Transcriptomic Analysis of Postnatal Rat Carotid Body Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. RNA Extraction, Illumine Library Preparation, and Sequencing
2.3. mRNA-Seq Bioinformatics Analysis
2.4. Functional Annotation Analyses with Identified DEGs
2.5. Protein–Protein Interactions between DEGs
2.6. QRT-PCR (Quantitative Reverse Transcription-PCR)
3. Results
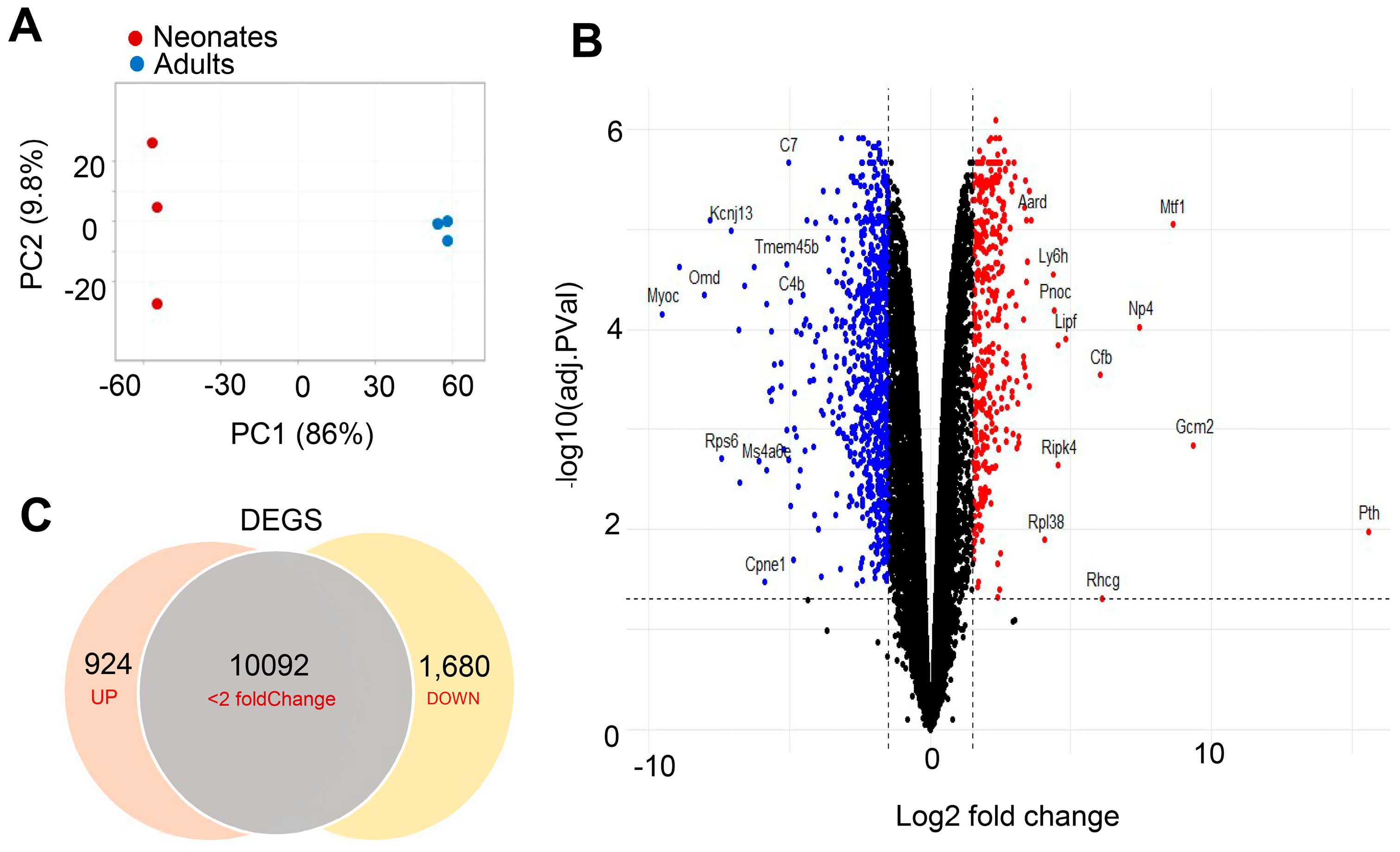
3.1. Generating RNA-Seq Data for Differential Expression Analysis from Neonatal and Adult CBs
3.2. Transcriptional Regulation Differences between Neonatal and Adult Carotid Bodies
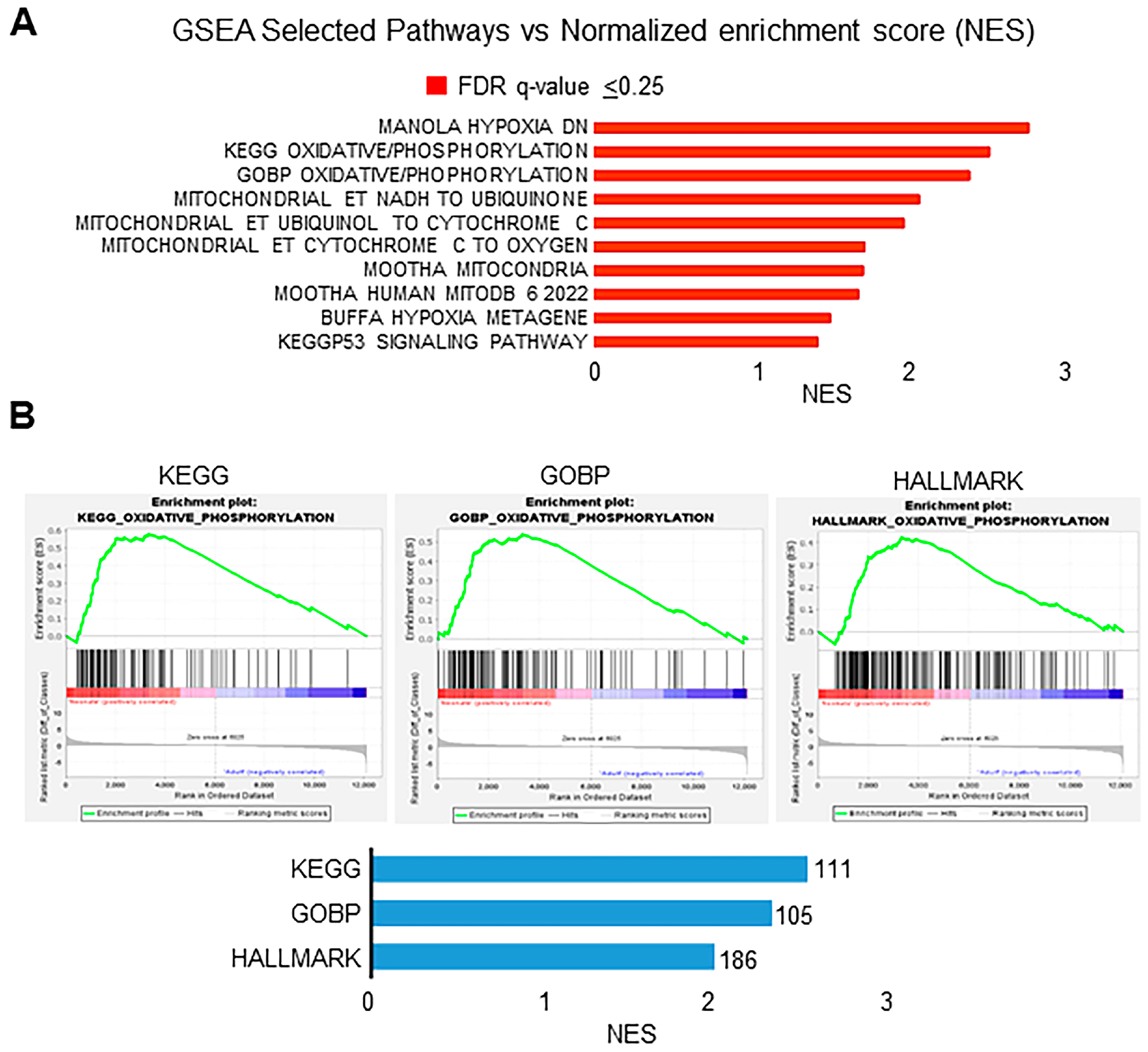
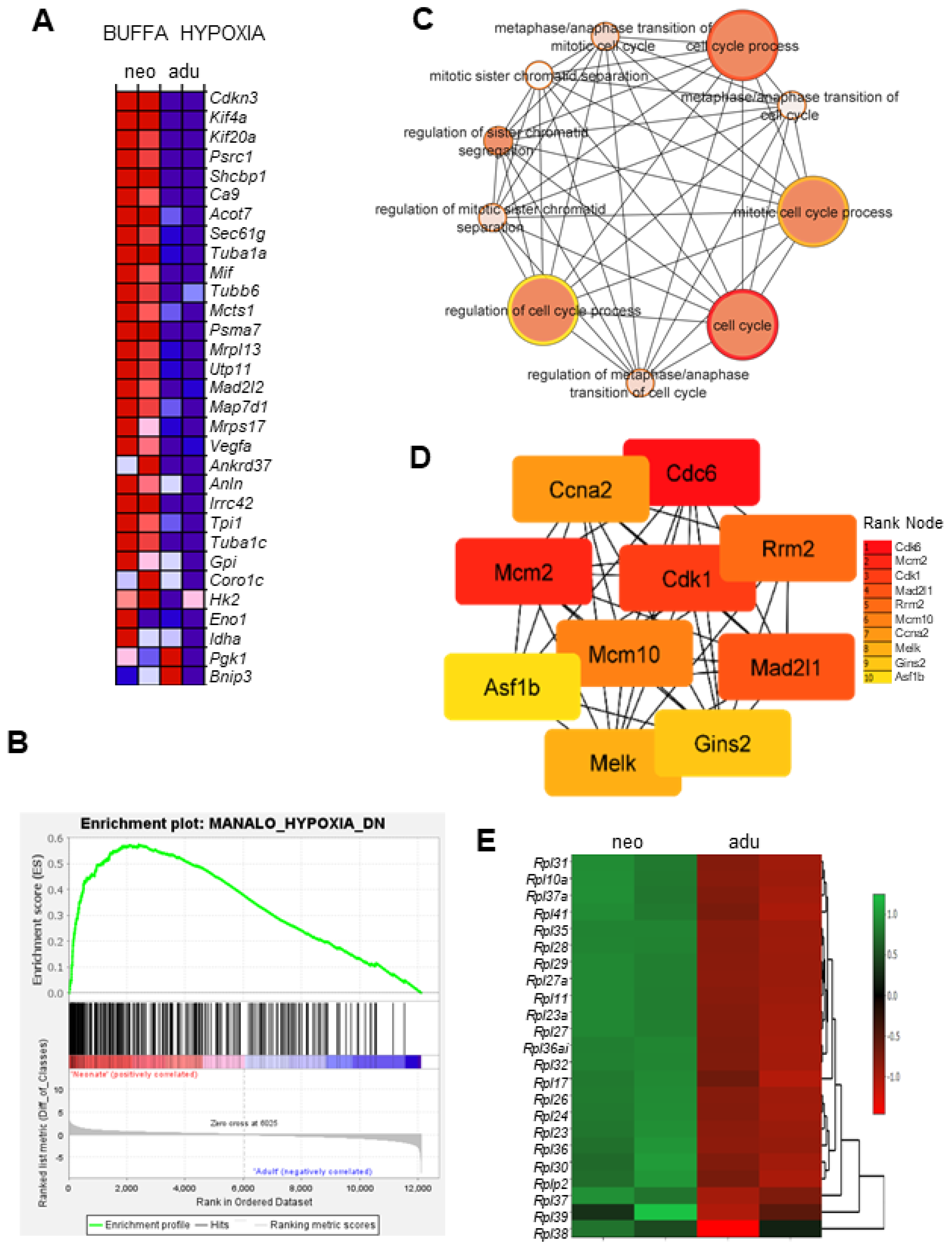
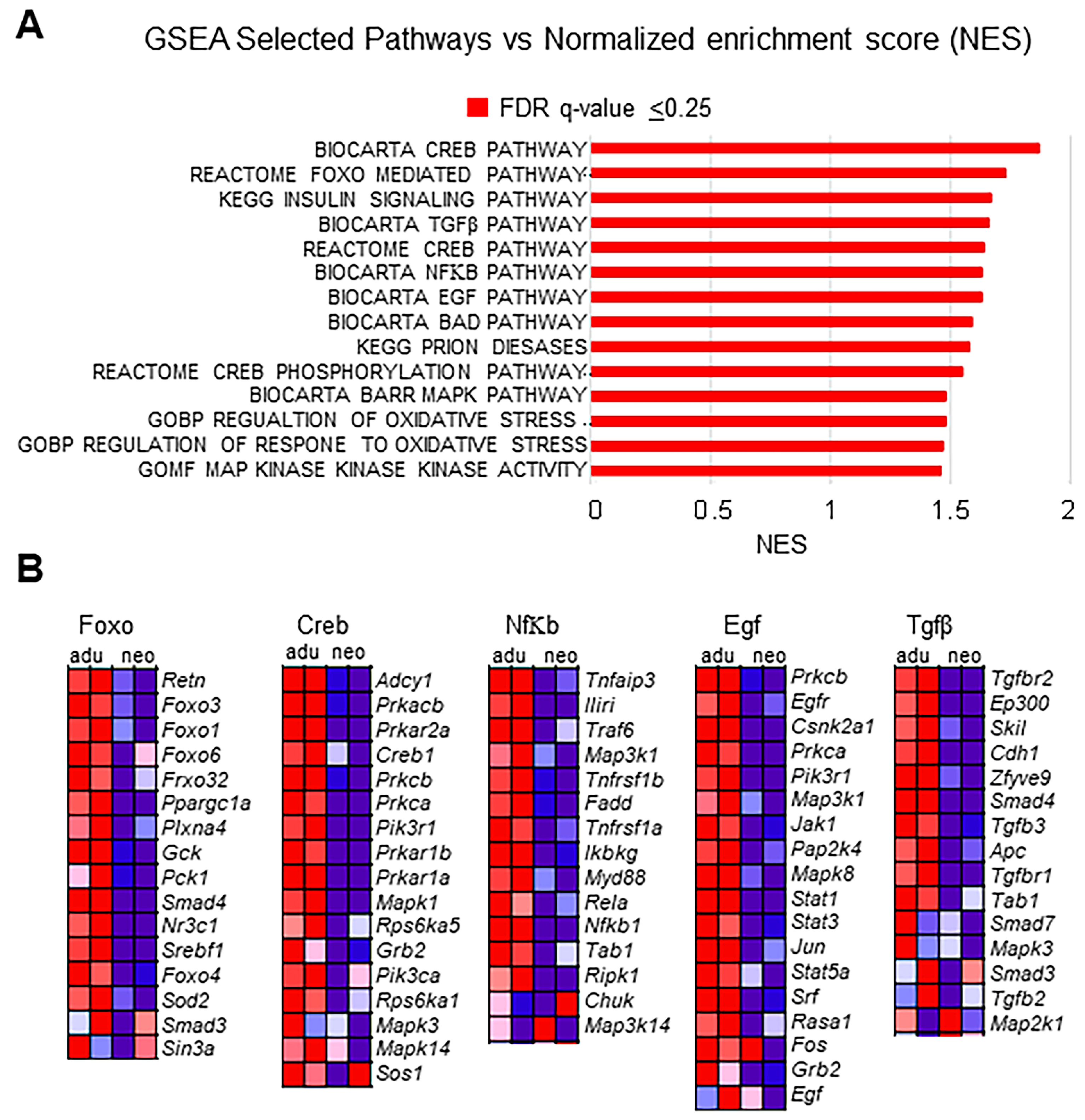
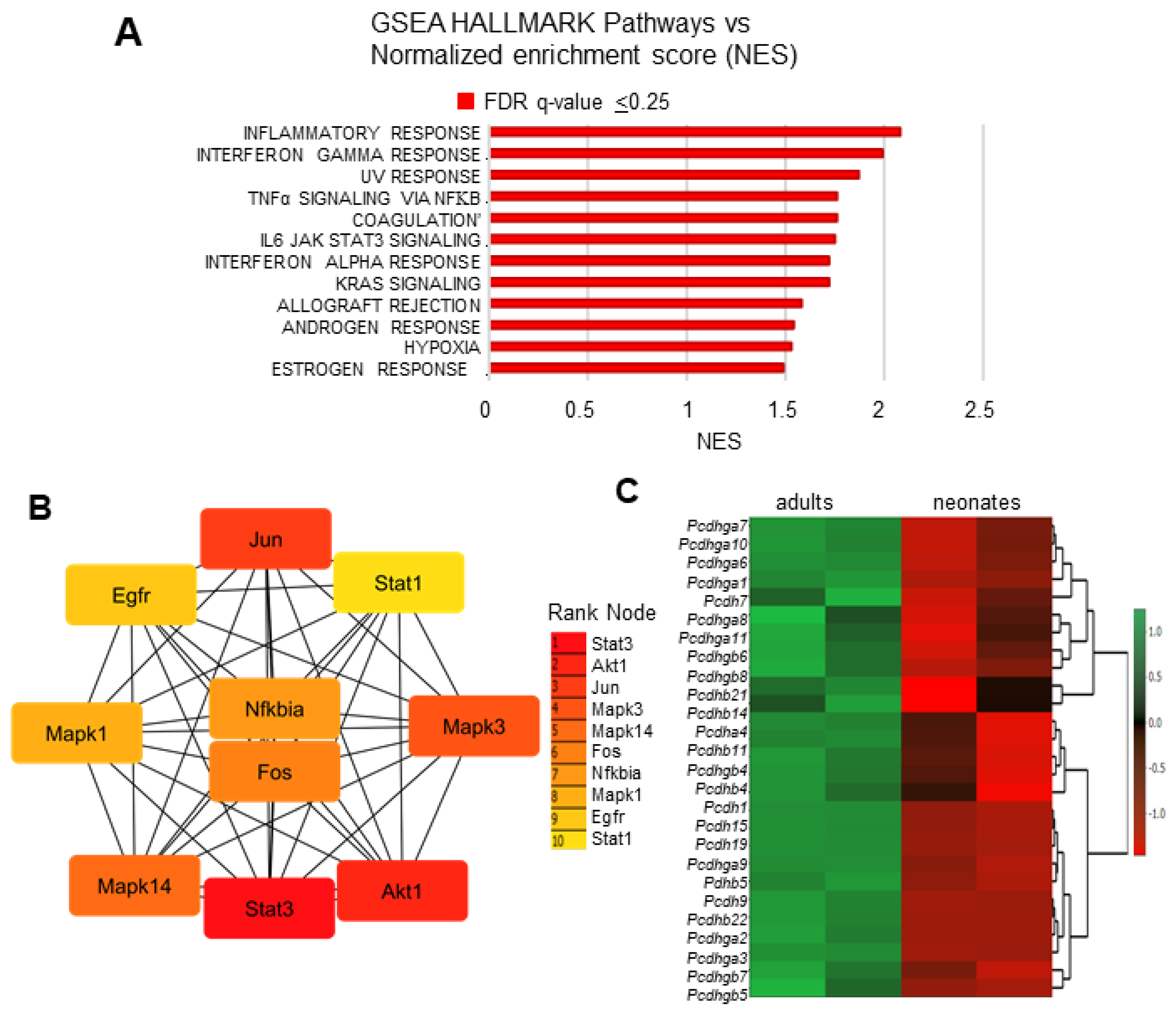
3.3. Transcriptional Signatures and DEG Responses Associated with Neonatal CBs
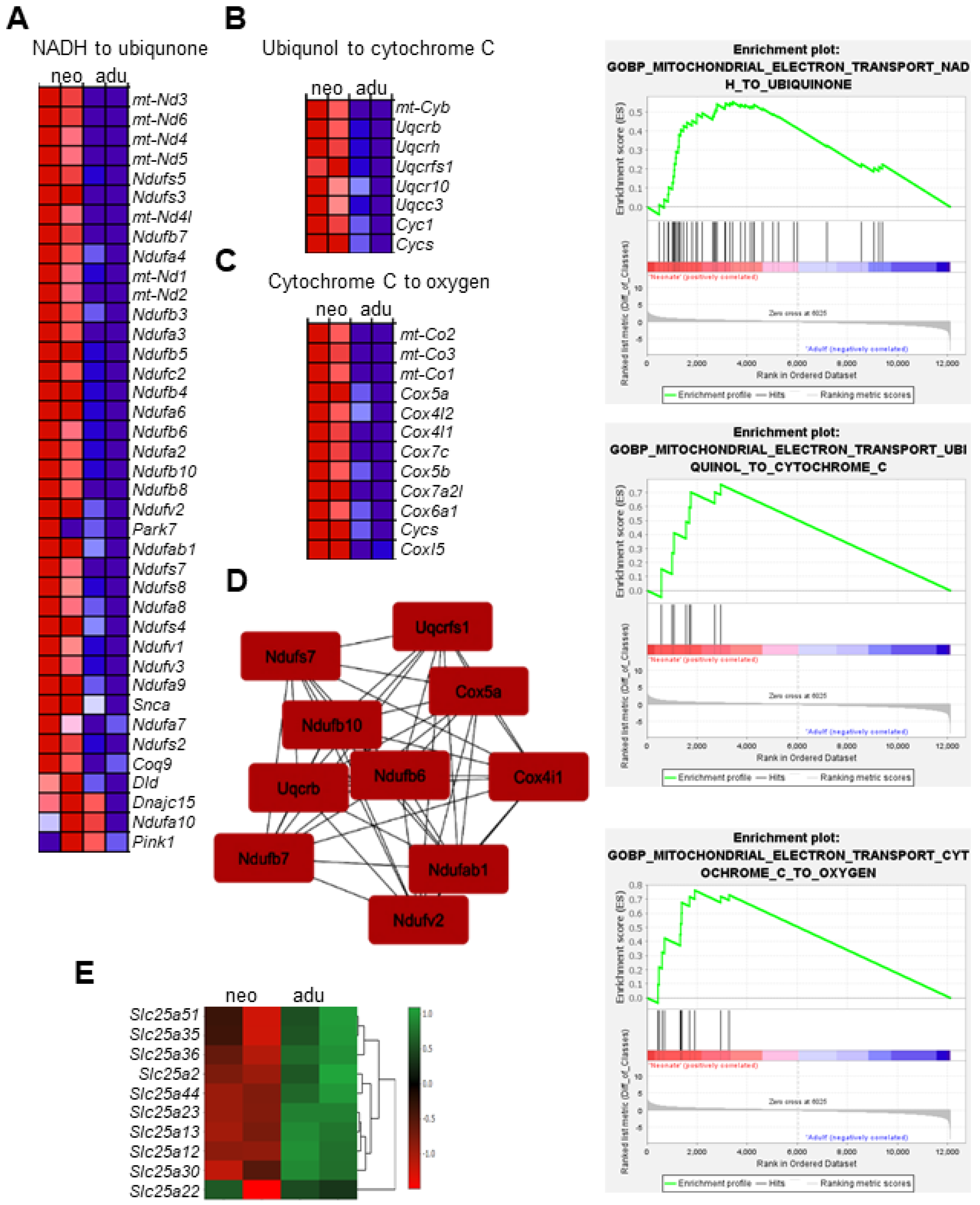
3.4. Transcriptional Signatures and DEG Responses Associated with Adult CBs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weir, E.K.; Lopez-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Prabhakar, N.R. Peripheral Chemoreceptors: Function and Plasticity of the Carotid Body. Compr. Physiol. 2012, 2, 141–219. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sáenz, P.; López-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.L.; Kim, I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir. Physiol. Neurobiol. 2005, 149, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Kholwadwala, D.; Donnelly, D.F. Maturation of carotid chemoreceptor sensitivity to hypoxia: In vitro studies in the newborn rat. J. Physiol. 1992, 453, 461–473. [Google Scholar] [CrossRef]
- Gauda, E.B.; Lawson, E.E. Developmental influences on carotid body responses to hypoxia. Respir. Physiol. 2000, 121, 199–208. [Google Scholar] [CrossRef]
- Hatton, C.J.; Carpenter, E.; Pepper, D.R.; Kumar, P.; Peers, C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J. Physiol. 1997, 501 Pt 1, 49–58. [Google Scholar] [CrossRef]
- Sterni, L.M.; Bamford, O.S.; Tomares, S.M.; Montrose, M.H.; Carroll, J.L. Developmental changes in intracellular Ca2+ response of carotid chemoreceptor cells to hypoxia. Am. J. Physiol. 1995, 268, L801–L808. [Google Scholar] [CrossRef]
- Donnelly, D.F.; Carroll, J.L. Mitochondrial function and carotid body transduction. High. Alt. Med. Biol. 2005, 6, 121–132. [Google Scholar] [CrossRef]
- Bairam, A.; Carroll, J.L. Neurotransmitters in carotid body development. Respir. Physiol. Neurobiol. 2005, 149, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Overholt, J.L.; Ficker, E.; Yang, T.; Shams, H.; Bright, G.R.; Prabhakar, N.R. HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body. J. Neurophysiol. 2000, 83, 1150–1157. [Google Scholar] [CrossRef]
- Arcangeli, A.; Rosati, B.; Cherubini, A.; Crociani, O.; Fontana, L.; Ziller, C.; Wanke, E.; Olivotto, M. HERG- and IRK-like inward rectifier currents are sequentially expressed during neuronal development of neural crest cells and their derivatives. Eur. J. Neurosci. 1997, 9, 2596–2604. [Google Scholar] [CrossRef]
- Kim, I.; Boyle, K.M.; Carroll, J.L. Postnatal development of E-4031-sensitive potassium current in rat carotid chemoreceptor cells. J. Appl. Physiol. 2005, 98, 1469–1477. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Pérez-García, M.T.; Gutiérrez, G.; Miguel-Velado, E.; López-López, J.R.; Marín, A.; Sánchez, D.; González, C. Comparative gene expression profile of mouse carotid body and adrenal medulla under physiological hypoxia. J. Physiol. 2005, 566, 491–503. [Google Scholar] [CrossRef]
- Balbir, A.; Lee, H.; Okumura, M.; Biswal, S.; Fitzgerald, R.S.; Shirahata, M. A search for genes that may confer divergent morphology and function in the carotid body between two strains of mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L704–L715. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, M.J.; Kåhlin, J.; Ebberyd, A.; Schulte, G.; Mkrtchian, S.; Eriksson, L.I. The human carotid body: Expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology 2010, 113, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chien, M.S.; Kaleem, S.; Matsunami, H. Single cell transcriptome analysis of mouse carotid body glomus cells. J. Physiol. 2016, 594, 4225–4251. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Nanduri, J.; Wang, N.; Yuan, G.; Khan, S.A.; Souvannakitti, D.; Peng, Y.J.; Kumar, G.K.; Garcia, J.A.; Prabhakar, N.R. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. USA 2009, 106, 1199–1204. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Zeller, M.; Malka, G.; Cottin, Y.; Vergely, C. Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy. Molecules 2020, 25, 2417. [Google Scholar] [CrossRef]
- Rueda, C.B.; Traba, J.; Amigo, I.; Llorente-Folch, I.; González-Sánchez, P.; Pardo, B.; Esteban, J.A.; del Arco, A.; Satrústegui, J. Mitochondrial ATP-Mg/Pi carrier SCaMC-3/Slc25a23 counteracts PARP-1-dependent fall in mitochondrial ATP caused by excitotoxic insults in neurons. J. Neurosci. 2015, 35, 3566–3581. [Google Scholar] [CrossRef]
- Gorgoglione, R.; Porcelli, V.; Santoro, A.; Daddabbo, L.; Vozza, A.; Monné, M.; Di Noia, M.A.; Palmieri, L.; Fiermonte, G.; Palmieri, F. The human uncoupling proteins 5 and 6 (UCP5/SLC25A14 and UCP6/SLC25A30) transport sulfur oxyanions, phosphate and dicarboxylates. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 724–733. [Google Scholar] [CrossRef]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef]
- Mills, E.; Jöbsis, F.F. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J. Neurophysiol. 1972, 35, 405–428. [Google Scholar] [CrossRef]
- Streller, T.; Huckstorf, C.; Pfeiffer, C.; Acker, H. Unusual cytochrome a592 with low PO2 affinity correlates as putative oxygen sensor with rat carotid body chemoreceptor discharge. FASEB J. 2002, 16, 1277–1279. [Google Scholar] [CrossRef]
- Fernández-Agüera, M.C.; Gao, L.; González-Rodríguez, P.; Pintado, C.O.; Arias-Mayenco, I.; García-Flores, P.; García-Pergañeda, A.; Pascual, A.; Ortega-Sáenz, P.; López-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22, 825–837. [Google Scholar] [CrossRef]
- Sommer, N.; Hüttemann, M.; Pak, O.; Scheibe, S.; Knoepp, F.; Sinkler, C.; Malczyk, M.; Gierhardt, M.; Esfandiary, A.; Kraut, S.; et al. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ. Res. 2017, 121, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Agani, F.; Iyer, N.; Kotch, L.; Laughner, E.; Leung, S.; Yu, A. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann. N. Y. Acad. Sci. 1999, 874, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Pickett, C.K.; Weil, J.V. Decreased carotid body hypoxic sensitivity in chronic hypoxia: Role of dopamine. Respir. Physiol. 1995, 101, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Katayama, P.L.; Leirão, I.P.; Kanashiro, A.; Menani, J.V.; Zoccal, D.B.; Colombari, D.S.A.; Colombari, E. The carotid body: A novel key player in neuroimmune interactions. Front. Immunol. 2022, 13, 1033774. [Google Scholar] [CrossRef]
- Conde, S.V.; Sacramento, J.F.; Martins, F.O. Immunity and the carotid body: Implications for metabolic diseases. Bioelectron. Med. 2020, 6, 24. [Google Scholar] [CrossRef]
- Mkrtchian, S.; Kahlin, J.; Ebberyd, A.; Gonzalez, C.; Sanchez, D.; Balbir, A.; Kostuk, E.W.; Shirahata, M.; Fagerlund, M.J.; Eriksson, L.I. The human carotid body transcriptome with focus on oxygen sensing and inflammation—A comparative analysis. J. Physiol. 2012, 590, 3807–3819. [Google Scholar] [CrossRef]
- Katayama, P.L.; Leirão, I.P.; Kanashiro, A.; Luiz, J.P.M.; Cunha, F.Q.; Navegantes, L.C.C.; Menani, J.V.; Zoccal, D.B.; Colombari, D.S.A.; Colombari, E. The carotid body detects circulating tumor necrosis factor-α to activate a sympathetic anti-inflammatory reflex. Brain Behav. Immun. 2022, 102, 370–386. [Google Scholar] [CrossRef]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Nanduri, J.; Wang, N.; Kumar, G.K.; Bindokas, V.; Paul, B.D.; Chen, X.; Fox, A.P.; Vignane, T.; Filipovic, M.R.; et al. Hypoxia sensing requires H. Sci. Adv. 2023, 9, eadf3026. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Peng, Y.-J.; Kang, W.; Hildreth, M.; Prabhakar, N.R.; Nanduri, J. Transcriptomic Analysis of Postnatal Rat Carotid Body Development. Genes 2024, 15, 302. https://doi.org/10.3390/genes15030302
Wang N, Peng Y-J, Kang W, Hildreth M, Prabhakar NR, Nanduri J. Transcriptomic Analysis of Postnatal Rat Carotid Body Development. Genes. 2024; 15(3):302. https://doi.org/10.3390/genes15030302
Chicago/Turabian StyleWang, Ning, Ying-Jie Peng, Wenjun Kang, Matthew Hildreth, Nanduri R. Prabhakar, and Jayasri Nanduri. 2024. "Transcriptomic Analysis of Postnatal Rat Carotid Body Development" Genes 15, no. 3: 302. https://doi.org/10.3390/genes15030302
APA StyleWang, N., Peng, Y.-J., Kang, W., Hildreth, M., Prabhakar, N. R., & Nanduri, J. (2024). Transcriptomic Analysis of Postnatal Rat Carotid Body Development. Genes, 15(3), 302. https://doi.org/10.3390/genes15030302





