A Simple Nonviral Method to Generate Human Induced Pluripotent Stem Cells Using SMAR DNA Vectors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning and Bacteria
2.2. Cell Culture
2.3. Cellular Transfection
2.4. Reprogramming NHDFs
2.5. iPSC Colony Picking
2.6. Alkaline Phosphatase Staining
2.7. Trilineage Differentiation
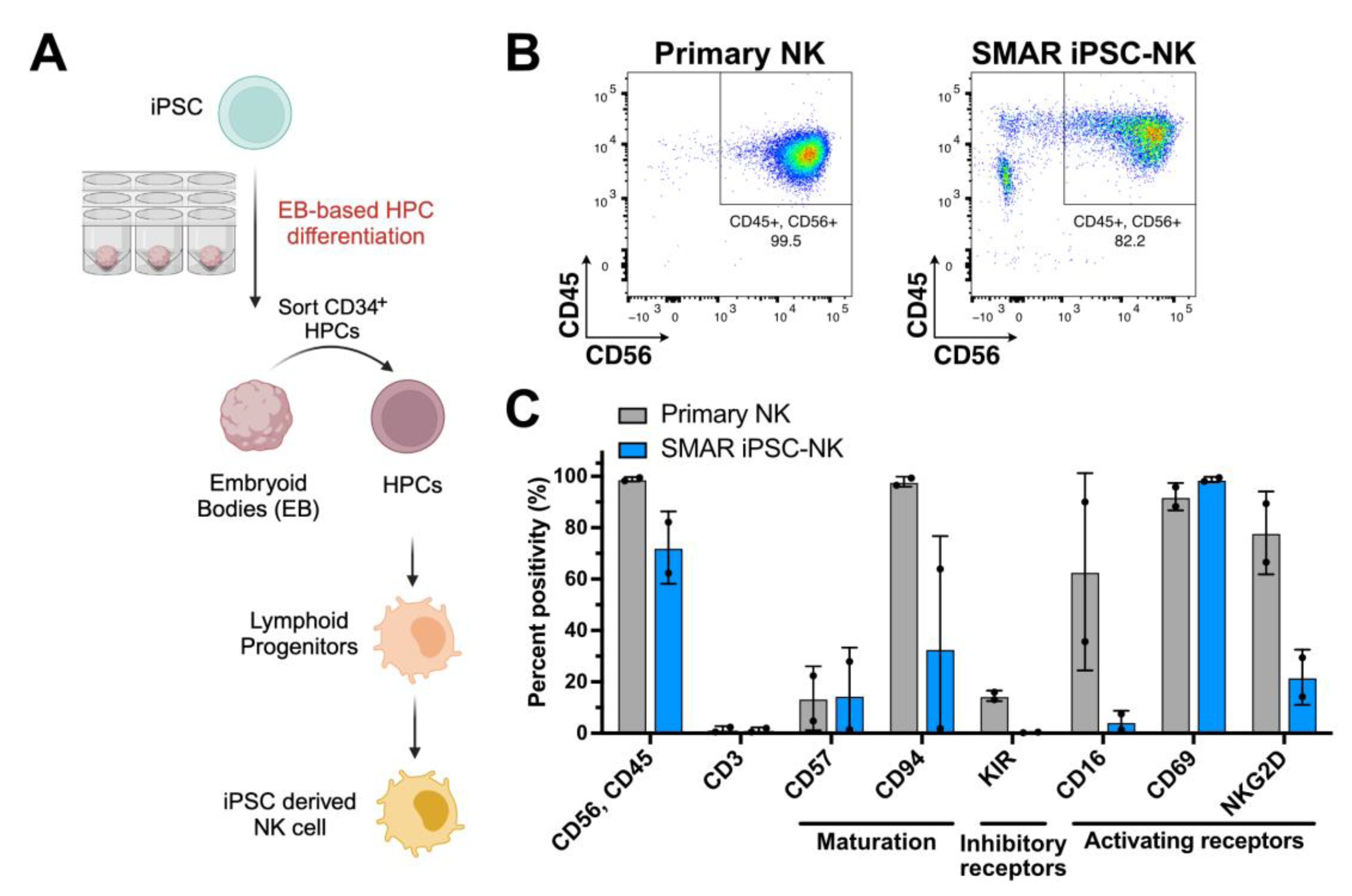
2.8. NK Cell Differentiation
2.9. PCR for Vector Retention
2.10. Microscopy
2.11. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.12. Western Blotting
2.13. Immunofluorescence
2.14. Statistical Analysis
3. Results
3.1. Generation of a Novel Nonviral Reprogramming Platform
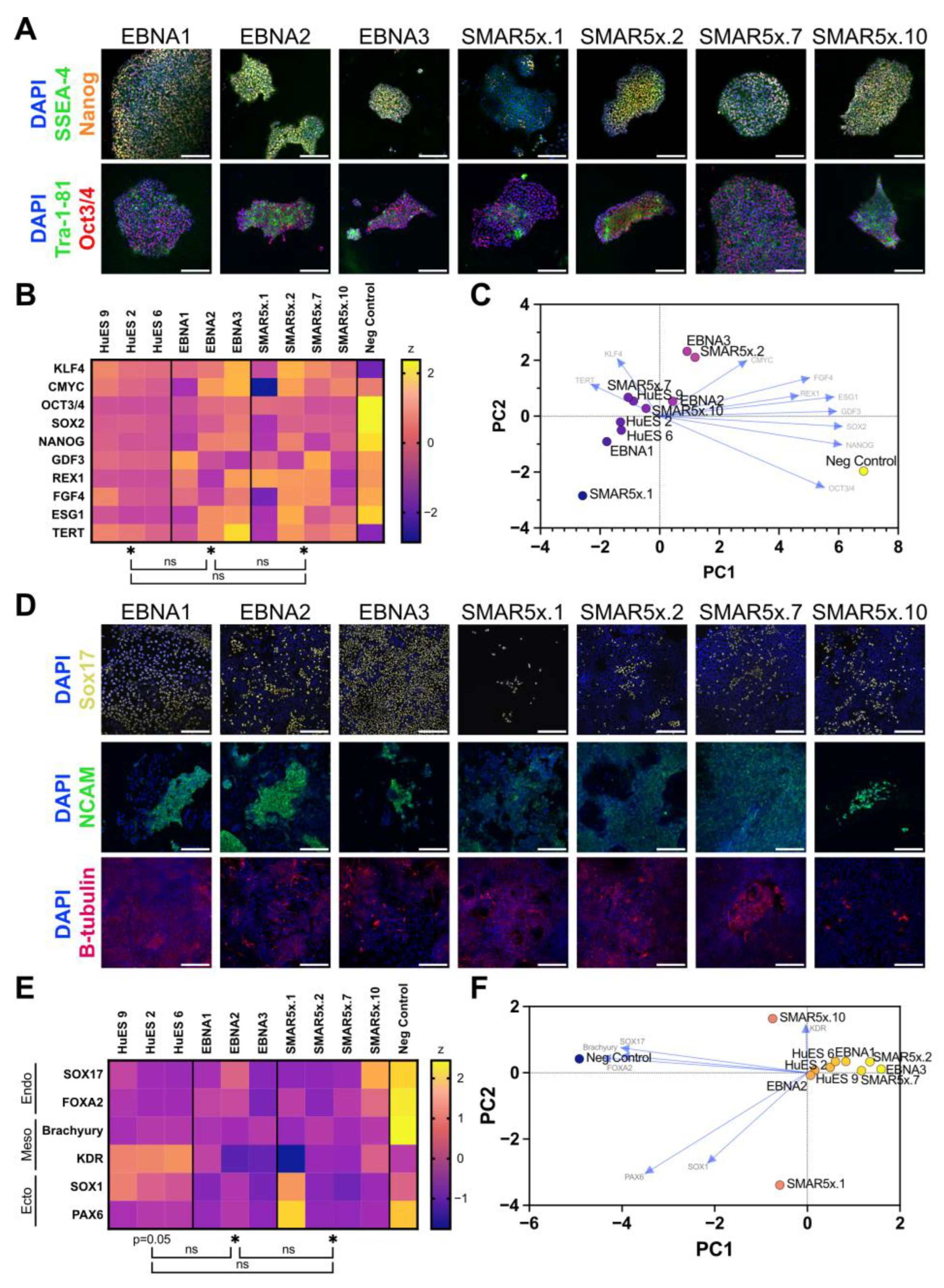
3.2. Characterisation of SMAR iPSCs
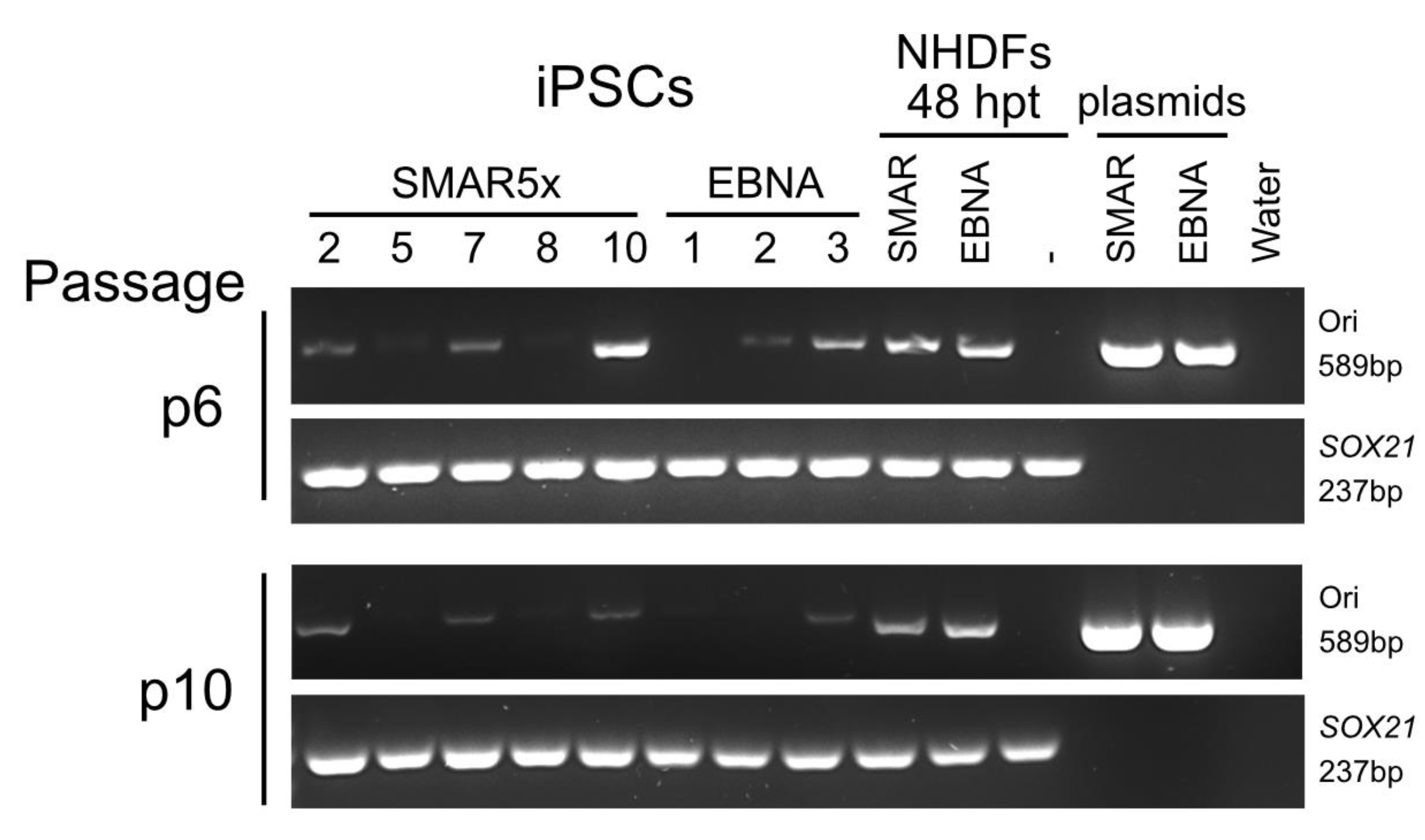
3.3. Generation of Vector-Free iPSCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Malik, N.; Rao, M.S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar] [CrossRef]
- Cesana, D.; Ranzani, M.; Volpin, M.; Bartholomae, C.; Duros, C.; Artus, A.; Merella, S.; Benedicenti, F.; Sergi Sergi, L.; Sanvito, F.; et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014, 22, 774–785. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Investigating Serious Risk of T-cell Malignancy Following BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T Cell Immunotherapies. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous (accessed on 1 December 2023).
- US Food and Drug Administration. FDA Roundup. 23 January 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-roundup-january-23-2024 (accessed on 7 February 2024).
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, A.; Hirohata, R.; Arreola, V.; Osawa, M.; Kato, T.M.; Nomura, M.; Kawaguchi, J.; Hara, H.; Kusano, K.; Takashima, Y.; et al. Improved Sendai viral system for reprogramming to naive pluripotency. Cell Rep. Methods 2022, 2, 100317. [Google Scholar] [CrossRef] [PubMed]
- Frappier, L. Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses 2012, 4, 1537–1547. [Google Scholar] [CrossRef]
- Humme, S.; Reisbach, G.; Feederle, R.; Delecluse, H.J.; Bousset, K.; Hammerschmidt, W.; Schepers, A. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. USA 2003, 100, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An Efficient Nonviral Method to Generate Integration-Free Human-Induced Pluripotent Stem Cells from Cord Blood and Peripheral Blood Cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Thornton, C.D.; Fielding, S.; Karbowniczek, K.; Roig-Merino, A.; Burrows, A.E.; FitzPatrick, L.M.; Sharaireh, A.; Tite, J.P.; Mole, S.E.; Harbottle, R.P.; et al. Safe and stable generation of induced pluripotent stem cells using doggybone DNA vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Yakubov, E.; Rechavi, G.; Rozenblatt, S.; Givol, D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010, 394, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Hong, Y.J.; Do, J.T. Cellular Reprogramming Using Protein and Cell-Penetrating Peptides. Int. J. Mol. Sci. 2017, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J. iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen. Ther. 2020, 13, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J. Clinical Trial for Parkinson’s Disease Gets a Green Light in the US. Cell Stem Cell 2021, 28, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Robbins, S.; Wang, X.; Virk, S.; Schuck, K.; Deveza, L.A.; Oo, W.M.; Carmichael, K.; Antony, B.; Eckstein, F.; et al. Efficacy and cost-effectiveness of Stem Cell injections for symptomatic relief and strUctural improvement in people with Tibiofemoral knee OsteoaRthritis: Protocol for a randomised placebo-controlled trial (the SCUlpTOR trial). BMJ Open 2021, 11, e056382. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of Mouse Induced Pluripotent Stem Cells without Viral Vectors. Science 2008, 322, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef]
- Yates, J.L.; Camiolo, S.M.; Bashaw, J.M. The minimal replicator of Epstein-Barr virus oriP. J. Virol. 2000, 74, 4512–4522. [Google Scholar] [CrossRef] [PubMed]
- De Belle, I.; Cai, S.; Kohwi-Shigematsu, T. The Genomic Sequences Bound to Special AT-rich Sequence-binding Protein 1 (SATB1) In Vivo in Jurkat T Cells Are Tightly Associated with the Nuclear Matrix at the Bases of the Chromatin Loops. J. Cell Biol. 1998, 141, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Jenke, B.H.; Fetzer, C.P.; Stehle, I.M.; Jonsson, F.; Fackelmayer, F.O.; Conradt, H.; Bode, J.; Lipps, H.J. An episomally replicating vector binds to the nuclear matrix protein SAF-A in vivo. EMBO Rep. 2002, 3, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, C.; Wong, S.P.; Harbottle, R.; Lipps, H.J. Scaffold/matrix attached region-based nonviral episomal vectors. Hum. Gene Ther. 2011, 22, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, F.; Hagedorn, C. Episomes and Transposases—Utilities to Maintain Transgene Expression from Nonviral Vectors. Genes. 2022, 13, 1872. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Lazaris, V.M.; Simantirakis, E.; Stavrou, E.F.; Verras, M.; Sgourou, A.; Keramida, M.K.; Vassilopoulos, G.; Athanassiadou, A. Non-Viral Episomal Vector Mediates Efficient Gene Transfer of the beta-Globin Gene into K562 and Human Haematopoietic Progenitor Cells. Genes 2023, 14, 1774. [Google Scholar] [CrossRef]
- Stavrou, E.F.; Lazaris, V.M.; Giannakopoulos, A.; Papapetrou, E.; Spyridonidis, A.; Zoumbos, N.C.; Gkountis, A.; Athanassiadou, A. The β-globin Replicator greatly enhances the potential of S/MAR based episomal vectors for gene transfer into human haematopoietic progenitor cells. Sci. Rep. 2017, 7, 40673. [Google Scholar] [CrossRef]
- Stavrou, E.F.; Simantirakis, E.; Verras, M.; Barbas, C.; Vassilopoulos, G.; Peterson, K.R.; Athanassiadou, A. Episomal vectors based on S/MAR and the β-globin Replicator, encoding a synthetic transcriptional activator, mediate efficient γ-globin activation in haematopoietic cells. Sci. Rep. 2019, 9, 19765. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Makkia, R.S.; Conley, S.M.; Cooper, M.J.; Naash, M.I. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Human. Mol. Genet. 2013, 22, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Toms, M.; Toualbi, L.; Almeida, P.V.; Harbottle, R.; Moosajee, M. Successful large gene augmentation of USH2A with non-viral episomal vectors. Mol. Ther. 2023, 31, 2755–2766. [Google Scholar] [CrossRef]
- Roig-Merino, A.; Urban, M.; Bozza, M.; Peterson, J.D.; Bullen, L.; Buchler-Schaff, M.; Stable, S.; van der Hoeven, F.; Muller-Decker, K.; McKay, T.R.; et al. An episomal DNA vector platform for the persistent genetic modification of pluripotent stem cells and their differentiated progeny. Stem Cell Rep. 2022, 17, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.; De Roia, A.; Correia, M.P.; Berger, A.; Tuch, A.; Schmidt, A.; Zornig, I.; Jager, D.; Schmidt, P.; Harbottle, R.P. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci. Adv. 2021, 7, eabf1333. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stehle, I.M.; Scinteie, M.F.; Baiker, A.; Jenke, A.C.; Lipps, H.J. Exploiting a minimal system to study the epigenetic control of DNA replication: The interplay between transcription and replication. Chromosome Res. 2003, 11, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.; Carnes, A.E.; Hodgson, C.P.; Williams, J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009, 27, 6454–6459. [Google Scholar] [CrossRef]
- Wernig, M.; Meissner, A.; Foreman, R.; Brambrink, T.; Ku, M.; Hochedlinger, K.; Bernstein, B.E.; Jaenisch, R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007, 448, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Domian, I.J. Persistent Integration of Reprogramming Factors Impairs the In Vitro Cardiogenic Potential of Induced Pluripotent Stem Cells. Circ. Cardiovasc. Genet. 2014, 7, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Herbst, F.; Ball, C.R.; Tuorto, F.; Nowrouzi, A.; Wang, W.; Zavidij, O.; Dieter, S.M.; Fessler, S.; van der Hoeven, F.; Kloz, U.; et al. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol. Ther. 2012, 20, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline Phosphatase in Stem Cells. Stem Cells Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [PubMed]
- Damjanov, I.; Andrews, P.W. Teratomas produced from human pluripotent stem cells xenografted into immunodeficient mice—A histopathology atlas. Int. J. Dev. Biol. 2016, 60, 337–419. [Google Scholar] [CrossRef] [PubMed]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Y.; Dong, X.; Liu, Z.; Sugimura, R.; Xie, G. IPSC-derived CAR-NK cells for cancer immunotherapy. Biomed. Pharmacother. 2023, 165, 115123. [Google Scholar] [CrossRef] [PubMed]
- Drozd, A.M.; Walczak, M.P.; Piaskowski, S.; Stoczynska-Fidelus, E.; Rieske, P.; Grzela, D.P. Generation of human iPSCs from cells of fibroblastic and epithelial origin by means of the oriP/EBNA-1 episomal reprogramming system. Stem Cell Res. Ther. 2015, 6, 122. [Google Scholar] [CrossRef]
- Warlich, E.; Kuehle, J.; Cantz, T.; Brugman, M.H.; Maetzig, T.; Galla, M.; Filipczyk, A.A.; Halle, S.; Klump, H.; Scholer, H.R.; et al. Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol. Ther. 2011, 19, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; Devine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M.; et al. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Samavarchi-Tehrani, P.; Golipour, A.; David, L.; Sung, H.-k.; Beyer, T.A.; Datti, A.; Woltjen, K.; Nagy, A.; Wrana, J.L. Functional Genomics Reveals a BMP-Driven Mesenchymal-to-Epithelial Transition in the Initiation of Somatic Cell Reprogramming. Cell Stem Cell 2010, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Parry, A.; Santos, F.; Okkenhaug, H.; Todd, C.D.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife 2022, 11, e71624. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Nakamura, M.; Narita, M.; Takahashi, K.; Yamanaka, S. Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts. Proc. Natl. Acad. Sci. USA 2013, 110, 12172–12179. [Google Scholar] [CrossRef]
- Toualbi, L.; Toms, M.; Almeida, P.V.; Harbottle, R.; Moosajee, M. Gene Augmentation of CHM Using Non-Viral Episomal Vectors in Models of Choroideremia. Int. J. Mol. Sci. 2023, 24, 15225. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.; Green, E.W.; Espinet, E.; De Roia, A.; Klein, C.; Vogel, V.; Offringa, R.; Williams, J.A.; Sprick, M.; Harbottle, R.P. Novel Non-integrating DNA Nano-S/MAR Vectors Restore Gene Function in Isogenic Patient-Derived Pancreatic Tumor Models. Mol. Ther. Methods Clin. Dev. 2020, 17, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, S.; Hagedorn, C.; Seruggia, D.; Magnusson, T.; Wagner, E.; Ogris, M.; Lipps, H.J. Controlled removal of a nonviral episomal vector from transfected cells. Gene 2010, 466, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ng, J.-H.; Heng, J.-C.D.; Ng, H.-H. Molecules that Promote or Enhance Reprogramming of Somatic Cells to Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 4, 301–312. [Google Scholar] [CrossRef]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chau, K.F.; Vodyanik, M.A.; Jiang, J.; Jiang, Y. Efficient Feeder-Free Episomal Reprogramming with Small Molecules. PLoS ONE 2011, 6, e17557. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Hong, H.; Takahashi, K.; Yamanaka, S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat. Protoc. 2010, 5, 418–428. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartley, A.; Burger, L.; Wincek, C.L.; Dons, L.; Li, T.; Grewenig, A.; Taşgın, T.; Urban, M.; Roig-Merino, A.; Ghazvini, M.; et al. A Simple Nonviral Method to Generate Human Induced Pluripotent Stem Cells Using SMAR DNA Vectors. Genes 2024, 15, 575. https://doi.org/10.3390/genes15050575
Hartley A, Burger L, Wincek CL, Dons L, Li T, Grewenig A, Taşgın T, Urban M, Roig-Merino A, Ghazvini M, et al. A Simple Nonviral Method to Generate Human Induced Pluripotent Stem Cells Using SMAR DNA Vectors. Genes. 2024; 15(5):575. https://doi.org/10.3390/genes15050575
Chicago/Turabian StyleHartley, Anna, Luisa Burger, Cornelia L. Wincek, Lieke Dons, Tracy Li, Annabel Grewenig, Toros Taşgın, Manuela Urban, Alicia Roig-Merino, Mehrnaz Ghazvini, and et al. 2024. "A Simple Nonviral Method to Generate Human Induced Pluripotent Stem Cells Using SMAR DNA Vectors" Genes 15, no. 5: 575. https://doi.org/10.3390/genes15050575
APA StyleHartley, A., Burger, L., Wincek, C. L., Dons, L., Li, T., Grewenig, A., Taşgın, T., Urban, M., Roig-Merino, A., Ghazvini, M., & Harbottle, R. P. (2024). A Simple Nonviral Method to Generate Human Induced Pluripotent Stem Cells Using SMAR DNA Vectors. Genes, 15(5), 575. https://doi.org/10.3390/genes15050575







