Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment
Abstract
1. Introduction
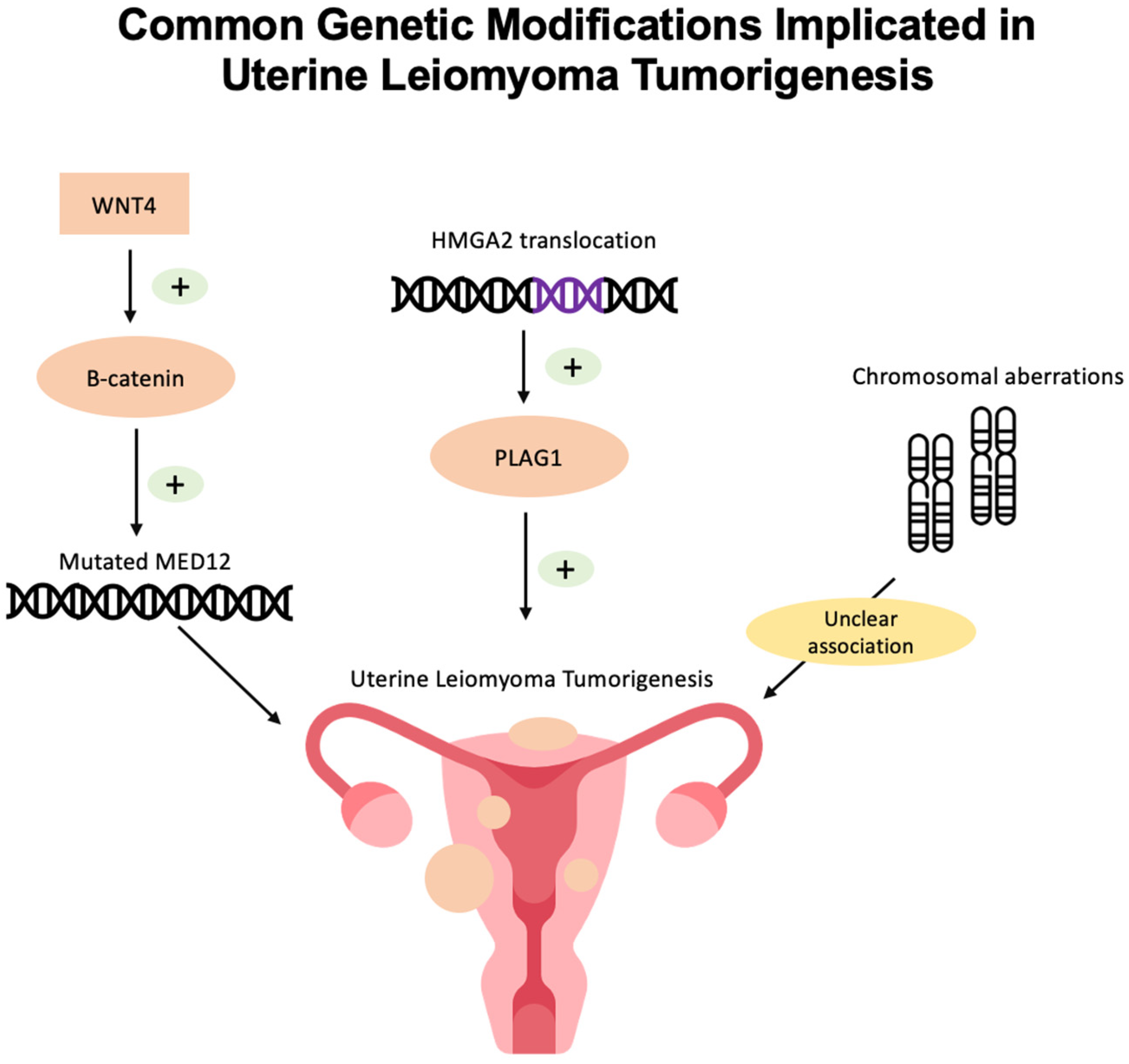
2. Genetic and Epigenetic Pathophysiology of Uterine Leiomyoma
2.1. MED12 Mutations
2.2. HMGA2 Overexpression
2.3. Chromosomal Aberrations
2.4. H19 Single-Nucleotide Polymorphism
2.5. Epigenetics of Uterine Leiomyoma
2.5.1. DNA Methylation and Demethylation Role in Uterine Leiomyoma Formation
2.5.2. Histone Modifications
2.5.3. miRNA
3. Genetic Epidemiology of Uterine Leiomyoma
3.1. Genome-Wide Association Studies in Uterine Leiomyoma
3.1.1. Genetic Drivers of Racial Disparities
3.1.2. Heritable Syndromes Related to Uterine Leiomyoma
4. Gene-Targeting Therapies in Uterine Leiomyoma
4.1. Adenovirus Vector
4.2. Suicide Gene Therapy
4.3. Ten-Eleven Translocation (TET) Enzymes
5. Future Directions
Funding
Conflicts of Interest
References
- Marsh, E.E.; Al-Hendy, A.; Kappus, D.; Galitsky, A.; Stewart, E.A.; Kerolous, M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. J. Women’s Health 2018, 27, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jose, J.; Manyonda, I. Clinical presentation of fibroids. Best. Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 615–626. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, M.S.D.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Vilos, G.A.; Allaire, C.; Laberge, P.-Y.; Leyland, N.; Vilos, A.G.; Murji, A.; Chen, I. The management of uterine leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Gonadotropin Releasing Hormone (GnRH) Analogues. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK547863/ (accessed on 27 February 2024).
- Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet. Gynecol. 2021, 137, e100–e115. [CrossRef] [PubMed]
- Commandeur, A.E.; Styer, A.K.; Teixeira, J.M. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum. Reprod. Update 2015, 21, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Medikare, V.; Kandukuri, L.R.; Ananthapur, V.; Deenadayal, M.; Nallari, P. The Genetic Bases of Uterine Fibroids; A Review. J. Reprod. Infertil. 2011, 12, 181–191. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3719293/ (accessed on 26 January 2024). [PubMed]
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, S.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine Fibroids: Pathogenesis and Interactions with Endometrium and Endomyometrial Junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Sabeh, M.E.; Saha, S.K.; Afrin, S.; Islam, M.S.; Borahay, M.A. Wnt/β-catenin Signalling Pathway in Uterine Leiomyoma: Role in Tumor Biology and Targeting Opportunities. Mol. Cell Biochem. 2021, 476, 3513–3536. [Google Scholar] [CrossRef]
- Je, E.M.; Kim, M.R.; Min, K.O.; Yoo, N.J.; Lee, S.H. Mutational analysis of MED12 exon 2 in uterine leiomyoma and other common tumors. Int. J. Cancer. 2012, 131, E1044–E1047. [Google Scholar] [CrossRef] [PubMed]
- Markowski, D.N.; Bartnitzke, S.; Löning, T.; Drieschner, N.; Helmke, B.M.; Bullerdiek, J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. Int. J. Cancer. 2012, 131, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Buyukcelebi, K.; Chen, X.; Abdula, F.; Duval, A.; Ozturk, H.; Seker-Polat, F.; Jin, Q.; Yin, P.; Feng, Y.; Wei, J.-J.; et al. Engineered MED12 mutations drive uterine fibroid-like transcriptional and metabolic programs by altering the 3D genome compartmentalization. Res Sq. 2023, Rs.3.rs-2537075. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.S.; Osinovskaya, N.S.; Yarmolinskaya, M.I. Pathogenomics of Uterine Fibroids Development. Int. J. Mol. Sci. 2019, 20, 6151. [Google Scholar] [CrossRef] [PubMed]
- Galindo, L.J.; Hernández-Beeftink, T.; Salas, A.; Jung, Y.; Reyes, R.; de Oca, F.M.; Hernández, M.; Almeida, T.A. HMGA2 and MED12 alterations frequently co-occur in uterine leiomyomas. Gynecol. Oncol. 2018, 150, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mehine, M.; Kaasinen, E.; Heinonen, H.-R.; Mäkinen, N.; Kämpjärvi, K.; Sarvilinna, N.; Aavikko, M.; Vähärautio, A.; Pasanen, A.; Bützow, R.; et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc. Natl. Acad. Sci. USA 2016, 113, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, N.; Kämpjärvi, K.; Frizzell, N.; Bützow, R.; Vahteristo, P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol. Cancer 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, E.; Qiang, W.; Zhang, Q.; Espona-Fiedler, M.; Druschitz, S.; Liu, Y.; Mittal, K.; Kong, B.; Kurita, T.; Wei, J.-J. MED12 and HMGA2 mutations: Two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod. Pathol. 2014, 27, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Leiomyoma. Cancer Genet. Cytogenet. 2005, 158, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xue, J.D.; Chao, F.; Jin, Y.F.; Fu, Q. Long non-coding RNA-H19 antagonism protects against renal fibrosis. Oncotarget 2016, 7, 51473–51481. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Liu, X.; Trottier, J.; Beaudoin, M.; Zhang, L.; Pope, C.; Peng, G.; Barbier, O.; Zhong, X.; et al. H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of epithelial cell adhesion molecule. Hepatology 2017, 66, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Guo, Z.; Xie, W.; Jin, W.; Zhu, D.; Chen, S.; Ren, T. The lncRNA H19 Mediates Pulmonary Fibrosis by Regulating the miR-196a/COL1A1 Axis. Inflammation 2018, 41, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Jiang, Y.; Wang, Z.; Zhang, N.; Al-Hendy, A.; Mamillapalli, R.; Kallen, A.N.; Kodaman, P.; Taylor, H.S.; Li, D.; et al. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene 2019, 38, 5356–5366. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mas, A.; Diamond, M.P.; Al-Hendy, A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod. Sci. 2016, 23, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Maekawa, R.; Yamagata, Y.; Tamura, I.; Lee, L.; Okada, M.; Jozaki, K.; Asada, H.; Tamura, H.; Sugino, N. Identification of uterine leiomyoma-specific marker genes based on DNA methylation and their clinical application. Sci. Rep. 2016, 6, 30652. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, P.; Xu, J.; Dotts, A.J.; Kujawa, S.A.; Coon V, J.S.; Zhao, H.; Shilatifard, A.; Dai, Y.; Bulun, S.E. Targeting DNA Methylation Depletes Uterine Leiomyoma Stem Cell–enriched Population by Stimulating Their Differentiation. Endocrinology 2020, 161, bqaa143. [Google Scholar] [CrossRef] [PubMed]
- George, J.W.; Fan, H.; Johnson, B.; Carpenter, T.J.; Foy, K.K.; Chatterjee, A.; Patterson, A.L.; Koeman, J.; Adams, M.; Madaj, Z.B.; et al. Integrated Epigenome, Exome, and Transcriptome Analyses Reveal Molecular Subtypes and Homeotic Transformation in Uterine Fibroids. Cell Rep. 2019, 29, 4069–4085.e6. [Google Scholar] [CrossRef]
- Carbajo-García, M.C.; Corachán, A.; Juárez-Barber, E.; Monleón, J.; Payá, V.; Trelis, A.; Quiñonero, A.; Pellicer, A.; Ferrero, H. Integrative analysis of the DNA methylome and transcriptome in uterine leiomyoma shows altered regulation of genes involved in metabolism, proliferation, extracellular matrix, and vesicles. J. Pathol. 2022, 257, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.N.; Grey, J.A.; Carpenter, T.J.; Madaj, Z.B.; Lau, K.H.; Givan, S.A.; Burns, G.W.; Chandler, R.L.; Wegienka, G.R.; Shen, H.; et al. Transcriptome and DNA methylome analyses reveal underlying mechanisms for the racial disparity in uterine fibroids. JCI Insight. 2022, 7, e160274. [Google Scholar] [CrossRef] [PubMed]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Carbajo-García, M.C.; de Miguel-Gómez, L.; Juárez-Barber, E.; Trelis, A.; Monleón, J.; Pellicer, A.; Flanagan, J.M.; Ferrero, H. Deciphering the Role of Histone Modifications in Uterine Leiomyoma: Acetylation of H3K27 Regulates the Expression of Genes Involved in Proliferation, Cell Signaling, Cell Transport, Angiogenesis and Extracellular Matrix Formation. Biomedicines 2022, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-García, M.C.; Juarez-Barber, E.; Segura-Benítez, M.; Faus, A.; Trelis, A.; Monleón, J.; Carmona-Antoñanzas, G.; Pellicer, A.; Flanagan, J.M.; Ferrero, H. H3K4me3 mediates uterine leiomyoma pathogenesis via neuronal processes, synapsis components, proliferation, and Wnt/β-catenin and TGF-β pathways. Reprod. Biol. Endocrinol. 2023, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Foster, R.; Karmon, A.E.; Lee, A.E.; Gatune, L.W.; Rueda, B.R.; Styer, A.K. MicroRNA 21a-5p overexpression impacts mediators of extracellular matrix formation in uterine leiomyoma. Reprod. Biol. Endocrinol. 2018, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.E.; Lin, Z.; Yin, P.; Milad, M.; Chakravarti, D.; Bulun, S.E. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil. Steril. 2008, 89, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xue, H.; Shao, W.; Wang, X.; Liao, H.; Ye, Y. Inhibiting effect of miR-29 on proliferation and migration of uterine leiomyoma via the STAT3 signaling pathway. Aging 2022, 14, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.E.; Steinberg, M.L.; Parker, J.B.; Wu, J.; Chakravarti, D.; Bulun, S.E. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil. Steril. 2016, 106, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2022, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, N.; Kuisma, H.; Pasanen, A.; Heikinheimo, O.; Sjöberg, J.; Bützow, R.; Sarvilinna, N.; Heinonen, H.-R.; Tolvanen, J.; Bramante, S.; et al. Genetic predisposition to uterine leiomyoma is determined by loci for genitourinary development and genome stability. eLife 2018, 7, e37110. [Google Scholar] [CrossRef] [PubMed]
- Rafnar, T.; Gunnarsson, B.; Stefansson, O.A.; Sulem, P.; Ingason, A.; Frigge, M.L.; Stefansdottir, L.; Sigurdsson, J.K.; Tragante, V.; Steinthorsdottir, V.; et al. Variants associating with uterine leiomyoma highlight genetic background shared by various cancers and hormone-related traits. Nat. Commun. 2018, 9, 3636. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Low, S.-K.; Akiyama, M.; Hirata, M.; Ueda, Y.; Matsuda, K.; Kimura, T.; Murakami, Y.; Kubo, M.; Kamatani, Y.; et al. GWAS of five gynecologic diseases and cross-trait analysis in Japanese. Eur. J. Hum. Genet. 2020, 28, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Tanikawa, C.; Hirasawa, A.; Chiyoda, T.; Yamagami, W.; Kataoka, F.; Susumu, N.; Terao, C.; Kamatani, Y.; Takahashi, A.; et al. Identification of a novel uterine leiomyoma GWAS locus in a Japanese population. Sci. Rep. 2020, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chen, L.; Guo, S.; Liu, Y.; Wu, H. Genetic Liability to Multiple Factors and Uterine Leiomyoma Risk: A Mendelian Randomization Study. Front. Endocrinol. 2023, 14. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2023.1133260 (accessed on 1 March 2024). [CrossRef] [PubMed]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Verzilina, I.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate Genes for Age at Menarche Are Associated with Uterine Leiomyoma. Front. Genet. 2020, 11, 512940. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.S.; Lin, R.T.; Lin, Y.C.; Wang, C.H.; Lin, S.H.; Imoto, S. Genome-wide causal mediation analysis identifies genetic loci associated with uterine fibroids mediated by age at menarche. Hum. Reprod. 2022, 37, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, C.; Han, Z.; Zhang, L.; Zhao, X.; Hao, Y.; Xiao, J.; Gallagher, C.S.; Kraft, P.; Morton, C.C.; et al. Investigating the shared genetic architecture of uterine leiomyoma and breast cancer: A genome-wide cross-trait analysis. Am. J. Hum. Genet. 2022, 109, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- McGrath, I.M.; Montgomery, G.W.; Mortlock, S. Insights from Mendelian randomization and genetic correlation analyses into the relationship between endometriosis and its comorbidities. Hum. Reprod. Update 2023, 29, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.S.; Mäkinen, N.; Harris, H.R.; Rahmioglu, N.; Uimari, O.; Cook, J.P.; Shigesi, N.; Ferreira, T.; Velez-Edwards, D.R.; Edwards, T.L.; et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019, 10, 4857. [Google Scholar] [CrossRef] [PubMed]
- Kho, P.F.; Mortlock, S.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Brinton, L.; et al. Genetic analyses of gynecological disease identify genetic relationships between uterine fibroids and endometrial cancer, and a novel endometrial cancer genetic risk region at the WNT4 1p36.12 locus. Hum. Genet. 2021, 140, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Bedaiwy, M.; Singh, S.S.; Bougie, O.; CAPTURE Registry Steering Committee. Influence of Ethnicity on Clinical Presentation and Quality of Life in Women with Uterine Fibroids: Results from a Prospective Observational Registry. J. Obstet. Gynaecol. Can. 2020, 42, 726–733.e1. [Google Scholar] [CrossRef]
- Hellwege, J.N.; Jeff, J.M.; Wise, L.A.; Gallagher, C.S.; Wellons, M.; Hartmann, K.E.; Jones, S.F.; Torstenson, E.S.; Dickinson, S.; Ruiz-Narváez, E.A.; et al. A multi-stage genome-wide association study of uterine fibroids in African Americans. Hum. Genet. 2017, 136, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.L.; Giri, A.; Hellwege, J.N.; Hartmann, K.E.; Stewart, E.A.; Jeff, J.M.; Bray, M.J.; Pendergrass, S.A.; Torstenson, E.S.; Keaton, J.M.; et al. A Trans-Ethnic Genome-Wide Association Study of Uterine Fibroids. Front. Genet. 2019, 10, 511. [Google Scholar] [CrossRef]
- Chan, I.; Wong, T.; Martinez-Mir, A.; Christiano, A.M.; McGrath, J.A. Familial multiple cutaneous and uterine leiomyomas associated with papillary renal cell cancer. Clin. Exp. Dermatol. 2005, 30, 75–78. [Google Scholar] [CrossRef]
- Tomlinson, I.P.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; Maher, E.R.; Schmidt, L.S.; Middelton, L.A.; Aittomäki, K.; Tomlinson, I.; Richard, S.; Linehan, W.M. Hereditary leiomyomatosis and renal cell cancer (HLRCC). Renal cancer risk, surveillance and treatment. Fam. Cancer 2014, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Karamurzin, Y.; Frizzell, N.; Garg, K.; Nonaka, D.; Chen, Y.-B.; A Soslow, R. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: Detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod. Pathol. 2014, 27, 1020–1027. [Google Scholar] [CrossRef][Green Version]
- Novel Mutations in FH and Expansion of the Spectrum of Phenotypes Expressed in Families with Hereditary Leiomyomatosis and Renal Cell Cancer|Journal of Medical Genetics. Available online: https://jmg.bmj.com/content/43/1/18 (accessed on 1 March 2024).
- Lehtonen, R.; Kiuru, M.; Vanharanta, S.; Sjöberg, J.; Aaltonen, L.-M.; Aittomäki, K.; Arola, J.; Butzow, R.; Eng, C.; Husgafvel-Pursiainen, K.; et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am. J. Pathol. 2004, 164, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Uterine Fibroids Gene Therapy: Targeted Adenovirus Vector (Ad-SSTR-RGD-TK/GCV) Provides Superior Inhibition of Human Leiomyoma Cells than Human Uterine Smooth Muscle Cells. Mol. Ther. 2011, 19, S95. [CrossRef]
- Abdelaziz, M.; Sherif, L.; ElKhiary, M.; Nair, S.; Shalaby, S.; Mohamed, S.; Eziba, N.; El-Lakany, M.; Curiel, D.; Ismail, N.; et al. Targeted Adenoviral Vector Demonstrates Enhanced Efficacy for In Vivo Gene Therapy of Uterine Leiomyoma. Reprod. Sci. 2016, 23, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Khatoon, N.; Curiel, D.T.; Hamada, F.M.; Arafa, H.M.; Al-Hendy, A. Toward gene therapy of uterine fibroids: Targeting modified adenovirus to human leiomyoma cells. Human. Reprod. 2008, 23, 514–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Freund, S.; Selutin, A.; Shved, N.; Selkov, S.; Kiselev, A. Serum-Resistant Ternary DNA Polyplexes for Suicide Gene Therapy of Uterine Leiomyoma. Int. J. Mol. Sci. 2023, 25, 34. [Google Scholar] [CrossRef]
- Duarte, S.; Carle, G.; Faneca, H.; de Lima, M.C.P.; Pierrefite-Carle, V. Suicide gene therapy in cancer: Where do we stand now? Cancer Lett. 2012, 324, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Zhang, D.; Salama, S.; Hamada, F.; Arafa, H.; Fouad, H.; Walker, C.; Al-Hendy, A. Towards fibroid gene therapy: Adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol. Obstet. Investig. 2009, 68, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G.; Ciebiera, M.; Ali, M.; Yang, Q.; Al-Hendy, A. Epigenetic Regulation in Uterine Fibroids—The Role of Ten-Eleven Translocation Enzymes and Their Potential Therapeutic Application. Int. J. Mol. Sci. 2022, 23, 2720. [Google Scholar] [CrossRef] [PubMed]
- An, J.; González-Avalos, E.; Chawla, A.; Jeong, M.; López-Moyado, I.F.; Li, W.; Goodell, M.A.; Chavez, L.; Ko, M.; Rao, A. Acute loss of TET function results in aggressive myeloid cancer in mice. Nat. Commun. 2015, 6, 10071. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaiyer, M.S.; Saad, E.; Kurt, I.; Borahay, M.A. Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment. Genes 2024, 15, 558. https://doi.org/10.3390/genes15050558
Ramaiyer MS, Saad E, Kurt I, Borahay MA. Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment. Genes. 2024; 15(5):558. https://doi.org/10.3390/genes15050558
Chicago/Turabian StyleRamaiyer, Malini S., Eslam Saad, Irem Kurt, and Mostafa A. Borahay. 2024. "Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment" Genes 15, no. 5: 558. https://doi.org/10.3390/genes15050558
APA StyleRamaiyer, M. S., Saad, E., Kurt, I., & Borahay, M. A. (2024). Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment. Genes, 15(5), 558. https://doi.org/10.3390/genes15050558





