SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency
Abstract
1. Introduction
2. Materials and Methods
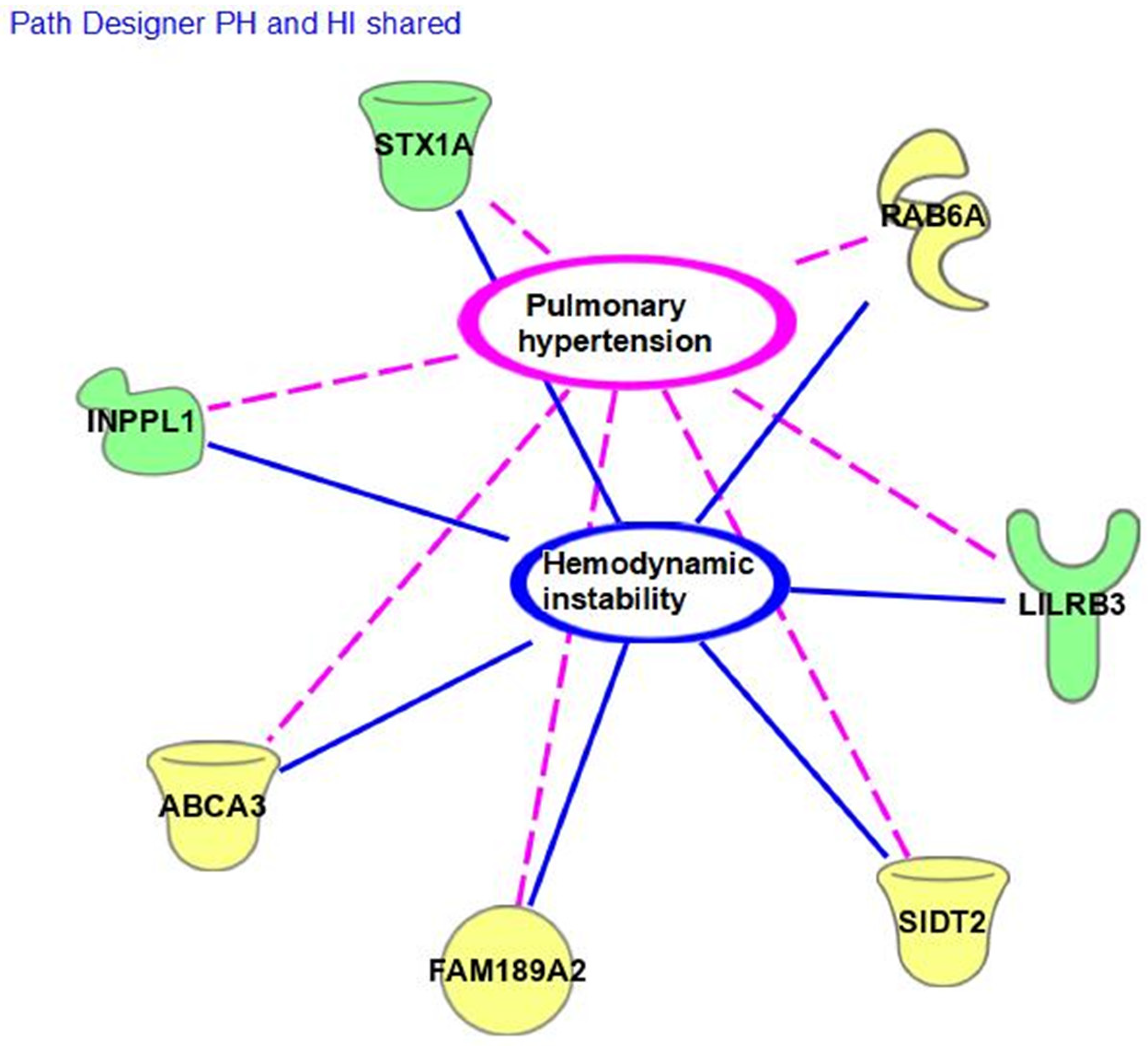
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, K.; McNally, J.D.; Choong, K.; Lawson, M.L.; Ramsay, T.; Wong, H.R. A cohort study of pediatric shock: Frequency of corticosteriod use and association with clinical outcomes. Shock 2015, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gajarski, R.J.; Stefanelli, C.B.; Graziano, J.N.; Kaciroti, N.; Charpie, J.R.; Vazquex, D. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr. Crit. Care Med. 2010, 11, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rivera, C.L.; Schwartz, S.M.; Sawyer, J.E.; Macrae, D.J.; Agus, M.S. Endocrinologic diseases in pediatric cardiac intensive care. Pediatr. Crit. Care Med. 2016, 17 (Suppl. S1), S296–S301. [Google Scholar] [CrossRef] [PubMed]
- Schulman, D.; Palmert, M.; Kemp, S. Adrenal insufficiency: Still a cause of morbidity and death in childhood. Pediatrics 2007, 119, e484–e494. [Google Scholar] [CrossRef] [PubMed]
- Schiller, O.; Dagan, O.; Birk, E.; Bitan, S.; Amir, G.; Frenkel, G.; Nahum, E. Adrenal insufficiency in children undergoing heart surgery does not correlate with more complex postoperative course. Pediatr. Cardiol. 2013, 34, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Wald, E.L.; Backer, C.L.; Dearani, J.A.; Li, Z.; Oliver, W.C.; Crow, S.S. Total and free cortisol responses and their relation to outcomes after cardiopulmonary bypass in infants. J. Thorac. Cardiov. Surg. 2017, 153, 1155–1163. [Google Scholar] [CrossRef]
- Zanas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-stress-epigenetic regulation of FKBP5: Clinical and translational implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Gibbison, B.; Upton, T.; Stoica, S.C.; Caputo, M.; Lightman, S.; Angelini, G.D. Corticosteroids in Pediatric Heart Surgery: Myth or Reality. Front. Pediatr. 2018, 20, 112. [Google Scholar] [CrossRef]
- Fudulu, D.P.; Angelini, G.D.; Papadopoulou, F.F.; Evans, J.; Walker-Smith, T.; Kema, I.; van Faassen, M.; Stoica, S.; Caputo, M.; Lightman, S.; et al. The Peacock study: Feasibility of the dynamic characterisation of the paediatric hypothalamic-pituitary-adrenal function during and after cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 245. [Google Scholar] [CrossRef]
- Scott, S.M.; Watterberg, K.L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 1995, 37, 112–116. [Google Scholar] [CrossRef]
- National Library of Medicine (NLM). Reference SNP (rs) Report rs1798718160. 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1798718160#frequency_tab (accessed on 1 December 2023).
- Virdi, M. Characterizing the Role of Syntaxin 1A in the Heart. Master’s Thesis, York University Graduate Program of Biology, Toronto, ON, Canada, 2019. [Google Scholar]
- Cao, F.; Hata, R.; Zhu, P.; Niinobe, M.; Sakanaka, M. Up-regulation of syntaxin1 in ischemic cortex after permanent focal ischemia in rats. Brain Res. 2009, 1272, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.; Zhang, Q.; Huang, C.; Shi, X. Syntaxin 1A mediates isoflurane but not hypoxia preconditioning-induced alleviation of hypoxia-reoxygenation injury in rat cardiomyocytes. Am. J. Transl. Res. 2015, 7, 1883–1895. [Google Scholar] [PubMed]
- Li, W.; Xia, Z.; Lei, S.; Zhan, L.; Zhao, B.; Liu, M. MiR-34a-5p mediates sevoflurane preconditioning-induced inhibition of hypoxia/reoxygenation injury through STX1A in cardiomyocytes. Biomed. Pharmacother. 2018, 102, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Xiao, Q.R.; Yang, Y.; Xu, J.L.; Zhang, F.; Liu, C.M.; Zhang, Z.M.; Lu, Y.Q.; Huang, N.P. MicroRNA-34a modulates the notch signaling pathway in mice with congenital heart disease and its role in heart development. J. Mol. Cell. Cardiol 2017, 114, 300–308. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Brueckner, M.; Chung, W.K.; Garg, V.; Lacro, R.V.; McGuire, A.L.; Mital, S.; Priest, J.R.; Pu, W.T.; Roberts, A.; et al. Genetic basis for congenital heart disease. Circulation 2018, 138, e653–e711. [Google Scholar] [CrossRef]
- Pober, B.R. Williams-Beuren syndrome. NEJM 2010, 362, 239–252. [Google Scholar] [CrossRef]
- INPPL1. GeneCards the Human Gene Database. INPPL1 Gene-Inositol Polyphosphate Phosphatase Like 1. 2022. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=INPPL1&keywords=INPPL1 (accessed on 26 January 2023).
- FAM189A/ENTREP1. GeneCards the Human Gene Database. ENTREP1 Gene-Endosomal Transmembrane Epsin Interactor 1. 2022. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=ENTREP1 (accessed on 10 January 2023).
- Charmet, R.; Duffy, S.; Keshavarzi, S.; Gyorgy, B.; Marre, M.; Rossing, P.; McKnight, A.J.; Maxwell, A.P.; Ahluwalia, T.V.S.; Paterson, A.D.; et al. Novel risk genes identified in a genome-wide association study for coronary artery disease in patients with type 1 diabetes. Cardiovasc. Diabetol. 2018, 17, 61. [Google Scholar] [CrossRef]
- National Library of Medicine (NLM). Reference SNP (rs) Report rs78542404. 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs78542404#frequency_tab (accessed on 1 December 2023).
- Yang, L.; Liu, G.; Li, X.; Xia, Z.; Wang, Y.; Lin, W.; Zhang, W.; Zhang, W.; Li, X. Small GTPase RAB6 deficiency promotes alveolar progenitor cell renewal and attenuates PM2.5-induced lung injury and fibrosis. Cell Death Dis. 2020, 11, 827. [Google Scholar] [CrossRef]
- León-Mimila, P.; Villamil-Ramírez, H.; Macías-Kauffer, L.R.; Jacobo-Albavera, L.; López-Contreras, B.E.; Posadas-Sánchez, R.; Posadas-Romero, C.; Romero-Hidalgo, S.; Morán-Ramos, S.; Domínguez-Pérez, M.; et al. Genome-wide association study identifies a functional SIDT2 variant associated with HDL-C (High-density lipoprotein cholesterol) levels and premature coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2494–2508. [Google Scholar] [CrossRef]
- Onnée, M.; Fanen, P.; Callebaut, I.; de Becdelièvre, A. Structure-based understanding of ABCA3 Variants. Int. J. Mol. Sci. 2021, 22, 10282. [Google Scholar] [CrossRef]
- Truong, A.D.; Hong, Y.; Lee, J.; Lee, K.; Tran, H.T.T.; Dang, H.V.; Nguyen, V.K.; Lillehoj, H.S.; Hong, Y.H. Chicken novel leukocyte immunoglobulin-like receptor subfamilies B1 and B3 are transcriptional regulators of major histocompatibility complex class I genes and signaling pathways. Asian-Aust. J. Anim. Sci. 2019, 32, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, S.; Kamoshita, N.; Nakayama, J.; Teramoto, R.; Pishesha, N.; Ohba, K.; Sato, N.; Kozawa, K.; Abe, H.; Semba, K.; et al. Epithelial cells remove precancerous cells by cell competition via MHC class I–LILRB3 interaction. Nat. Immunol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
| n (%) or Median (IQR) | Cohort (n = 16) | CIRCI (n = 8) | Non-CIRCI (n = 8) | p-Value |
|---|---|---|---|---|
| Male gender (%) | 13 (81.25) | 7 (87.5) | 6 (75) | 0.500 |
| Race | ||||
| White (%) Black (%) Multi-racial (%) | 14 (87.5) 1 (6.25) 1 (6.25) | 7 (87.5) 1 (12.5) 0 | 1 (12.5) 0 1 (12. 5) | 0.767 |
| Hispanic ethnicity (%) | 3 (18.75) | 2 (25) | 1 (12.5) | 0.500 |
| Gestational age (weeks) | 39 (1) | 39 (0.5) | 39 (1) | 0.505 |
| Single ventricle cardiac diagnosis (%) | 13 (81.25) | 7 (87.5) | 6 (75) | 0.500 |
| Cardiac surgery type | ||||
| Central/BTT Shunt (%) COA/Aortic Arch (%) Hybrid (%) Norwood (%) TAPVR/Shunt (%) | 8 (50) 2 (12.5) 2 (12.5) 3 (18.75) 1 (6.25) | 3 (37.5) 0 2 (25) 2 (25) 1 (12.5) | 5 (62.5) 2 (25) 0 1 (12.5) 0 | 0.825 |
| Age at surgery (days) | 10 (7.25) | 10 (6.5) | 9.5 (5) | 0.328 |
| Weight at surgery (kg) | 3 (0.66) | 3.25 (0.65) | 3.42 (0.75) | 0.798 |
| Intubated pre-operatively (%) | 4 (25) | 3 (37.5) | 1(4.25) | 0.285 |
| STAT category | ||||
| 1 (%) 2 (%) 3 (%) 4 (%) 5 (%) | 1 (6.25) - - 8 (50) 7 (43.75) | - - - 4 (50) 4 (50) | 1 (12.5) - - 4 (50) 3 (37.5) | 0.48 |
| Cortisol level | 5.45 (2.95) | 2.15 (3.3) | 5.95 (9.98) | <0.0001 |
| Time of day cortisol drawn (00:00) | 12:54 (8:33) | 15:32 (6:11) | 10:32 (10:11) | 0.234 |
| Lowest cortisol level drawn (days) | 2 (2.55) | 2.71 (2.44) | 1.11 (2.4) | 0.505 |
| Hydrocortisone started after level (%) | 15 (93.75) | 8 (100) | 7 (87.5) | 0.500 |
| Length of hydrocortisone (days) | 10 (11.25) | 10 (7.5) | 6 (11) | 0.491 |
| ECMO required post-operatively (%) | 4 (25) | 2 (25) | 2 (25) | 0.715 |
| Total mechanical ventilation (days) | 11 (12.25) | 13 (11.5) | 9.5 (7) | 0.574 |
| Reintubation after initial extubation (%) | 9 (56.25) | 6 (75) | 3 (37.5) | 0.442 |
| CBP time (minutes) | 123 (29.5) | 118.5 (23) | 127 (27) | 0.394 |
| Open-chest post-operatively | 9 (56.25) | 5 (62.5) | 4 (50) | 0.500 |
| Post-operative chest closure (days) | 2.5 (8.5) | 6 (10.25) | 1 (4.25) | 0.442 |
| Max VIS first post-operative day | 10.5 (12.75) | 13 (10.5) | 8 (14.75) | 0.721 |
| Max Sv02 first post-operative day | 48.15 (29.85) | 44.3 (18.35) | 60.75 (31.2) | 0.328 |
| Min right rS02 first post-operative day | 36.5 (16) | 36.5 (15.25) | 35 (24.5) | 0.382 |
| Min left rS02 first post-operative day | 51.5 (21.25) | 48 (16.25) | 53 (17) | 1.00 |
| Max lactate first post-operative day | 3.65 (3.1) | 2.8 (1.63) | 4.6 (1.63) | 0.328 |
| Highest BUN post-operatively | 9 (0.25) | 9 (0) | 9 (1) | 0.574 |
| Highest creatinine post-operatively | 0.40 (0.24) | 0.35 (0.13) | 0.43 (0.26) | 0.328 |
| Length of CICU stay (days) | 26.5 (16) | 33 (22.25) | 19.5 (12.75) | 0.083 |
| Length of neonatal stay (days) | 60 (97.25) | 59.5 (91.75) | 54.5 (90.25) | 0.645 |
| Transplant-free survival discharge (%) | 13 (81.25) | 6 (75) | 7 (87.5) | 0.500 |
| Status | Alteration | Impact/Function | ||
|---|---|---|---|---|
| CIRCI | Non-CIRCI | |||
| STX1A | `x x x x x x x x | - - - - - - - - | c.541-8A>C | Splicing/Loss |
| RAB6A | `- x X X X x X X | - - - - - - - - | c.496-9T>C | Unknown |
| ABCA3 | `x x x x - x x - | - - - - - - - - | c.2695A>C (p.T899P) | Missense/Normal |
| SIDT2 | `- x x x x x - x | - - - - - - - - | c. 1015 + 17T>G | Unknown |
| LILRB3 | `x - x x - x x x | - - - - - - - - | c.940G>Tp.D314Y | Missense/Normal |
| INPPL1 | `x x x x - -x x | - - - - - x - - | c.2329T>Ap.Y777N | Splicing/Loss |
| FAM189A2 | `- x x x x x x x | x - - - - - - - | c.742T>Ap.S248T | Missense/Normal |
| `x - x x x x x - | - - x - x - - - | c.704-3C>A | Splicing/Loss | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehl, N.; Kibiryeva, N.; Marshall, J.; Tsai, S.L.; Farias, J.S.; Silva-Gburek, J.; Erickson, L.A. SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency. Genes 2024, 15, 128. https://doi.org/10.3390/genes15010128
Diehl N, Kibiryeva N, Marshall J, Tsai SL, Farias JS, Silva-Gburek J, Erickson LA. SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency. Genes. 2024; 15(1):128. https://doi.org/10.3390/genes15010128
Chicago/Turabian StyleDiehl, Nicholas, Natalia Kibiryeva, Jennifer Marshall, Sarah L. Tsai, Juan S. Farias, Jaime Silva-Gburek, and Lori A. Erickson. 2024. "SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency" Genes 15, no. 1: 128. https://doi.org/10.3390/genes15010128
APA StyleDiehl, N., Kibiryeva, N., Marshall, J., Tsai, S. L., Farias, J. S., Silva-Gburek, J., & Erickson, L. A. (2024). SNARE-ing the Reason for Post-Cardiac Surgery Critical Illness-Related Corticosteroid Insufficiency. Genes, 15(1), 128. https://doi.org/10.3390/genes15010128





