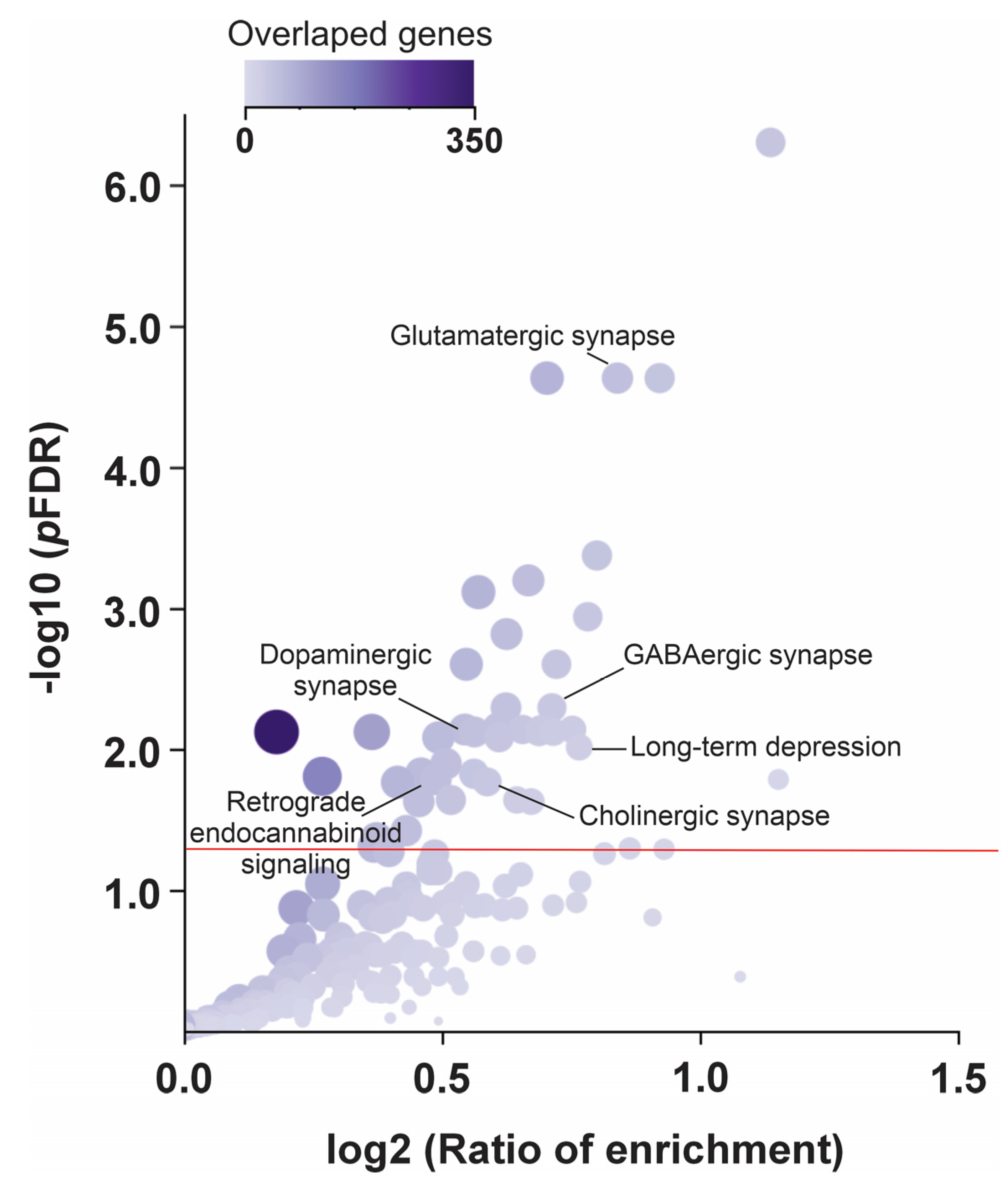
2.6. Functional and Pathway Enrichment Analyses
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Study Population
3.2. Genome-Wide Association Analysis
3.3. In Silico Functional Analysis
3.4. Pathway Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, D.J.; Palk, A.C.; Kendler, K.S. What Is a Mental Disorder? An Exemplar-Focused Approach. Psychol. Med. 2021, 51, 894–901. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Depression and Other Common Mental Disorders: Global Health Estimates. WHO-MSD-MER-2017.2. 2017. Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 25 November 2024).
- Pan American Health Organization (PAHO). The Burden of Mental Disorders in the Region of the Americas. 2018. Available online: https://iris.paho.org/handle/10665.2/49578 (accessed on 25 November 2024).
- Lu, W. Adolescent Depression: National Trends, Risk Factors, and Healthcare Disparities. Am. J. Health Behav. 2019, 43, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bang, Y.R.; Kim, C.K. Sex and Age Differences in Psychiatric Disorders among Children and Adolescents: High-Risk Students Study. Psychiatry Investig. 2014, 11, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Lu, J.; Ran, H.; Che, Y.; Fang, D.; Liang, X.; Sun, H.; Chen, L.; Peng, J.; et al. Treatment Rates for Mental Disorders Among Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2338174. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Huang, Y.; Han, C.; Li, Y.; Xu, Y.; Liu, Y.; He, X. Alarming changes in the global burden of mental disorders in children and adolescents from 1990 to 2019: A systematic analysis for the Global Burden of Disease study. Eur. Child Adolesc. Psychiatry 2022, 31, 1827–1845. [Google Scholar] [CrossRef]
- Costello, E.J.; Egger, H.L.; Angold, A. The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 631–648. [Google Scholar] [CrossRef]
- Beidel, D.C.; Turner, S.M.; Morris, T.L. Behavioral treatment of childhood social phobia. J. Consult. Clin. Psychol. 2000, 68, 1072–1080. [Google Scholar] [CrossRef]
- La Maison, C.; Munhoz, T.N.; Santos, I.S.; Anselmi, L.; Barros, F.C.; Matijasevich, A. Prevalence and Risk Factors of Psychiatric Disorders in Early Adolescence: 2004 Pelotas (Brazil) Birth Cohort. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 685–697. [Google Scholar] [CrossRef]
- Christ, C.; de Waal, M.M.; Dekker, J.J.M.; van Kuijk, I.; van Schaik, D.J.F.; Kikkert, M.J.; Goudriaan, A.E.; Beekman, A.T.F.; Messman-Moore, T.L. Linking Childhood Emotional Abuse and Depressive Symptoms: The Role of Emotion Dysregulation and Interpersonal Problems. PLoS ONE 2019, 14, e0211882. [Google Scholar] [CrossRef]
- Covey, H.C.; Grubb, L.M.; Franzese, R.J.; Menard, S. Adolescent Exposure to Violence and Adult Anxiety, Depression, and PTSD. Crim. Justice Rev. 2020, 45, 185–201. [Google Scholar] [CrossRef]
- Reiss, F.; Meyrose, A.K.; Otto, C.; Lampert, T.; Klasen, F.; Ravens-Sieberer, U. Socioeconomic Status, Stressful Life Situations, and Mental Health Problems in Children and Adolescents: Results of the German BELLA Cohort Study. PLoS ONE 2019, 14, e0213700. [Google Scholar] [CrossRef] [PubMed]
- Ensink, J.B.M.; de Moor, M.H.M.; Zafarmand, M.H.; de Laat, S.; Uitterlinden, A.; Vrijkotte, T.G.M.; Lindauer, R.; Middeldorp, C.M. Maternal Environmental Risk Factors and the Development of Internalizing and Externalizing Problems in Childhood: The Complex Role of Genetic Factors. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Franic, S.; Dolan, C.V.; Borsboom, D.; van Beijsterveldt, C.E.; Boomsma, D.I. Three-and-a-Half-Factor Model? The Genetic and Environmental Structure of the CBCL/6–18 Internalizing Grouping. Behav. Genet. 2014, 44, 254–268. [Google Scholar] [PubMed]
- Nivard, M.G.; Dolan, C.V.; Kendler, K.S.; Kan, K.J.; Willemsen, G.; van Beijsterveldt, C.E.; Lindauer, R.J.; van Beek, J.H.; Geels, L.M.; Bartels, M.; et al. Stability in Symptoms of Anxiety and Depression as a Function of Genotype and Environment: A Longitudinal Twin Study from Ages 3 to 63 Years. Psychol. Med. 2015, 45, 1039–1049. [Google Scholar] [CrossRef]
- van Sprang, E.D.; Maciejewski, D.F.; Milaneschi, Y.; Elzinga, B.M.; Beekman, A.T.F.; Hartman, C.A.; van Hemert, A.M.; Penninx, B.W.J.H. Familial Risk for Depressive and Anxiety Disorders: Associations with Genetic, Clinical, and Psychosocial Vulnerabilities. Psychol. Med. 2022, 52, 696–706. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-Wide Association Study of Depression Phenotypes in UK Biobank Identifies Variants in Excitatory Synaptic Pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M. Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Genome-Wide Association Analyses Identify 44 Risk Variants and Refine the Genetic Architecture of Major Depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- Meng, X.; Navoly, G.; Giannakopoulou, O. Multi-Ancestry Genome-Wide Association Study of Major Depression Aids Locus Discovery, Fine Mapping, Gene Prioritization, and Causal Inference. Nat. Genet. 2024, 56, 222–233. [Google Scholar] [CrossRef]
- Friligkou, E.; Løkhammer, S.; Cabrera-Mendoza, B.; Shen, J.; He, J.; Deiana, G.; Zanoaga, M.D.; Asgel, Z.; Pilcher, A.; Di Lascio, L.; et al. Gene discovery and biological insights into anxiety disorders from a large-scale multi-ancestry genome-wide association study. Nat. Genet. 2024, 56, 2036–2045. [Google Scholar] [CrossRef]
- Benke, K.S.; Nivard, M.G.; Velders, F.P.; Walters, R.K.; Pappa, I.; Scheet, P.A.; Xiao, X.; Ehli, E.A.; Palmer, L.J.; Whitehouse, A.J.; et al. A Genome-Wide Association Meta-Analysis of Preschool Internalizing Problems. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 667–676.e7. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.S.; Hammerschlag, A.R.; Ip, H.F.; Allegrini, A.G.; Benyamin, B.; Border, R.; Diemer, E.W.; Jiang, C.; Karhunen, V.; Lu, Y.; et al. Genome-Wide Association Meta-Analysis of Childhood and Adolescent Internalizing Symptoms. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.L.; Cunha, S.S.; Alcântara-Neves, N.; Carvalho, L.P.; Cruz, A.A.; Stein, R.T.; Genser, B.; Cooper, P.J.; Rodrigues, L.C. Risk Factors and Immunological Pathways for Asthma and Other Allergic Diseases in Children: Background and Methodology of a Longitudinal Study in a Large Urban Center in Northeastern Brazil (Salvador-SCAALA Study). BMC Pulm. Med. 2006, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms & Profiles; ASEBA: Burlington, VT, USA, 2001. [Google Scholar]
- Bordin, I.A.S.; Mari, J.J.; Caeiro, M.F. Validação da Versão Brasileira do “Child Behavior Checklist” (CBCL) (Inventário de Comportamentos da Infância e Adolescência): Dados Preliminares. Rev. ABP-APAL 1995, 17, 55–66. [Google Scholar]
- Magalhães, W.C.S.; Araujo, N.M.; Leal, T.P.; Araujo, G.S.; Viriato, P.J.S.; Kehdy, F.S.; Costa, G.N.; Barreto, M.L.; Horta, B.L.; Lima-Costa, M.F.; et al. EPIGEN-Brazil Initiative Resources: A Latin American Imputation Panel and the Scientific Workflow. Genome Res. 2018, 28, 1090–1095. [Google Scholar] [CrossRef]
- Delaneau, O.; Marchini, J.; Zagury, J.-F. A Linear Complexity Phasing Method for Thousands of Genomes. Nat. Methods 2012, 9, 179–181. [Google Scholar] [CrossRef]
- Howie, B.N.; Donnelly, P.; Marchini, J. A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef]
- Kehdy, F.S.G.; Gouveia, M.H.; Machado, M.; Magalhães, W.C.; Horimoto, A.R.; Horta, B.L.; Moreira, R.G.; Leal, T.P.; Scliar, M.O.; Soares-Souza, G.B.; et al. Origin and Dynamics of Admixture in Brazilians and Its Effect on the Pattern of Deleterious Mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 8696–8701. [Google Scholar] [CrossRef]
- Bernstein, H.-G.; Hölzl, G.; Dobrowolny, H.; Hildebrandt, J.; Trübner, K.; Krohn, M.; Bogerts, B.; Pahnke, J. Vascular and Extravascular Distribution of the ATP-Binding Cassette Transporters ABCB1 and ABCC1 in Aged Human Brain and Pituitary. Mech. Ageing Dev. 2014, 141–142, 12–21. [Google Scholar] [CrossRef]
- Devine, K.; Villalobos, E.; Kyle, C.J.; Andrew, R.; Reynolds, R.M.; Stimson, R.H.; Nixon, M.; Walker, B.R. The ATP-Binding Cassette Proteins ABCB1 and ABCC1 as Modulators of Glucocorticoid Action. Nat. Rev. Endocrinol. 2023, 19, 112–124. [Google Scholar] [CrossRef]
- Pahnke, J.; Langer, O.; Krohn, M. Alzheimer’s and ABC Transporters—New Opportunities for Diagnostics and Treatment. Neurobiol. Dis. 2014, 72, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-Y.; Hsiao, I.-T.; Chen, C.-S.; Chen, C.-H.; Hsieh, C.-J.; Wai, Y.-Y.; Chang, C.-J.; Tseng, H.-J.; Yen, T.-C.; Liu, C.-Y.; et al. Increased Brain Amyloid Deposition in Patients with a Lifetime History of Major Depression: Evidenced on 18F-Florbetapir (AV-45/Amyvid) Positron Emission Tomography. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Scherrmann, J.M. Expression and Function of Multidrug Resistance Transporters at the Blood-Brain Barriers. Expert Opin. Drug Metab. Toxicol. 2005, 1, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Kyle, C.J.; Nixon, M.; Homer, N.Z.M.; Morgan, R.A.; Andrew, R.; Stimson, R.H.; Walker, B.R. ABCC1 Modulates Negative Feedback Control of the Hypothalamic-Pituitary-Adrenal Axis in Vivo in Humans. Metabolism 2022, 128, 155419. [Google Scholar] [CrossRef]
- Guerry, J.D.; Hastings, P.D. In Search of HPA Axis Dysregulation in Child and Adolescent Depression. Clin. Child Fam. Psychol. Rev. 2011, 14, 135–160. [Google Scholar] [CrossRef]
- Buitelaar, J.K. The Role of the HPA-Axis in Understanding Psychopathology: Cause, Consequence, Mediator, or Moderator? Eur. Child Adolesc. Psychiatry 2013, 22, 387–389. [Google Scholar] [CrossRef][Green Version]
- Cao, C.; Rijlaarsdam, J. Childhood Parenting and Adolescent Internalizing and Externalizing Symptoms: Moderation by Multilocus Hypothalamic–Pituitary–Adrenal Axis-Related Genetic Variation. Dev. Psychopathol. 2023, 35, 524–536. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, M.-S.; Lee, J.H.; Kim, S.W.; Kang, R.-H.; Choi, M.-J.; Park, S.J.; Kim, S.J.; Lee, J.M.; Cole, S.P.C.; et al. MRP1 Polymorphisms Associated with Citalopram Response in Patients with Major Depression. J. Clin. Psychopharmacol. 2010, 30, 116–125. [Google Scholar] [CrossRef]
- Lotta, L.A.; Pietzner, M.; Stewart, I.D.; Wittemans, L.B.L.; Li, C.; Bonelli, R.; Raffler, J.; Biggs, E.K.; Oliver-Williams, C.; Auyeung, V.P.W.; et al. A Cross-Platform Approach Identifies Genetic Regulators of Human Metabolism and Health. Nat. Genet. 2021, 53, 54–64. [Google Scholar] [CrossRef]
- Hysi, P.G.; Mangino, M.; Christofidou, P.; Falchi, M.; Karoly, E.D.; NIHR Bioresource Investigators; Mohney, R.P.; Valdes, A.M.; Spector, T.D.; Menni, C. Metabolome Genome-Wide Association Study Identifies 74 Novel Genomic Regions Influencing Plasma Metabolite Levels. Metabolites 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic Atlas of the Plasma Metabolome Prioritizes Metabolites Implicated in Human Diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Virmani, A.; Binienda, Z. Role of Carnitine Esters in Brain Neuropathology. Mol. Asp. Med. 2004, 25, 533–549. [Google Scholar] [CrossRef]
- Lamhonwah, A.M.; Hawkins, C.E.; Tam, C.; Wong, J.; Mai, L.; Tein, I. Expression Patterns of the Organic Cation/Carnitine Transporter Family in Adult Murine Brain. Brain Dev. 2008, 30, 31–42. [Google Scholar] [CrossRef]
- Liu, R.; Pagliaccio, D.; Herbstman, J.B.; Fox, N.A.; Margolis, A.E. Prenatal Exposure to Air Pollution and Childhood Internalizing Problems: Roles of Shyness and Anterior Cingulate Cortex Activity. J. Child Psychol. Psychiatry 2023, 64, 1037–1044. [Google Scholar] [CrossRef]
- Freo, U.; Brugnatelli, V.; Turco, F.; Zanette, G. Analgesic and Antidepressant Effects of the Clinical Glutamate Modulators Acetyl-L-Carnitine and Ketamine. Front. Neurosci. 2021, 15, 584649. [Google Scholar] [CrossRef]
- Massart, R.; Guilloux, J.P.; Mignon, V.; Sokoloff, P.; Diaz, J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur. J. Neurosci. 2009, 30, 397–414. [Google Scholar] [CrossRef]
- Hasler, G.; Drevets, W.C.; Manji, H.K.; Charney, D.S. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004, 29, 1765–1781. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology 2010, 35, 192–216. [Google Scholar] [CrossRef]
- Okbay, A.; Wu, Y.; Wang, N.; Jayashankar, H.; Bennett, M.; Nehzati, S.M.; Sidorenko, J.; Kweon, H.; Goldman, G.; Gjorgjieva, T.; et al. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat. Genet. 2022, 54, 437–449. [Google Scholar] [CrossRef]
- Ben Hamida, S.; Sengupta, S.M.; Clarke, E.; McNicholas, M.; Moroncini, E.; Darcq, E.; Ter-Stepanian, M.; Fortier, M.È.; Grizenko, N.; Joober, R.; et al. The orphan receptor GPR88 controls impulsivity and is a risk factor for Attention-Deficit/Hyperactivity Disorder. Mol. Psychiatry 2022, 27, 4662–4672. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Schlaaff, K.; Dobrowolny, H.; Frodl, T.; Mawrin, C.; Gos, T.; Steiner, J.; Bogerts, B. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav. Immun. 2020, 88, 497–506. [Google Scholar] [CrossRef]
- Vogl, A.M.; Brockmann, M.M.; Giusti, S.A.; Maccarrone, G.; Vercelli, C.A.; Bauder, C.A.; Richter, J.S.; Roselli, F.; Hafner, A.S.; Dedic, N.; et al. Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition. Nat. Neurosci. 2015, 18, 239–251. [Google Scholar] [CrossRef]
- Brockmann, M.M.; Döngi, M.; Einsfelder, U.; Körber, N.; Refojo, D.; Stein, V. Neddylation regulates excitatory synaptic transmission and plasticity. Sci. Rep. 2019, 9, 17935. [Google Scholar] [CrossRef]
- Duman, R.S. Pathophysiology of Depression and Innovative Treatments: Remodeling Glutamatergic Synaptic Connections. Dialogues Clin. Neurosci. 2014, 16, 11–27. [Google Scholar] [CrossRef]
- Moriguchi, S.; Takamiya, A.; Noda, Y.; Horita, N.; Wada, M.; Tsugawa, S.; Plitman, E.; Sano, Y.; Tarumi, R.; ElSalhy, M.; et al. Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 2019, 24, 952–964. [Google Scholar] [CrossRef]
- Li, C.T.; Yang, K.C.; Lin, W.C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence from Clinical Neuroimaging Studies. Front. Psychiatry 2019, 9, 767. [Google Scholar] [CrossRef]
- Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef]
- Luscher, B.; Fuchs, T. GABAergic control of depression-related brain states. Adv. Pharmacol. 2015, 73, 97–144. [Google Scholar]
- Mineur, Y.S.; Cahuzac, E.L.; Mose, T.N.; Bentham, M.P.; Plantenga, M.E.; Thompson, D.C.; Picciotto, M.R. Interaction between Noradrenergic and Cholinergic Signaling in Amygdala Regulates Anxiety- and Depression-Related Behaviors in Mice. Neuropsychopharmacology 2018, 43, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Song, H.; Chu, G.; Zhan, X.; Liu, B.; Mu, Y.; Wang, J.-Z.; Lu, Y. Basal Forebrain Cholinergic Innervation Induces Depression-Like Behaviors through Ventral Subiculum Hyperactivation. Neurosci. Bull. 2023, 39, 617–630. [Google Scholar] [CrossRef]
- Bekhbat, M.; Li, Z.; Mehta, N.D.; Treadway, M.T.; Lucido, M.J.; Woolwine, B.J.; Haroon, E.; Miller, A.H.; Felger, J.C. Functional Connectivity in Reward Circuitry and Symptoms of Anhedonia as Therapeutic Targets in Depression with High Inflammation: Evidence from a Dopamine Challenge Study. Mol. Psychiatry 2022, 27, 4113–4121. [Google Scholar] [CrossRef]
- Xu, H.; Li, T.; Gong, Q.; Xu, H.; Hu, Y.; Lü, W.; Yang, X.; Li, J.; Xu, W.; Kuang, W. Genetic Variations in the Retrograde Endocannabinoid Signaling Pathway in Chinese Patients with Major Depressive Disorder. Front. Neurol. 2023, 14, 1208546. [Google Scholar] [CrossRef]
- Atwood, B.K.; Lovinger, D.M.; Mathur, B.N. Presynaptic Long-Term Depression Mediated by Gi/o-Coupled Receptors. Trends Neurosci. 2014, 37, 663–673. [Google Scholar] [CrossRef]
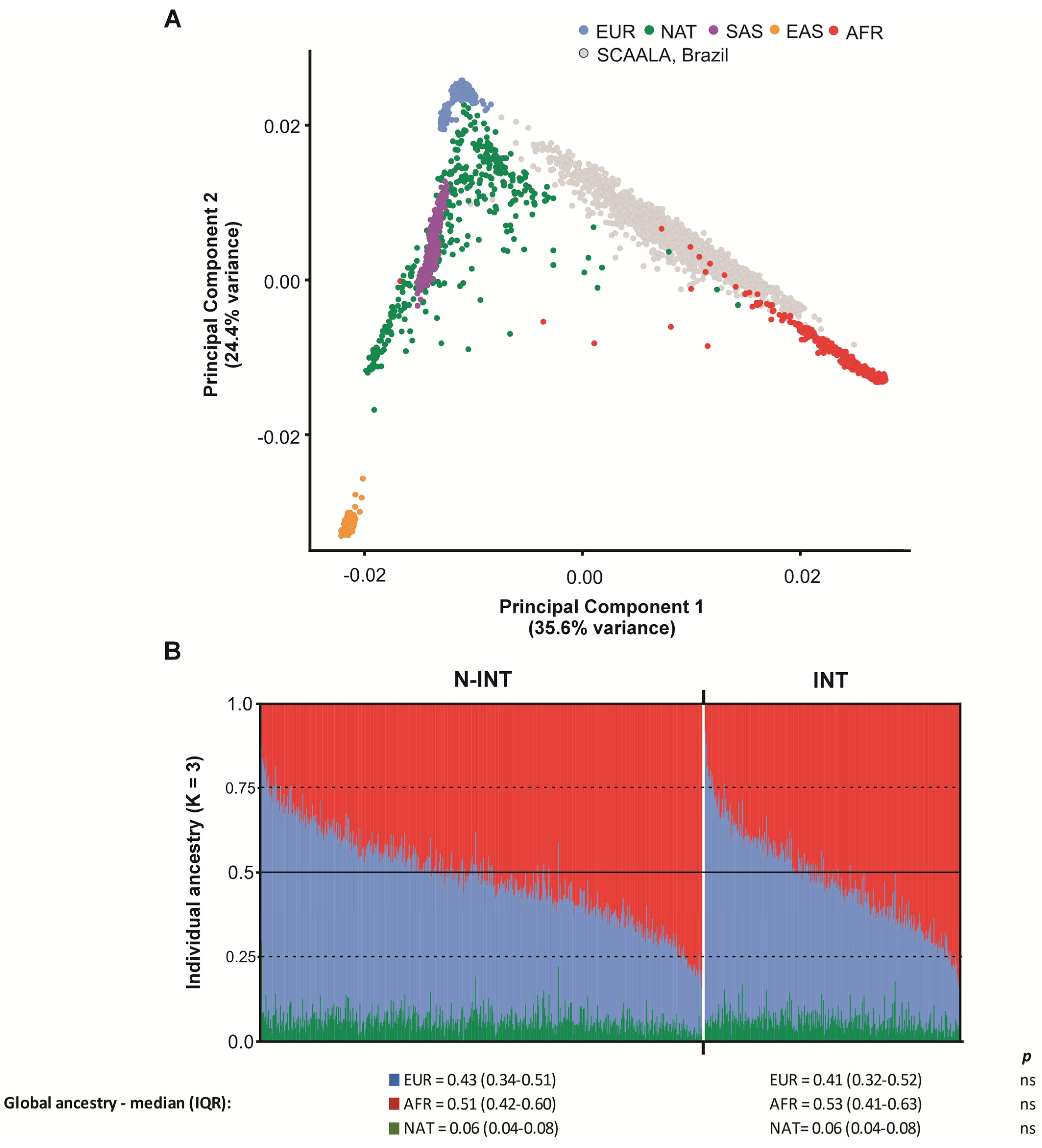


| N-INT | INT | p | |
|---|---|---|---|
| Number of children (%) | 794 (63.8) | 450 (36.2) | - |
| Sex, female (%) | 390 (49.1) | 178 (39.6) | <0.05 |
| Median age, years (IQR) | 8 (6–9) | 8 (7–9) | ns |
| SNP | Coordinate a | Locus | A1 | MAF | OR | 95% CI | p |
|---|---|---|---|---|---|---|---|
| rs7196970 | 16:16002935 | 16p13.11 | G | 0.49 | 0.61 | 0.51–0.73 | 4.5 × 10−8 |
| rs11847624 | 14:90740595 | 14q32.11 | G | 0.21 | 1.66 | 1.36–2.02 | 5.5 × 10−7 |
| rs1728386 | 16:86384206 | 16q24.1 | T | 0.24 | 1.65 | 1.35–2.00 | 5.8 × 10−7 |
| rs138365190 | 12:128452629 | 12q24.32 | A | 0.03 | 3.13 | 1.98–4.93 | 8.9 × 10−7 |
| rs405792 | 21:14494814 | 21q11.2 | A | 0.25 | 0.61 | 0.50–0.75 | 1.5 × 10−6 |
| rs11166475 | 1:100556556 | 1p21.2 | A | 0.41 | 1.52 | 1.28–1.81 | 1.8 × 10−6 |
| rs1526415 | 7:125439821 | 7q31.33 | A | 0.06 | 2.33 | 1.64–3.30 | 2.0 × 10−6 |
| rs33973779 | 11:98784980 | 11q22.1 | G | 0.09 | 1.96 | 1.48–2.59 | 2.3 × 10−6 |
| rs57145395 | 7:125377403 | 7q31.33 | G | 0.06 | 2.19 | 1.58–3.03 | 2.4 × 10−6 |
| rs74894866 | 12:19026261 | 12p12.3 | T | 0.20 | 0.58 | 0.47–0.73 | 2.6 × 10−6 |
| rs79063512 | 4:11194663 | 4p16.1 | T | 0.07 | 2.11 | 1.54–2.89 | 3.3 × 10−6 |
| rs152168 | 16:66852656 | 16q22.1 | A | 0.30 | 0.64 | 0.53–0.77 | 3.3 × 10−6 |
| rs156426 | 7:23267856 | 7p15.3 | C | 0.07 | 2.13 | 1.55–2.94 | 3.4 × 10−6 |
| rs2082027 | 4:147412952 | 4q31.22 | C | 0.34 | 0.66 | 0.55–0.78 | 3.9 × 10−6 |
| rs115631938 | 6:37537257 | 6p21.2 | C | 0.04 | 2.69 | 1.77–4.08 | 3.9 × 10−6 |
| rs2179654 | 1:209434426 | 1p32.2 | T | 0.25 | 1.57 | 1.29–1.90 | 4.2 × 10−6 |
| rs115162927 | 1:234572600 | 1q42.2 | A | 0.02 | 4.16 | 2.27–7.64 | 4.2 × 10−6 |
| rs57279798 | 9:109082533 | 9q31.3 | A | 0.08 | 2.00 | 1.49–2.68 | 4.3 × 10−6 |
| rs73092035 | 20:8440666 | 20p12.3 | C | 0.18 | 0.57 | 0.45–0.73 | 4.6 × 10−6 |
| 1:206062625_T | 1:206062625 | 1q32.1 | C | 0.41 | 1.48 | 1.25–1.76 | 4.7 × 10−6 |
| rs1924622 | 13:28522605 | 13q12.3 | C | 0.07 | 2.04 | 1.51–2.78 | 4.8 × 10−6 |
| rs76680358 | 1:212556035 | 1q32.3 | T | 0.16 | 1.69 | 1.35–2.12 | 4.9 × 10−6 |
| rs113284492 | 7:125396505 | 7q31.33 | T | 0.02 | 3.79 | 2.14–6.71 | 4.9 × 10−6 |
| rs6665232 | 1:209512951 | 1p32.2 | A | 0.48 | 0.68 | 0.57–0.80 | 5.1 × 10−6 |
| rs12682188 | 8:15772149 | 8p22 | C | 0.39 | 0.66 | 0.56–0.79 | 5.3 × 10−6 |
| rs10220411 | 14:68985371 | 14q24.1 | G | 0.36 | 1.49 | 1.25–1.77 | 5.9 × 10−6 |
| rs626337 | 3:173442741 | 3q26.31 | A | 0.47 | 0.68 | 0.58–0.80 | 6.1 × 10−6 |
| rs1959485 | 14:70487504 | 14q24.2 | T | 0.40 | 0.67 | 0.57–0.80 | 6.2 × 10−6 |
| rs515683 | 1:58183297 | 1p32.2 | A | 0.37 | 0.67 | 0.56–0.80 | 6.4 × 10−6 |
| rs78294387 | 7:23289364 | 7p15.3 | A | 0.10 | 1.85 | 1.41–2.41 | 7.3 × 10−6 |
| 2:78015566_C | 2:78015566 | 2p12 | C | 0.27 | 1.54 | 1.27–1.86 | 7.8 × 10−6 |
| rs73466526 | 15:99736749 | 15q26.3 | A | 0.12 | 1.73 | 1.36–2.21 | 8.6 × 10−6 |
| rs7011010 | 8:116114085 | 8q23.3 | C | 0.36 | 1.48 | 1.25–1.76 | 8.7 × 10−6 |
| rs1983270 | 3:86304891 | 3p12.1 | T | 0.23 | 1.58 | 1.29–1.93 | 8.8 × 10−6 |
| rs4945142 | 11:77095476 | 11q13.5 | T | 0.35 | 1.48 | 1.25–1.76 | 9.0 × 10−6 |
| rs11054328 | 12:11510821 | 12p13.2 | T | 0.15 | 1.69 | 1.34–2.13 | 9.7 × 10−6 |
| rs701379 | 9:98124543 | 9q22.33 | T | 0.26 | 0.64 | 0.53–0.78 | 9.9 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britto, G.d.S.G.; Moreira, A.O.; Bispo Amaral, E.H.; Santos, D.E.; São Pedro, R.B.; Barreto, T.M.M.; Feitosa, C.A.; Neves dos Santos, D.; Tarazona-Santos, E.; Barreto, M.L.; et al. Genome-Wide Insights into Internalizing Symptoms in Admixed Latin American Children. Genes 2025, 16, 63. https://doi.org/10.3390/genes16010063
Britto GdSG, Moreira AO, Bispo Amaral EH, Santos DE, São Pedro RB, Barreto TMM, Feitosa CA, Neves dos Santos D, Tarazona-Santos E, Barreto ML, et al. Genome-Wide Insights into Internalizing Symptoms in Admixed Latin American Children. Genes. 2025; 16(1):63. https://doi.org/10.3390/genes16010063
Chicago/Turabian StyleBritto, Gabriela de Sales Guerreiro, Alberto O. Moreira, Edson Henrique Bispo Amaral, Daniel Evangelista Santos, Raquel B. São Pedro, Thaís M. M. Barreto, Caroline Alves Feitosa, Darci Neves dos Santos, Eduardo Tarazona-Santos, Maurício Lima Barreto, and et al. 2025. "Genome-Wide Insights into Internalizing Symptoms in Admixed Latin American Children" Genes 16, no. 1: 63. https://doi.org/10.3390/genes16010063
APA StyleBritto, G. d. S. G., Moreira, A. O., Bispo Amaral, E. H., Santos, D. E., São Pedro, R. B., Barreto, T. M. M., Feitosa, C. A., Neves dos Santos, D., Tarazona-Santos, E., Barreto, M. L., Figueiredo, C. A. V. d., Costa, R. d. S., Godard, A. L. B., & Oliveira, P. R. S. (2025). Genome-Wide Insights into Internalizing Symptoms in Admixed Latin American Children. Genes, 16(1), 63. https://doi.org/10.3390/genes16010063






