AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice
Abstract
1. Background
2. Methods
2.1. Mouse Strains
2.2. AOM/DSS-Induced CRC
2.3. Tumor Analysis and Colon Sample Collection
2.4. Histopathological Analysis
2.5. Cell Culture
2.6. Immunoblotting Analysis
2.7. Immunohistochemical Staining
2.8. Quantitative Reverse-Transcriptase (RT)-PCR
2.9. α-Ketoglutarate Assays
2.10. Methylated DNA Immunoprecipitation
2.11. Statistical Analyses
3. Results
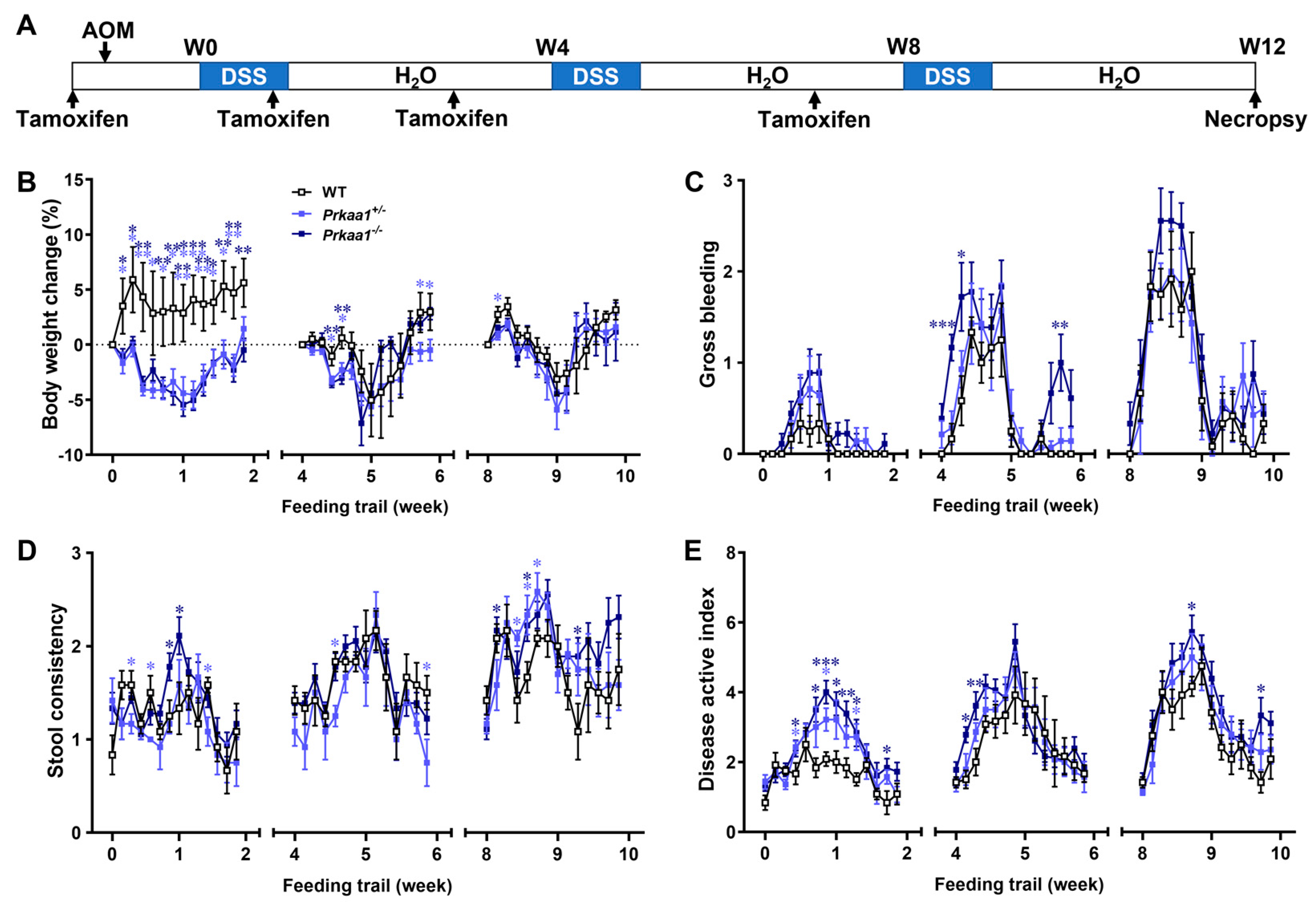
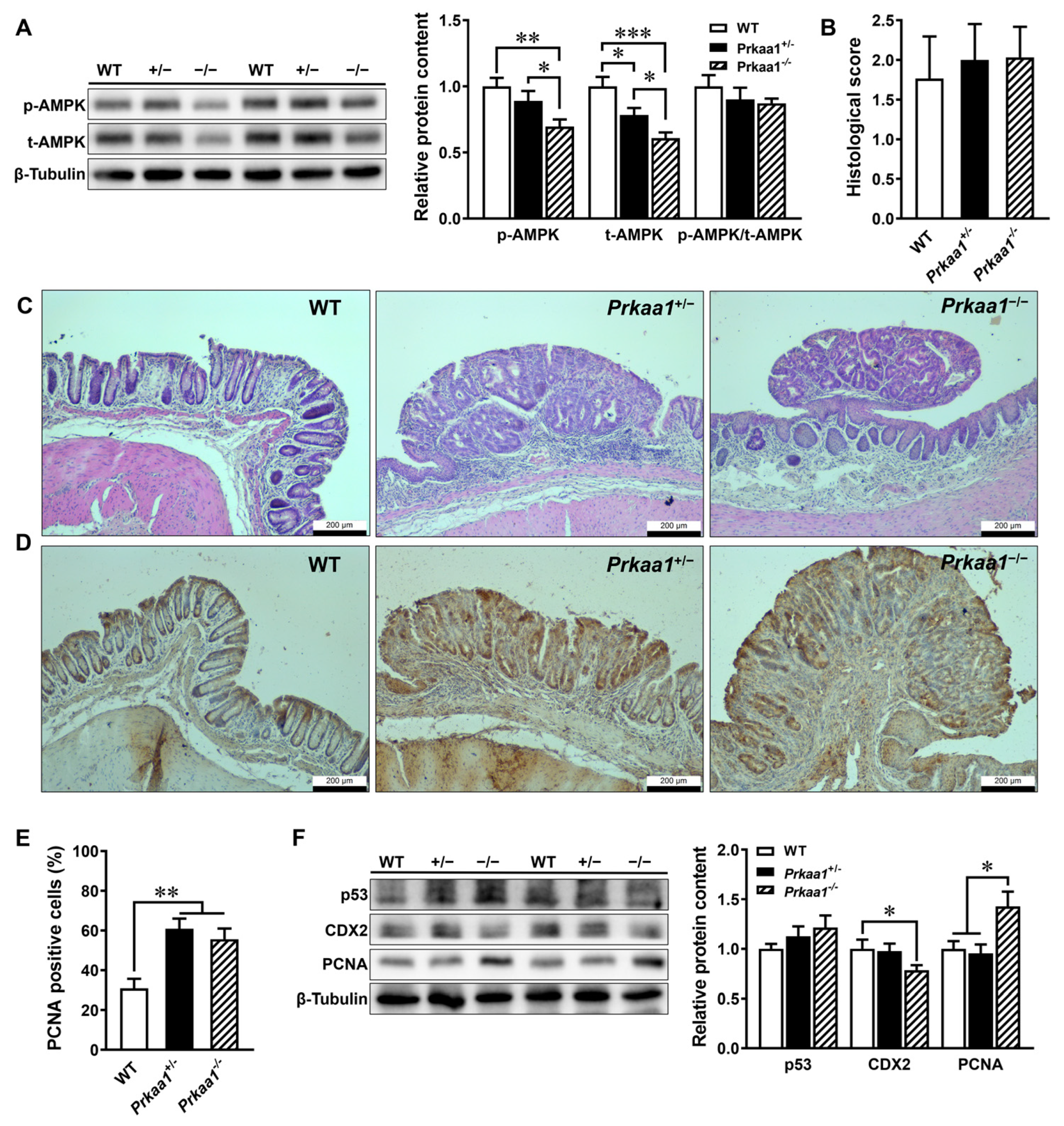
3.1. AMPK Deficiency Aggravates Colorectal Tumorigenesis
3.2. AMPK Ablation-Induced CRC Is Associated with Decreased α-Ketoglutarate Production in Colonic Tissues
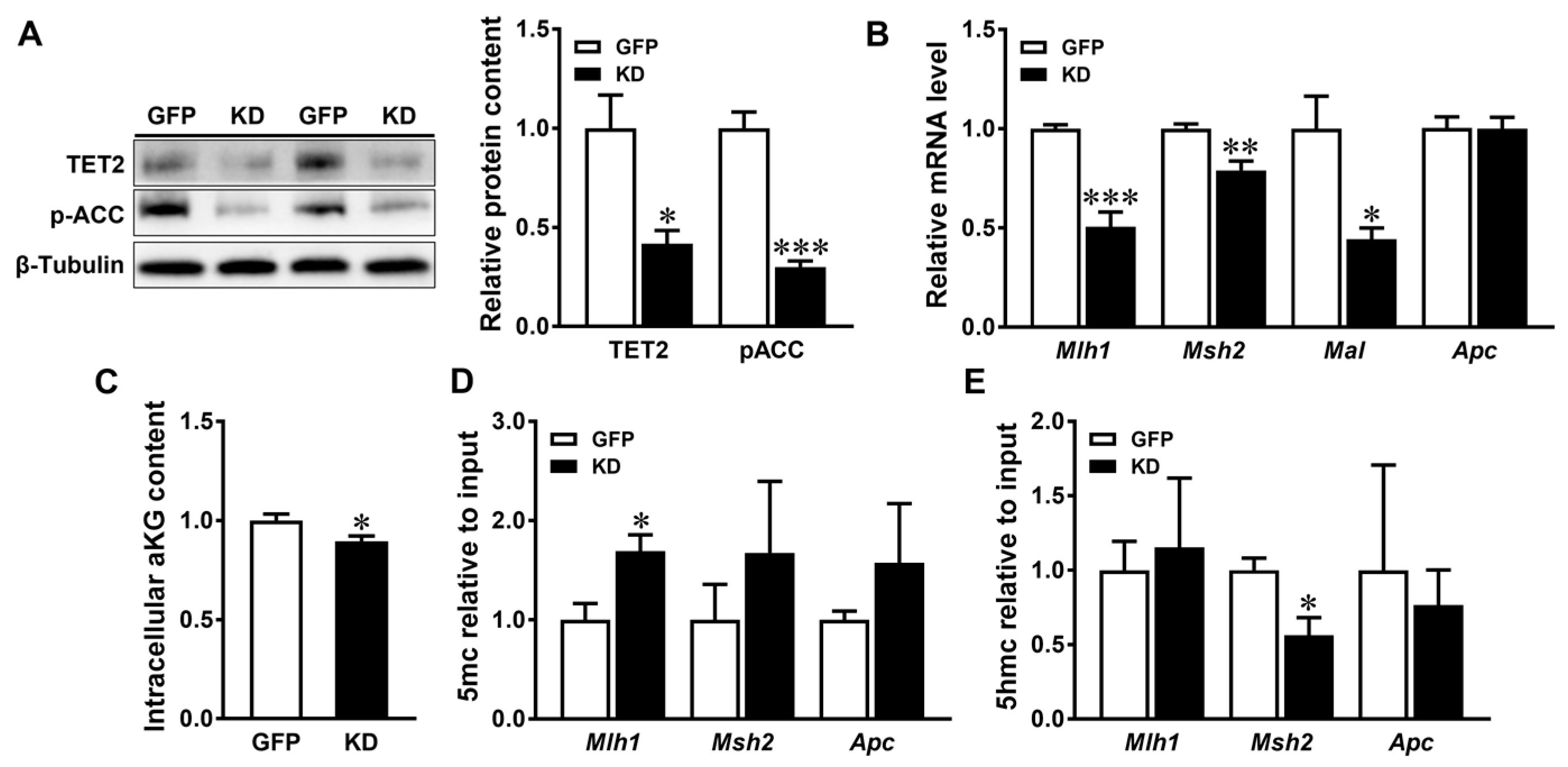
3.3. AMPK Regulates the DNA Methylation of Tumor Suppressive Genes in Caco-2 Cells
4. Discussion
4.1. AMPK Deficiency Promotes Colorectal Carcinogenesis
4.2. AMPK KO-Induced α-Ketoglutarate Deficiency Induces Epigenetic Alterations Associated with CRC
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zheng, X.B.; Zong, X.Y.; Li, Z.T.; Li, N.; Hur, J.; Fritz, C.D.L.; Chapman, W.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, J.; Sidransky, D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010, 29, 181–206. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, T.; Lind, G.E.; Costa, V.L.; Meling, G.I.; Vatn, M.; Hoff, G.S.; Rognum, T.O.; Skotheim, R.I.; Thiis-Evensen, E.; Lothe, R.A. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol. Cancer 2008, 7, 94. [Google Scholar] [CrossRef]
- Li, M.; Gao, F.; Xia, Y.D.; Tang, Y.; Zhao, W.; Jin, C.C.; Luo, H.J.; Wang, J.W.; Li, Q.S.; Wang, Y.L. Filtrating colorectal cancer associated genes by integrated analyses of global DNA methylation and hydroxymethylation in cancer and normal tissue. Sci. Rep. 2016, 6, 31826. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Umar, A.; Polyak, K.; Graff, J.R.; Ahuja, N.; Issa, J.P.J.; Markowitz, S.; Willson, J.K.V.; Hamilton, S.R.; Kinzler, K.W.; et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 6870–6875. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Liu, L.M.; Chen, X.J.; Shen, J.J.; Shan, J.J.; Xu, Y.M.; Yang, Z.; Wu, L.; Xia, F.; Bie, P.; et al. Decrease of 5-hydroxymethylcytosine is associated with progression of hepatocellular carcinoma through downregulation of TET1. PLoS ONE 2013, 8, e62828. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.G.; Xu, Y.F.; Ceol, C.; Wu, F.Z.; Larson, A.; Dresser, K.; Xu, W.Q.; Tan, L.; Hu, Y.G.; Zhan, Q.; et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef]
- Kudo, Y.; Tateishi, K.; Yamamoto, K.; Yamamoto, S.; Asaoka, Y.; Ijichi, H.; Nagae, G.; Yoshida, H.; Aburatani, H.; Koike, K. Loss of 5-hydroxymethylcytosine is accompanied with malignant cellular transformation. Cancer Sci. 2012, 103, 670–676. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.L.; McLendon, R.; Rasheed, B.A.; Yuan, W.S.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Katada, S.; Imhof, A.; Sassone-Corsi, P. Connecting threads: Epigenetics and metabolism. Cell 2012, 148, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Thompson, C.B. Metabolic regulation of epigenetics. Cell Metab. 2012, 16, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Teperino, R.; Schoonjans, K.; Auwerx, J. Histone methyl transferases and demethylases; Can they link metabolism and transcription? Cell Metab. 2010, 12, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Lee, W.S.; Park, O.J. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp. Mol. Med. 2009, 41, 201–207. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, M.S.; Cha, B.Y.; Lee, J.S.; Sul, J.Y.; Song, I.S.; Kim, J.Y. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol. Pharm. Bull. 2012, 35, 1614–1620. [Google Scholar] [CrossRef]
- Li, W.D.; Hua, B.J.; Saud, S.M.; Lin, H.S.; Hou, W.; Matter, M.S.; Jia, L.B.; Colburn, N.H.; Young, M.R. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinog. 2015, 54, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Iniguez, A.B.; Tian, Q.; Du, M.; Zhu, M.-J. PGC-1α in mediating mitochondrial biogenesis and intestinal epithelial differentiation promoted by purple potato extract. J. Funct. Foods 2022, 98, 105291. [Google Scholar] [CrossRef]
- Sun, X.F.; Yang, Q.Y.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- El Marjou, F.; Janssen, K.P.; Chang, B.H.J.; Li, M.; Hindie, V.; Chan, L.; Louvard, D.; Chambon, P.; Metzger, D.; Robine, S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004, 39, 186–193. [Google Scholar] [CrossRef]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Kang, Y.F.; Xue, Y.S.; Du, M.; Zhu, M.J. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Xu, Z.X.; Sun, X.F.; Deavila, J.; Du, M.; Zhu, M.J. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J. Nutr. Biochem. 2020, 84, 108443. [Google Scholar] [CrossRef]
- Young, N.P.; Kamireddy, A.; Van Nostrand, J.L.; Eichner, L.J.; Shokhirev, M.N.; Dayn, Y.; Shaw, R.J. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev. 2016, 30, 535–552. [Google Scholar] [CrossRef]
- Hofman, F. Immunohistochemistry. Curr. Protoc. Immunol. 2002, 49, 21.24.21–21.24.23. [Google Scholar] [CrossRef]
- Xue, Y.S.; Zhang, H.Y.; Sun, X.F.; Zhu, M.J. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS ONE 2016, 11, e0168670. [Google Scholar] [CrossRef]
- Sun, Q.; Bibi, S.; Xue, Y.; Du, M.; Chew, B.; Zhu, M.-J. Dietary purple potato supplement attenuates DSS-induced colitis in mice: Impact on mitochondrial function. J. Nutr. Biochem. 2024, 126, 109585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Liang, X.W.; Sun, X.F.; Zhang, L.P.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.M.; Wang, S.B.; Wang, B.; et al. AMPK/alpha-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Mohn, F.; Weber, M.; Schübeler, D.; Roloff, T.-C. Methylated DNA immunoprecipitation (medip). DNA Methylation Methods Protoc. 2009, 507, 55–64. [Google Scholar]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, D.; Chen, H.; Shi, G.M.; Fetahu, I.S.; Wu, F.Z.; Rabidou, K.; Fang, R.; Tan, L.; Xu, S.Y.; et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018, 559, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Nunez, N.P.; Pennisi, P.; Brodt, P.; Sun, H.; Fallavollita, L.; Zhao, H.; Scavo, L.; Novosyadlyy, R.; Kurshan, N.; et al. Increased tumor growth in mice with diet-induced obesity: Impact of ovarian hormones. Endocrinology 2006, 147, 5826–5834. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; Garcia-Canaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim. Et Biophys. Acta-Proteins Proteom. 2010, 1804, 581–591. [Google Scholar] [CrossRef]
- Zheng, B.; Jeong, J.H.; Asara, J.M.; Yuan, Y.Y.; Granters, S.R.; Chin, L.; Cantley, L.C. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 2009, 33, 237–247. [Google Scholar] [CrossRef]
- Arciuch, V.G.A.; Russo, M.A.; Kang, K.S.; Di Cristofano, A. Inhibition of AMPK and krebs cycle gene expression drives metabolic remodeling of PTEN-deficient preneoplastic thyroid cells. Cancer Res. 2013, 73, 5459–5472. [Google Scholar] [CrossRef]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef]
- Imamura, K.; Ogura, T.; Kishimoto, A.; Kaminishi, M.; Esumi, H. Cell cycle regulation via p53 phosphorylation by a 5’-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 2001, 287, 562–567. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Uchiyama, T.; Suzuki, K.; Nozaki, Y.; Yoneda, K.; Fujita, K.; Yoneda, M.; et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol. Carcinog. 2010, 49, 662–671. [Google Scholar] [CrossRef]
- Tomimoto, A.; Endo, H.; Sugiyama, M.; Fujisawa, T.; Hosono, K.; Takahashi, H.; Nakajima, N.; Nagashima, Y.; Wada, K.; Nakagama, H.; et al. Metformin suppresses intestinal polyp growth in Apc(Min/+) mice. Cancer Sci. 2008, 99, 2136–2141. [Google Scholar] [CrossRef]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Ko, S.H.; Kim, J.S. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 2014, 29, 502–510. [Google Scholar] [CrossRef]
- Schneider, M.B.; Matsuzaki, H.; Haorah, J.; Ulrich, A.; Standop, J.; Ding, X.Z.; Adrian, T.E.; Pour, P.M. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001, 120, 1263–1270. [Google Scholar] [CrossRef]
- Memmott, R.M.; Mercado, J.R.; Maier, C.R.; Kawabata, S.; Fox, S.D.; Dennis, P.A. Metformin prevents tobacco carcinogen-induced lung tumorigenesis. Cancer Prev. Res. 2010, 3, 1066–1076. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Laurent, K.; Loubat, A.; Giorgetti-Peraldi, S.; Colosetti, P.; Auberger, P.; Tanti, J.F.; Le Marchand-Brustel, Y.; Bost, F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008, 27, 3576–3586. [Google Scholar] [CrossRef]
- Losman, J.A.; Koivunen, P.; Kaelin, W.G. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 2020, 20, 710–726. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Harris, W.A. Metabolism in physiological cell proliferation and differentiation. Trends Cell Biol. 2013, 23, 484–492. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Liang, G.; Lenz, H.J. DNA methylation aberrancies delineate clinically distinct subsets of colorectal cancer and provide novel targets for epigenetic therapies. Oncogene 2018, 37, 566–577. [Google Scholar] [CrossRef]
- Prensner, J.R.; Chinnaiyan, A.M. Metabolism unhinged: IDH mutations in cancer. Nat. Med. 2011, 17, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.M.; Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef]
- Cimmino, L.; Abdel-Wahab, O.; Levine, R.L.; Aifantis, I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell 2011, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; McCabe, N.; Mullarkey, M.; Cummins, R.; Burgess, D.J.; Nakabeppu, Y.; Oka, S.; Kay, E.; Lord, C.J.; Ashworth, A. DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell 2010, 17, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, Y.; Zhao, J.X.; Rogers, C.J.; Zhu, M.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity downregulates microRNA let-7g expression, a possible mechanism for enhanced adipogenesis during ovine fetal skeletal muscle development. Int. J. Obes. 2013, 37, 568–575. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Tian, Q.; Bravo Iniguez, A.; Sun, X.; Zhang, H.; Deavila, J.; Du, M.; Zhu, M.-J. AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice. Genes 2024, 15, 835. https://doi.org/10.3390/genes15070835
Sun Q, Tian Q, Bravo Iniguez A, Sun X, Zhang H, Deavila J, Du M, Zhu M-J. AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice. Genes. 2024; 15(7):835. https://doi.org/10.3390/genes15070835
Chicago/Turabian StyleSun, Qi, Qiyu Tian, Alejandro Bravo Iniguez, Xiaofei Sun, Hui Zhang, Jeanene Deavila, Min Du, and Mei-Jun Zhu. 2024. "AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice" Genes 15, no. 7: 835. https://doi.org/10.3390/genes15070835
APA StyleSun, Q., Tian, Q., Bravo Iniguez, A., Sun, X., Zhang, H., Deavila, J., Du, M., & Zhu, M.-J. (2024). AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice. Genes, 15(7), 835. https://doi.org/10.3390/genes15070835





