A Rare Pathological Phenotype of Endometrioid Serous and Clear-Cell Ovarian Cancer with PIK3CA Mutations in Relation to The Excellent Response of Alpelisib
Abstract
:1. Introduction
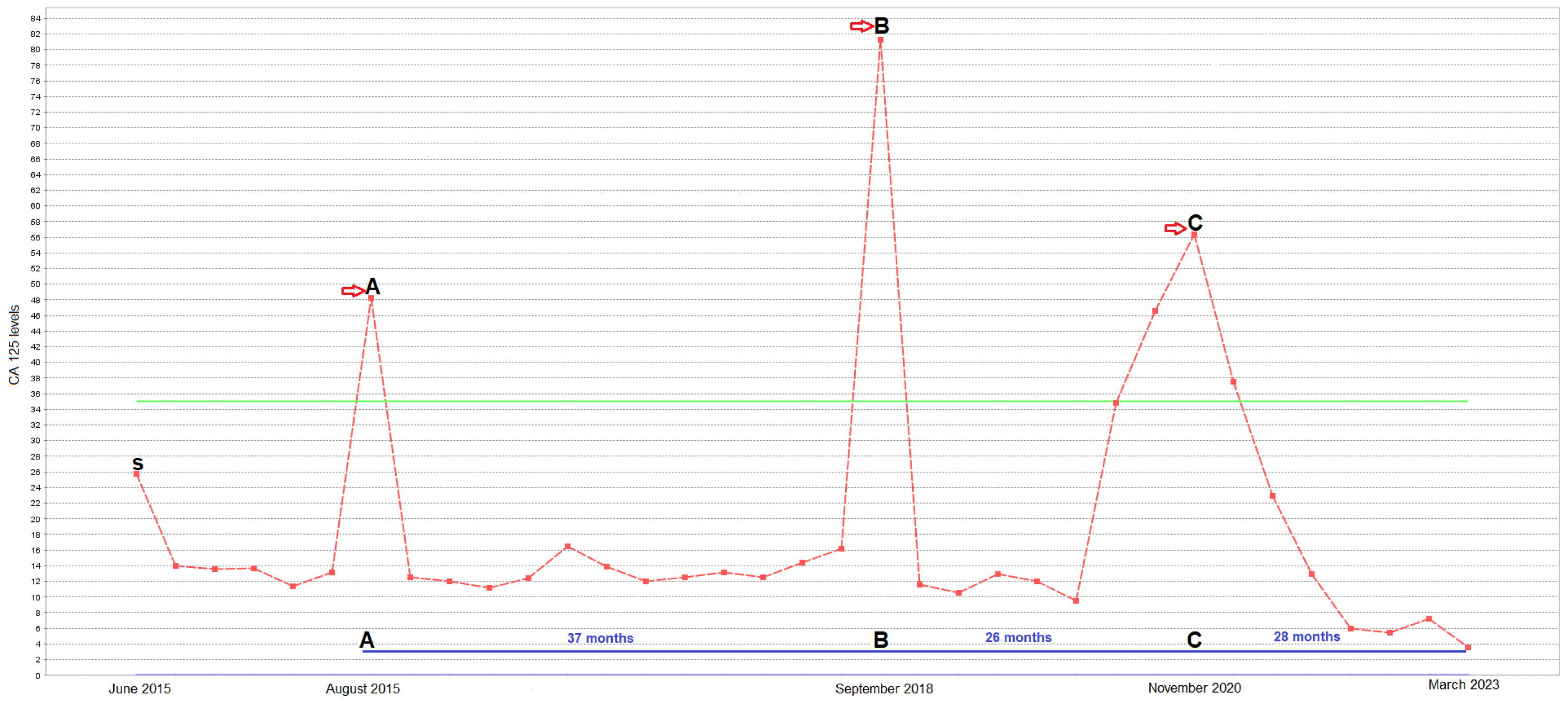
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goff, B.A.; Mandel, L.; Muntz, H.G.; Melancon, C.H. Ovarian carcinoma diagnosis. Cancer 2000, 89, 2068–2075. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Turkyılmaz, M.; Hacıkamiloglu, E.; Baran Deniz, E.; Boztas, G.; Dundar, S.; Kavak Ergun, A.; Sevinc, A.; Tutuncu, S.; Atik, E. Cancer Statistics in Turkey 2015; T.C Saglik Bakanligi, Halk Saglıgı Genel Mudurlugu: Ankara, Turkey, 2018.
- Bristow, R.E.; Puri, I.; Chi, D.S. Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis. Gynecol. Oncol. 2009, 112, 265–274. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Xu, H.; Wang, K.; Fu, Z.; Jiang, Y.; Yao, Z. RASAL2 down-regulation in ovarian cancer promotes epithelial-mesenchymal transition and metastasis. Oncotarget 2014, 5, 6734–6745. [Google Scholar] [CrossRef]
- Crumbaker, M.; Khoja, L.; Joshua, A.M. AR Signaling and the PI3K Pathway in Prostate Cancer. Cancers 2017, 9, 34. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Y.; Ma, W.L.; Lu, Y.S. Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives. Ther. Clin. Risk Manag. 2021, 17, 193–207. [Google Scholar] [CrossRef]
- Fritsch, C.; Huang, A.; Chatenay-Rivauday, C.; Schnell, C.; Reddy, A.; Liu, M.; Kauffmann, A.; Guthy, D.; Erdmann, D.; De Pover, A.; et al. Characterization of the Novel and Specific PI3Kα Inhibitor NVP-BYL719 and Development of the Patient Stratification Strategy for Clinical TrialsNVP-BYL719 PI3Kα Inhibitor and Predictive Response Modeling. Mol. Cancer Ther. 2014, 13, 1117–1129. [Google Scholar] [CrossRef]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, Cd005343. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Prenzler, A.; Eils, R.; Graf von der Schulenburg, J.M. Genome sequencing: A systematic review of health economic evidence. Health Econ. Rev. 2013, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Catasus, L.; Gallardo, A.; Cuatrecasas, M.; Prat, J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod. Pathol. 2008, 21, 131–139. [Google Scholar] [CrossRef]
- Rahman, M.; Nakayama, K.; Rahman, M.T.; Nakayama, N.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, S.; Otsuki, Y.; Shih Ie, M.; et al. Clinicopathologic and biological analysis of PIK3CA mutation in ovarian clear cell carcinoma. Hum. Pathol. 2012, 43, 2197–2206. [Google Scholar] [CrossRef]
- Passarelli, A.; Ventriglia, J.; Pisano, C.; Cecere, S.C.; Napoli, M.D.; Rossetti, S.; Tambaro, R.; Tarotto, L.; Fiore, F.; Farolfi, A.; et al. The way to precision medicine in gynecologic cancers: The first case report of an exceptional response to alpelisib in a PIK3CA-mutated endometrial cancer. Front. Oncol. 2022, 12, 1088962. [Google Scholar] [CrossRef]
- Dirican, E.; Akkiprik, M.; Özer, A. Mutation distributions and clinical correlations of PIK3CA gene mutations in breast cancer. Tumor Biol. 2016, 37, 7033–7045. [Google Scholar] [CrossRef]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127, 923–928. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef]
- Stein, R.C. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr. Relat. Cancer 2001, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Zhang, J. Molecularly targeting the PI3K-Akt-mTOR pathway can sensitize cancer cells to radiotherapy and chemotherapy. Cell. Mol. Biol. Lett. 2014, 19, 233–242. [Google Scholar] [CrossRef]
- Mutão, T.S.; Lopes, M.D.S.M.; de Menezes Ayoub, E.G.; Viecili, F. Alpelisib Monotherapy in PIK3CA-Mutated Efficacy on Triple-Negative Metastatic Breast Cancer in Subsequent Lines: A Case Report. J. Pharm. Pharmacol. 2023, 11, 26–30. [Google Scholar]
- Ye, Y.; Huang, Z.; Zhang, M.; Li, J.; Zhang, Y.; Lou, C. Synergistic therapeutic potential of alpelisib in cancers (excluding breast cancer): Preclinical and clinical evidences. Biomed. Pharmacother. 2023, 159, 114183. [Google Scholar] [CrossRef]
- Bogani, G.; Chiappa, V.; Bini, M.; Ronzulli, D.; Indini, A.; Conca, E.; Raspagliesi, F. BYL719 (alpelisib) for the treatment of PIK3CA-mutated, recurrent/advanced cervical cancer. Tumori J. 2023, 109, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Barry, W.T.; Birrer, M.; Westin, S.N.; Cadoo, K.A.; Shapiro, G.I.; Mayer, E.L.; O’Cearbhaill, R.E.; Coleman, R.L.; Kochupurakkal, B.; et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: A dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019, 20, 570–580. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Gonzalez-Martin, A.; Cruz, F.M.; Friedlander, M.; Glasspool, R.; Lorusso, D.; Marth, C.; Monk, B.J.; Kim, J.W.; Hinson, P.; et al. EPIK-O/ENGOT-OV61: Alpelisib plus olaparib vs cytotoxic chemotherapy in high-grade serous ovarian cancer (phase III study). Future Oncol. 2022, 18, 3481–3492. [Google Scholar] [CrossRef]
- Batalini, F.; Moulder, S.L.; Winer, E.P.; Rugo, H.S.; Lin, N.U.; Wulf, G.M. Response of Brain Metastases From PIK3CA-Mutant Breast Cancer to Alpelisib. JCO Precis Oncol. 2020, 4, PO.19.00403. [Google Scholar] [CrossRef]
- Sheth, H.; Kumar, P.; Shreenivas, A.; Sambath, J.; Pragya, R.; Madre, C.; Athikari, N.; Khandare, H.; Peshattiwar, V.; Datar, R.; et al. Excellent Response With Alpelisib and Bicalutamide for Advanced Salivary Duct Carcinoma With PIK3CA Mutation and High Androgen Receptor Expression—A Case Report. JCO Precis Oncol. 2021, 5, 744–750. [Google Scholar] [CrossRef]


| Research (NCT Number) | Disease | Targetable Mutations | Treatment | Response |
|---|---|---|---|---|
| Multicenter, open-label, phase Ib trial [28], NCT01623349 | recurrent ovarian, fallopian tube, primary peritoneal, or breast cancer | BRCA | The starting oral dose level: 1 × 250 mg/day alpelisib + 2 × 100 mg/day olaparib. Level 1: 1 × 250 mg/day alpelisib + 2 × 200 mg/day olaparib. Level 2: 1 × 300 mg/day alpelisib + 2 × 200 mg/day olaparib. Level 3: 1 × 200 mg/day alpelisib + 2 × 200mg/day olaparib. | The response rate of olaparib and alpelisib was 30% in all patients with germline BRCA mutations and 33% in those who also had platinum-resistant disease. |
| Randomized, open-label, multicenter phase III trial [29], NCT04729387 | platinum-resistant or -refractory high-grade serous ovarian cancer | no germline BRCA mutation | Oral 1 × 200 mg/day alpelisib and 2 × 200 mg/day olaparib. | In patients with platinum-resistant or –refractory, the combination of alpelisib/olaparib may offer improved efficacy compared to that of single-agent cytotoxic chemotherapy. |
| Phase II, NCT05238831 | Locally advanced ovarian cancer | Oral 2 × 200 mg/day alpelisib. | Study results not yet published. | |
| Our present case-study | Mixed-type ovarian cancer | PIK3CA (E542K) | Oral 2 × 200 mg/day alpelisib. | CR (complete response) over 2 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayram, E.; Khatib, G.; Guney, B.; Kilicbagir, E.; Gulec, H.R.; Boga, I.; Paydas, S. A Rare Pathological Phenotype of Endometrioid Serous and Clear-Cell Ovarian Cancer with PIK3CA Mutations in Relation to The Excellent Response of Alpelisib. Genes 2023, 14, 1632. https://doi.org/10.3390/genes14081632
Bayram E, Khatib G, Guney B, Kilicbagir E, Gulec HR, Boga I, Paydas S. A Rare Pathological Phenotype of Endometrioid Serous and Clear-Cell Ovarian Cancer with PIK3CA Mutations in Relation to The Excellent Response of Alpelisib. Genes. 2023; 14(8):1632. https://doi.org/10.3390/genes14081632
Chicago/Turabian StyleBayram, Ertugrul, Ghanim Khatib, Burak Guney, Emine Kilicbagir, Huru Rabia Gulec, Ibrahim Boga, and Semra Paydas. 2023. "A Rare Pathological Phenotype of Endometrioid Serous and Clear-Cell Ovarian Cancer with PIK3CA Mutations in Relation to The Excellent Response of Alpelisib" Genes 14, no. 8: 1632. https://doi.org/10.3390/genes14081632
APA StyleBayram, E., Khatib, G., Guney, B., Kilicbagir, E., Gulec, H. R., Boga, I., & Paydas, S. (2023). A Rare Pathological Phenotype of Endometrioid Serous and Clear-Cell Ovarian Cancer with PIK3CA Mutations in Relation to The Excellent Response of Alpelisib. Genes, 14(8), 1632. https://doi.org/10.3390/genes14081632







