Genetic and Epigenetic Mechanisms of Psoriasis
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Genetics
3.1.1. Antigen Presentation
3.1.2. Th1 Signaling Pathway
3.1.3. Th17 Signaling Pathway
3.1.4. Innate Immunity
3.1.5. Skin Barrier Function
3.1.6. Genetics of Generalized Pustular Psoriasis
3.1.7. Genetics in Psoriatic Arthritis
3.1.8. Genetics and Psoriasis Treatment
3.2. Epigenetics
3.2.1. Non Coding RNA
3.2.2. DNA Methylation
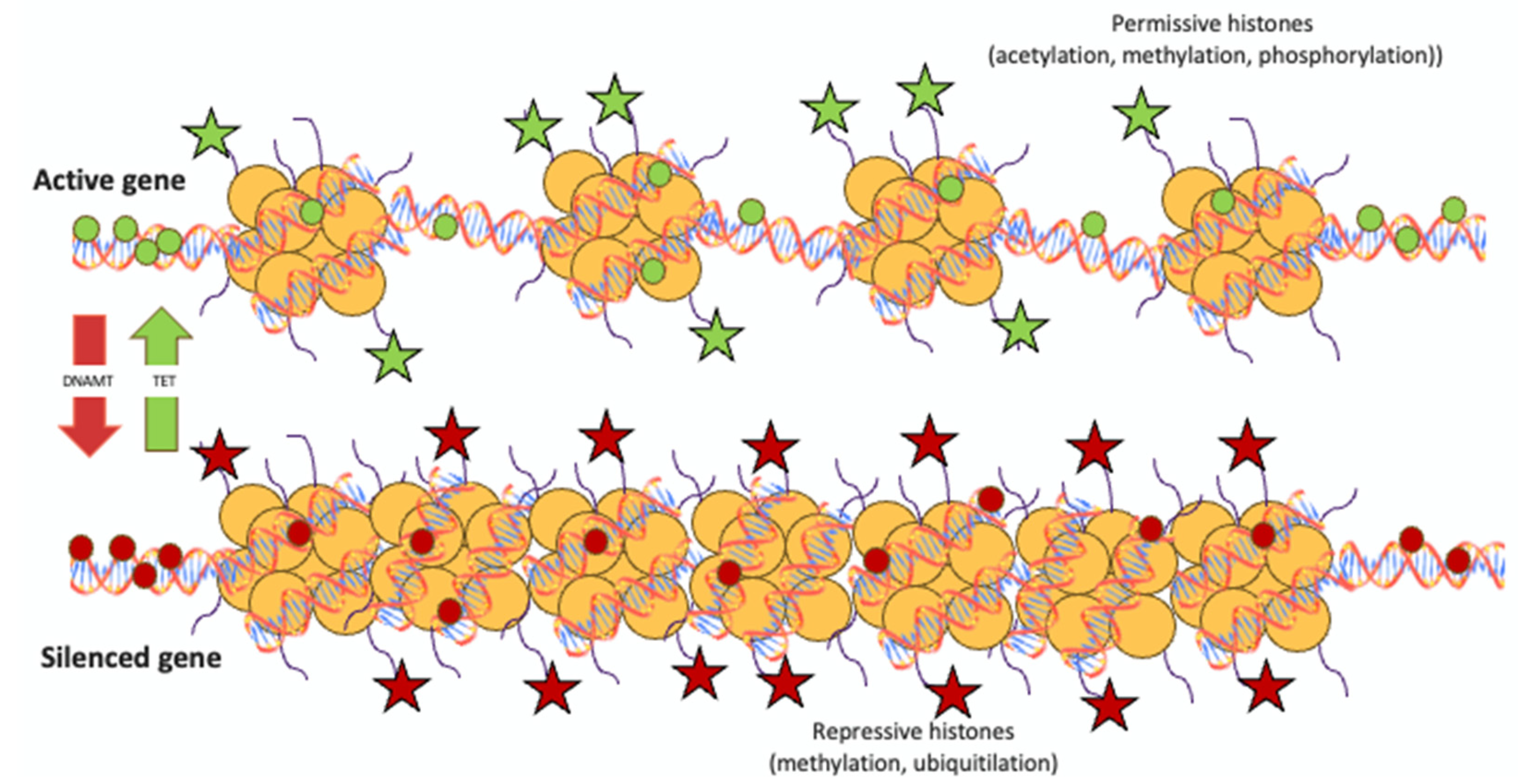
3.2.3. Histone Modification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Zeng, C.; Tsoi, L.C.; Gudjonsson, J.E. Dysregulated Epigenetic Modifications in Psoriasis. Exp. Dermatol. 2021, 30, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Bohannan, B.; Mburu, S.; Coates, L.C.; Ogdie, A.; Alarcon, I.; Kasparek, T.; Frade, S.; Barrio, S.F.; Augustin, M. Patient Perspectives on Psoriatic Disease Burden: Results from the Global Psoriasis and Beyond Survey. Dermatology 2023, 239, 621. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef]
- Conic, R.R.Z.; Damiani, G.; Schrom, K.P.; Ramser, A.E.; Zheng, C.; Xu, R.; McCormick, T.S.; Cooper, K.D. Psoriasis and Psoriatic Arthritis Cardiovascular Disease Endotypes Identified by Red Blood Cell Distribution Width and Mean Platelet Volume. J. Clin. Med. 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Rizzi, M.; Radovanovic, D.; Airoldi, A.; Cristiano, A.; Conic, R.; Petrou, S.; Pigatto, P.D.M.; Bragazzi, N.; Colombo, D.; et al. Psoriasis and Respiratory Comorbidities: The Added Value of Fraction of Exhaled Nitric Oxide as a New Method to Detect, Evaluate, and Monitor Psoriatic Systemic Involvement and Therapeutic Efficacy. Biomed. Res. Int. 2018, 2018, 3140682. [Google Scholar] [CrossRef]
- Dand, N.; Mahil, S.K.; Capon, F.; Smith, C.H.; Simpson, M.A.; Barker, J.N. Psoriasis and Genetics. Acta Derm. Venereol. 2020, 100, 54–64. [Google Scholar] [CrossRef]
- Babaie, F.; Omraninava, M.; Gorabi, A.M.; Khosrojerdi, A.; Aslani, S.; Yazdchi, A.; Torkamandi, S.; Mikaeili, H.; Sathyapalan, T.; Sahebkar, A. Etiopathogenesis of Psoriasis from Genetic Perspective: An Updated Review. Curr. Genom. 2022, 23, 163–174. [Google Scholar] [CrossRef]
- Gibson, F.; Hanly, A.; Grbic, N.; Grunberg, N.; Wu, M.; Collard, M.; Alani, R.M. Epigenetic Dysregulation in Autoimmune and Inflammatory Skin Diseases. Clin. Rev. Allergy Immunol. 2022, 63, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chiou, M.J.; Yang, S.F.; Kuo, C.F. The Effect of Paternal Psoriasis on Neonatal Outcomes: A Nationwide Population-Based Study. Front. Immunol. 2023, 14, 1172274. [Google Scholar] [CrossRef]
- Lønnberg, A.S.; Skov, L.; Skytthe, A.; Kyvik, K.O.; Pedersen, O.B.; Thomsen, S.F. Heritability of Psoriasis in a Large Twin Sample. Br. J. Dermatol. 2013, 169, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.T.; Henseler, T.; Christophers, E.; Voorhees, J.J.; Nair, R.P. Of Genes and Antigens: The Inheritance of Psoriasis. J. Investig. Dermatol. 1994, 103 (Suppl. S5), 150S–153S. [Google Scholar] [CrossRef]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The Immunogenetics of Psoriasis: A Comprehensive Review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Veal, C.D.; Capon, F.; Allen, M.H.; Heath, E.K.; Evans, J.C.; Jones, A.; Patel, S.; Burden, D.; Tillman, D.; Barker, J.N.W.N.; et al. Family-Based Analysis Using a Dense Single-Nucleotide Polymorphism-Based Map Defines Genetic Variation at PSORS1, the Major Psoriasis-Susceptibility Locus. Am. J. Hum. Genet. 2002, 71, 554–564. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Zhu, J.; Li, C. Multi-Omics Research Strategies for Psoriasis and Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 8018. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Spain, S.L.; Ellinghaus, E.; Stuart, P.E.; Capon, F.; Knight, J.; Tejasvi, T.; Kang, H.M.; Allen, M.H.; Lambert, S.; et al. Enhanced Meta-Analysis and Replication Studies Identify Five New Psoriasis Susceptibility Loci. Nat. Commun. 2015, 6, 7001. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large Scale Meta-Analysis Characterizes Genetic Architecture for Common Psoriasis Associated Variants. Nat. Commun. 2017, 8, 15382. [Google Scholar] [CrossRef]
- Kuiper, J.J.W.; Prinz, J.C.; Stratikos, E.; Kuśnierczyk, P.; Arakawa, A.; Springer, S.; Mintoff, D.; Padjen, I.; Shumnalieva, R.; Vural, S.; et al. EULAR Study Group on “MHC-I-Opathy”: Identifying Disease-Overarching Mechanisms across Disciplines and Borders. Ann. Rheum. Dis. 2023, 82, 887–896. [Google Scholar] [CrossRef]
- Ray-Jones, H.; Eyre, S.; Barton, A.; Warren, R.B. One SNP at a Time: Moving beyond GWAS in Psoriasis. J. Investig. Dermatol. 2016, 136, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Reeves, E.; Vollmer, S.; Arakawa, Y.; He, M.; Galinski, A.; Stöhr, J.; Dornmair, K.; James, E.; Prinz, J.C. ERAP1 Controls the Autoimmune Response against Melanocytes in Psoriasis by Generating the Melanocyte Autoantigen and Regulating Its Amount for HLA-C*06:02 Presentation. J. Immunol. 2021, 207, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Siewert, K.; Stöhr, J.; Besgen, P.; Kim, S.M.; Rühl, G.; Nickel, J.; Vollmer, S.; Thomas, P.; Krebs, S.; et al. Melanocyte Antigen Triggers Autoimmunity in Human Psoriasis. J. Exp. Med. 2015, 212, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.J.; Sun, L.D.; Soltani-Arabshahi, R.; Bowcock, A.M.; Nair, R.P.; Stuart, P.; Elder, J.T.; Schrodi, S.J.; Begovich, A.B.; Abecasis, G.R.; et al. Multiple Loci within the Major Histocompatibility Complex Confer Risk of Psoriasis. PLoS Genet. 2009, 5, e1000606. [Google Scholar] [CrossRef]
- Kagami, S.; Rizzo, H.L.; Lee, J.J.; Koguchi, Y.; Blauvelt, A. Circulating Th17, Th22, and Th1 Cells Are Increased in Psoriasis. J. Investig. Dermatol. 2010, 130, 1373–1383. [Google Scholar] [CrossRef]
- Nair, R.P.; Ruether, A.; Stuart, P.E.; Jenisch, S.; Tejasvi, T.; Hiremagalore, R.; Schreiber, S.; Kabelitz, D.; Lim, H.W.; Voorhees, J.J.; et al. Polymorphisms of the IL12B and IL23R Genes Are Associated with Psoriasis. J. Investig. Dermatol. 2008, 128, 1653–1661. [Google Scholar] [CrossRef]
- Strange, A.; Capon, F.; Spencer, C.C.A.; Knight, J.; Weale, M.E.; Allen, M.H.; Barton, A.; Band, G.; Bellenguez, C.; Bergboer, J.G.M.; et al. A Genome-Wide Association Study Identifies New Psoriasis Susceptibility Loci and an Interaction between HLA-C and ERAP1. Nat. Genet. 2010, 42, 985–990. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Spain, S.L.; Knight, J.; Ellinghaus, E.; Stuart, P.E.; Capon, F.; Ding, J.; Li, Y.; Tejasvi, T.; Gudjonsson, J.E.; et al. Identification of 15 New Psoriasis Susceptibility Loci Highlights the Role of Innate Immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Chen, Y.; Tato, C.M.; Laurence, A.; Joyce-Shaikh, B.; Blumenschein, W.M.; McClanahan, T.K.; O’Shea, J.J.; Cua, D.J. The Interleukin 23 Receptor Is Essential for the Terminal Differentiation of Interleukin 17-Producing Effector T Helper Cells in Vivo. Nat. Immunol. 2009, 10, 314–324. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Guttman-Yassky, E.; Suárez-Farĩas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative Responses to IL-17 and TNF-α in Human Keratinocytes Account for Key Inflammatory Pathogenic Circuits in Psoriasis. J. Investig. Dermatol. 2011, 131, 677–687. [Google Scholar] [CrossRef]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.G.; Civello, D.; Catanese, J.J.; et al. A Large-Scale Genetic Association Study Confirms IL12B and Leads to the Identification of IL23R as Psoriasis-Risk Genes. Am. J. Hum. Genet. 2007, 80, 273–290. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Ellinghaus, E.; Nair, R.P.; Stuart, P.E.; Esko, T.; Metspalu, A.; Debrus, S.; Raelson, J.V.; Tejasvi, T.; Belouchi, M.; et al. Combined Analysis of Genome-Wide Association Studies for Crohn Disease and Psoriasis Identifies Seven Shared Susceptibility Loci. Am. J. Hum. Genet. 2012, 90, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.; Yang, Z.; Wang, W.; Li, B.; Bai, M.; Wu, J.; Ge, H.; Dong, Z.; Shen, J.; Tang, H.; et al. Genetic Study on Small Insertions and Deletions in Psoriasis Reveals a Role in Complex Human Diseases. J. Investig. Dermatol. 2019, 139, 2302.e14–2312.e14. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.; Bouchlaka-Souissi, C.; Zaraa, I.; Helms, C.; Doss, N.; Bouazizi, F.; Dhaoui, R.; Ben Ossman, A.; Gaied, A.B.A.-E.; Mokni, M. Family-Based Association Study in Tunisian Familial Psoriasis. Int. J. Dermatol. 2012, 51, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Prans, E.; Kingo, K.; Traks, T.; Silm, H.; Vasar, E.; Kõks, S. Copy Number Variations in IL22 Gene Are Associated with Psoriasis Vulgaris. Hum. Immunol. 2013, 74, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, M.; Migdalska-Sęk, M.; Brzeziańska-Lasota, E.; Zelga, P.; Woźniacka, A. Analysis of Molecular Markers as IL-12, IL-22 and IFN-γ in Correlation with a Clinical Course in Patients with Psoriasis. Int. J. Occup. Med. Environ. Health 2020, 33, 635–647. [Google Scholar] [CrossRef]
- Chiang, C.C.; Cheng, W.J.; Korinek, M.; Lin, C.Y.; Hwang, T.L. Neutrophils in Psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Czerwińska, J.; Owczarczyk-Saczonek, A. The Role of the Neutrophilic Network in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2022, 23, 1840. [Google Scholar] [CrossRef]
- Lambert, S.; Hambro, C.A.; Johnston, A.; Stuart, P.E.; Tsoi, L.C.; Nair, R.P.; Elder, J.T. Neutrophil Extracellular Traps Induce Human Th17 Cells: Effect of Psoriasis-Associated TRAF3IP2 Genotype. J. Investig. Dermatol. 2019, 139, 1245. [Google Scholar] [CrossRef]
- Purzycka-Bohdan, D.; Nedoszytko, B.; Sobalska-Kwapis, M.; Zabłotna, M.; Żmijewski, M.A.; Wierzbicka, J.; Gleń, J.; Strapagiel, D.; Szczerkowska-Dobosz, A.; Nowicki, R.J. Assessment of the Potential Role of Selected Single Nucleotide Polymorphisms (SNPs) of Genes Related to the Functioning of Regulatory T Cells in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 6061. [Google Scholar] [CrossRef]
- Kim, J.; Moreno, A.; Krueger, J.G. The Imbalance between Type 17 T-Cells and Regulatory Immune Cell Subsets in Psoriasis Vulgaris. Front. Immunol. 2022, 13, 1005115. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Luo, N.; Zhong, X.; Xu, T.; Hao, P. The Immunoregulatory Effects of Natural Products on Psoriasis via Its Action on Th17 Cells versus Regulatory T Cells Balance. Int. Immunopharmacol. 2022, 110, 109032. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Z.; Zhao, Z.; Yu, Y.; Fan, H.; Xu, X.; Bu, X.; Gu, J. IL-21 Induces an Imbalance of Th17/Treg Cells in Moderate-to-Severe Plaque Psoriasis Patients. Front. Immunol. 2019, 10, 1865. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-ΚB: An Essential Transcription Factor in Psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Stuart, P.E.; Nair, R.P.; ELinghaus, E.; Ding, J.; Tejasvi, T.; GudjonSon, J.E.; Li, Y.; Weidinger, S.; Eberlein, B.; Gieger, C.; et al. Genome-Wide Association Analysis Identifies Three Psoriasis Susceptibility Loci. Nat. Genet. 2010, 42, 1000–1004. [Google Scholar] [CrossRef]
- Hüffmeier, U.; Uebe, S.; Ekici, A.B.; Bowes, J.; Giardina, E.; Korendowych, E.; Juneblad, K.; Apel, M.; McManus, R.; Ho, P.; et al. Common Variants at TRAF3IP2 Are Associated with Susceptibility to Psoriatic Arthritis and Psoriasis. Nat. Genet. 2010, 42, 996–999. [Google Scholar] [CrossRef]
- Jordan, C.T.; Cao, L.; Roberson, E.D.O.; Pierson, K.C.; Yang, C.F.; Joyce, C.E.; Ryan, C.; Duan, S.; Helms, C.A.; Liu, Y.; et al. PSORS2 Is Due to Mutations in CARD14. Am. J. Hum. Genet. 2012, 90, 784–795. [Google Scholar] [CrossRef]
- Sun, L.D.; Cheng, H.; Wang, Z.X.; Zhang, A.P.; Wang, P.G.; Xu, J.H.; Zhu, Q.X.; Zhou, H.S.; ELinghaus, E.; Zhang, F.R.; et al. Association Analyses Identify Six New Psoriasis Susceptibility Loci in the Chinese Population. Nat. Genet. 2010, 42, 1005–1009. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-KappaB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Prens, E.P.; Kant, M.; Van Dijk, G.; Van Der Wel, L.I.; Mourits, S.; Van Der Fits, L. IFN-α Enhances Poly-IC Responses in Human Keratinocytes by Inducing Expression of Cytosolic Innate RNA Receptors: Relevance for Psoriasis. J. Investig. Dermatol. 2008, 128, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hollox, E.J.; Huffmeier, U.; Zeeuwen, P.L.J.M.; Palla, R.; Lascorz, J.; Rodijk-Olthuis, D.; Van De Kerkhof, P.C.M.; Traupe, H.; De Jongh, G.; Den Heijer, M.; et al. Psoriasis Is Associated with Increased β-Defensin Genomic Copy Number. Nat. Genet. 2008, 40, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Y.; Chen, G.; Yang, Y.; Zhou, D.; Zhang, Z.; Zhang, D.; Chen, Y.; Lu, Z.; He, L.; et al. Deletion of the Late Cornified Envelope Genes LCE3C and LCE3B Is Associated with Psoriasis in a Chinese Population. J. Investig. Dermatol. 2011, 131, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Young, K.Z.; Sarkar, M.K.; Gudjonsson, J.E. Pathophysiology of Generalized Pustular Psoriasis. Exp. Dermatol. 2023. [Google Scholar] [CrossRef]
- Mahil, S.K.; Twelves, S.; Farkas, K.; Setta-Kaffetzi, N.; Burden, A.D.; Gach, J.E.; Irvine, A.D.; Képíró, L.; Mockenhaupt, M.; Oon, H.H.; et al. AP1S3 Mutations Cause Skin Autoinflammation by Disrupting Keratinocyte Autophagy and Up-Regulating IL-36 Production. J. Investig. Dermatol. 2016, 136, 2251–2259. [Google Scholar] [CrossRef]
- Onitsuka, M.; Farooq, M.; Iqbal, M.N.; Yasuno, S.; Shimomura, Y. A Homozygous Loss-of-Function Variant in the MPO Gene Is Associated with Generalized Pustular Psoriasis. J. Dermatol. 2023, 50, 664–671. [Google Scholar] [CrossRef]
- Vergnano, M.; Mockenhaupt, M.; Benzian-Olsson, N.; Paulmann, M.; Grys, K.; Mahil, S.K.; Chaloner, C.; Barbosa, I.A.; August, S.; Burden, A.D.; et al. Loss-of-Function Myeloperoxidase Mutations Are Associated with Increased Neutrophil Counts and Pustular Skin Disease. Am. J. Hum. Genet. 2021, 108, 757. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-Wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of Pathogenesis in Psoriatic Arthritis: Genotype Determines Clinical Phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, L.; Zhang, H.; Xu, F.; Zhou, X.; Yu, L.; Sun, J.; Chen, J.; Ying, H.; Xu, X.; et al. Mendelian Randomization and Clinical Trial Evidence Supports TYK2 Inhibition as a Therapeutic Target for Autoimmune Diseases. EBioMedicine 2023, 89, 104488. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Strafella, C.; Cosio, T.; Lanna, C.; Campione, E.; Novelli, G.; Giardina, E.; Cascella, R. Pharmacogenomics: An Update on Biologics and Small-Molecule Drugs in the Treatment of Psoriasis. Genes 2021, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Antonatos, C.; Asmenoudi, P.; Panoutsopoulou, M.; Vasilopoulos, Y. Pharmaco-Omics in Psoriasis: Paving the Way towards Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 7090. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, C.W.; Chen, C.B.; Chen, W.T.; Chang, Y.C.; Hui, R.C.Y.; Chung, W.H. Pharmacogenomics on the Treatment Response in Patients with Psoriasis: An Updated Review. Int. J. Mol. Sci. 2023, 24, 7329. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Saklatvala, J.; Rispens, T.; Loeff, F.C.; de Vries, A.; Allen, M.H.; Barbosa, I.A.; Baudry, D.; Dasandi, T.; Duckworth, M.; et al. Development of Antidrug Antibodies against Adalimumab Maps to Variation within the HLA-DR Peptide-Binding Groove. JCI Insight 2023, 8, e156643. [Google Scholar] [CrossRef]
- Al-Janabi, A.; Eyre, S.; Foulkes, A.C.; Khan, A.R.; Dand, N.; Burova, E.; DeSilva, B.; Makrygeorgou, A.; Davies, E.; Smith, C.H.; et al. Atopic Polygenic Risk Score Is Associated with Paradoxical Eczema Developing in Patients with Psoriasis Treated with Biologics. J. Investig. Dermatol. 2023, 143, 1470.e1–1478.e1. [Google Scholar] [CrossRef]
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The Role of Epigenetic Factors in Psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [Google Scholar] [CrossRef]
- Roszkiewicz, M.; Dopytalska, K.; Szymańska, E.; Jakimiuk, A.; Walecka, I. Environmental Risk Factors and Epigenetic Alternations in Psoriasis. Ann. Agric. Environ. Med. 2020, 27, 335–342. [Google Scholar] [CrossRef]
- Frischknecht, L.; Vecellio, M.; Selmi, C. The Role of Epigenetics and Immunological Imbalance in the Etiopathogenesis of Psoriasis and Psoriatic Arthritis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19886505. [Google Scholar] [CrossRef]
- Xiang, Z.; Yang, Y.; Chang, C.; Lu, Q. The Epigenetic Mechanism for Discordance of Autoimmunity in Monozygotic Twins. J. Autoimmun. 2017, 83, 43–50. [Google Scholar] [CrossRef]
- Gervin, K.; Vigeland, M.D.; Mattingsdal, M.; Hammerø, M.; Nygård, H.; Olsen, A.O.; Brandt, I.; Harris, J.R.; Undlien, D.E.; Lyle, R. DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes. PLoS Genet. 2012, 8, e1002454. [Google Scholar] [CrossRef] [PubMed]
- Vecellio, M.; Paraboschi, E.M.; Ceribelli, A.; Isailovic, N.; Motta, F.; Cardamone, G.; Robusto, M.; Asselta, R.; Brescianini, S.; Sacrini, F.; et al. DNA Methylation Signature in Monozygotic Twins Discordant for Psoriatic Disease. Front. Cell Dev. Biol. 2021, 9, 778677. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Ganguly, T.; Chatterjee, R. Emerging Roles of Non-Coding RNAs in Psoriasis Pathogenesis. Funct. Integr. Genom. 2023, 23, 129. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Folkersen, L.; Biskup, E.; Xu, N.; Manfe, V.; Niazi, O.; Gniadecki, R. Ubiquitin-Specific Peptidase 2 as a Potential Link between MicroRNA-125b and Psoriasis. Br. J. Dermatol. 2017, 176, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Huang, Y.; Zhu, X.; Lin, X.; Luo, D. MiR-125b-mediated Regulation of Cell Proliferation through the Jagged-1/Notch Signaling Pathway by Inhibiting BRD4 Expression in Psoriasis. Mol. Med. Rep. 2019, 19, 5227–5236. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, R.; Ma, R.; Tang, X.; Gong, Y.; Yu, Z.; Shi, Y. The Role of MicroRNA in Psoriasis: A Review. Exp. Dermatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, H.; Wang, C.; Zeng, B.; Tang, X.; Zhang, Y.; Peng, Y.; Luo, M.; Huang, P.; Yang, Z. MiR-203 Promotes HaCaT Cell Overproliferation through Targeting LXR-α and PPAR-γ. Cell Cycle 2020, 19, 1928–1940. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Jin, M.; Li, H.; Li, S. MiR-383 Reduces Keratinocyte Proliferation and Induces the Apoptosis in Psoriasis via Disruption of LCN2-Dependent JAK/STAT Pathway Activation. Int. Immunopharmacol. 2021, 96, 107587. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, F.; Tian, Q.; Dong, J.; Chen, L.; Hu, R. Involvement of MiR-214-3p/FOXM1 Axis During the Progression of Psoriasis. Inflammation 2022, 45, 267–278. [Google Scholar] [CrossRef]
- Qu, S.; Liu, Z.; Wang, B. EZH2 Is Involved in Psoriasis Progression by Impairing MiR-125a-5p Inhibition of SFMBT1 and Leading to Inhibition of the TGFβ/SMAD Pathway. Ther. Adv. Chronic. Dis. 2021, 12, 2040622320987348. [Google Scholar] [CrossRef]
- Xia, P.; Pasquali, L.; Gao, C.; Srivastava, A.; Khera, N.; Freisenhausen, J.C.; Luo, L.; Rosén, E.; van Lierop, A.; Homey, B.; et al. MiR-378a Regulates Keratinocyte Responsiveness to Interleukin-17A in Psoriasis. Br. J. Dermatol. 2022, 187, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, Z.; Lou, F.; Zhang, L.; Ke, F.; Bai, J.; Liu, Z.; Liu, J.; Wang, H.; Zhu, H.; et al. NF-ΚB-Induced MicroRNA-31 Promotes Epidermal Hyperplasia by Repressing Protein Phosphatase 6 in Psoriasis. Nat. Commun. 2015, 6, 7652. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zeng, J.; Yuan, J.; Deng, X.; Huang, Y.; Chen, L.; Zhang, P.; Feng, H.; Liu, Z.; Wang, Z.; et al. MicroRNA-210 Overexpression Promotes Psoriasis-like Inflammation by Inducing Th1 and Th17 Cell Differentiation. J. Clin. Invest. 2018, 128, 2551–2568. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; D’Agostino, M.; Sileno, S.; Di Vito, L.; Uras, C.; Abeni, D.; Martino, F.; Barillà, F.; Madonna, S.; Albanesi, C.; et al. The Oxidative Stress-Induced MiR-200c Is Upregulated in Psoriasis and Correlates with Disease Severity and Determinants of Cardiovascular Risk. Oxid. Med. Cell. Longev. 2019, 2019, 8061901. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zeng, J.; Li, W.; Lin, L.; Zhou, X.; Tian, X.; Liu, W.; Zhang, L.; Zhang, X. Silencing of MiR-155 Suppresses Inflammatory Responses in Psoriasis through Inflammasome NLRP3 Regulation. Int. J. Mol. Med. 2018, 42, 1086–1095. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, G.; Wang, M.; Chen, C.; Zhang, M.; Liu, M.; Shao, Y.; Zheng, Y. LncRNA RP6-65G23.1 Accelerates Proliferation and Inhibits Apoptosis via p-ERK1/2/p-AKT Signaling Pathway on Keratinocytes. J. Cell. Biochem. 2020, 121, 4580–4589. [Google Scholar] [CrossRef]
- Gao, J.; Chen, F.; Hua, M.; Guo, J.; Nong, Y.; Tang, Q.; Zhong, F.; Qin, L. Knockdown of LncRNA MIR31HG Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Qiao, M.; Li, R.; Zhao, X.; Yan, J.; Sun, Q. Up-Regulated LncRNA-MSX2P1 Promotes the Growth of IL-22-Stimulated Keratinocytes by Inhibiting MiR-6731-5p and Activating S100A7. Exp. Cell Res. 2018, 363, 243–254. [Google Scholar] [CrossRef]
- Xiang, S.; Wu, X.; Xiang, Y. Sinomenine Suppressed Keratinocyte Proliferation and Imiquimod-Induced Psoriasis-Like Dermatitis by Regulating LncRNA XIST. Skin Pharmacol. Physiol. 2022, 35, 328–342. [Google Scholar] [CrossRef]
- Huang, S.; Zhen, Y.; Yin, X.; Yang, Z.; Li, X.; Wang, R.; Wen, H.; Zhong, H.; Yan, J.; Sun, Q. KMT2C Induced by FABP5P3 Aggravates Keratinocyte Hyperproliferation and Psoriasiform Skin Inflammation by Upregulating the Transcription of PIK3R3. J. Investig. Dermatol. 2023, 143, 37.e8–47.e8. [Google Scholar] [CrossRef]
- Yin, X.; Yang, Z.; Zhu, M.; Chen, C.; Huang, S.; Li, X.; Zhong, H.; Wen, H.; Sun, Q.; Yu, X.; et al. ILF2 Contributes to Hyperproliferation of Keratinocytes and Skin Inflammation in a KLHDC7B-DT-Dependent Manner in Psoriasis. Front. Genet. 2022, 13, 890624. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yang, Z.; Zhu, M.; Chen, C.; Sun, Q. Role of the Long Non-Coding RNA, SPRR2C, Based on an in Vitro Psoriatic Keratinocyte Cell Model. Eur. J. Dermatol. 2022, 32, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Zhang, K.; Lu, W.J.; Xu, G.W.; Zhang, J.F.; Tang, Z.L. LncRNA MEG3 Influences the Proliferation and Apoptosis of Psoriasis Epidermal Cells by Targeting MiR-21/Caspase-8. BMC Mol. Cell Biol. 2019, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Shehata, W.; Maraee, A.; Abd El Monem Ellaithy, M.; Tayel, N.; Abo-Ghazala, A.; Mohammed El-Hefnawy, S. Circulating Long Noncoding RNA Growth Arrest-Specific Transcript 5 as a Diagnostic Marker and Indicator of Degree of Severity in Plaque Psoriasis. Int. J. Dermatol. 2021, 60, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.Y.; Tawfik, N.Z.; Soliman, N.H.; Eldeen, L.A.T. The LncRNA PRINS-MiRNA-MRNA Axis Gene Expression Profile as a Circulating Biomarker Panel in Psoriasis. Mol. Diagn. Ther. 2022, 26, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cheng, S.; Zou, G.; Ding, X. Paeoniflorin Inhibits Proliferation and Migration of Psoriatic Keratinocytes via the LncRNA NEAT1/MiR-3194-5p/Galectin-7 Axis. Anticancer Drugs 2022, 33, E423–E433. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The Emerging Roles and Functions of Circular RNAs and Their Generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Li, Y.; Yu, N.; Ding, Y.; Shi, Y. CircRAB3B Suppresses Proliferation, Motility, Cell Cycle Progression and Promotes the Apoptosis of IL-22-Induced Keratinocytes Depending on the Regulation of MiR-1228-3p/PTEN Axis in Psoriasis. Autoimmunity 2021, 54, 303–312. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, X.; Chen, C.; Huang, S.; Li, X.; Yan, J.; Sun, Q. CircOAS3 Regulates Keratinocyte Proliferation and Psoriatic Inflammation by Interacting with Hsc70 via the JNK/STAT3/NF-ΚB Signaling Pathway. Inflammation 2022, 45, 1924–1935. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Z.; Yin, X.; Huang, S.; Yan, J.; Sun, Q. CircEIF5 Contributes to Hyperproliferation and Inflammation of Keratinocytes in Psoriasis via P-NFκB and p-STAT3 Signalling Pathway. Exp. Dermatol. 2022, 31, 1145–1153. [Google Scholar] [CrossRef]
- Shi, Q.; Luo, J.; Chen, W.; He, Q.; Long, J.; Zhang, B. Circ_0060531 Knockdown Ameliorates IL-22-Induced Keratinocyte Damage by Binding to MiR-330-5p to Decrease GAB1 Expression. Autoimmunity 2022, 55, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, C.; Bai, X.; Xiao, C.; Dang, E.; Wang, G. Hsa_circ_0003738 Inhibits the Suppressive Function of Tregs by Targeting MiR-562/IL-17A and MiR-490-5p/IFN-γ Signaling Pathway. Mol. Ther. Nucleic. Acids. 2020, 21, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ding, J.; Yan, J.; Li, R.; Jiao, J.; Sun, Q. Circular RNA Expression Profile and Analysis of Their Potential Function in Psoriasis. Cell Physiol. Biochem. 2018, 50, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Alagia, A.; Gullerova, M. The Methylation Game: Epigenetic and Epitranscriptomic Dynamics of 5-Methylcytosine. Front. Cell Dev. Biol. 2022, 10, 915685. [Google Scholar] [CrossRef]
- Luo, Y.; Qu, K.; Kuai, L.; Ru, Y.; Huang, K.; Yan, X.; Xing, M. Epigenetics in Psoriasis: Perspective of DNA Methylation. Mol. Genet. Genom. 2021, 296, 1027–1040. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, W.; Shen, C.; Li, H.; Zuo, X.; Zheng, X.; Yue, M.; Zhang, C.; Yu, L.; Chen, M.; et al. Epigenome-Wide Association Analysis Identified Nine Skin DNA Methylation Loci for Psoriasis. J. Investig. Dermatol. 2016, 136, 779–787. [Google Scholar] [CrossRef]
- Chandra, A.; Senapati, S.; Roy, S.; Chatterjee, G.; Chatterjee, R. Epigenome-Wide DNA Methylation Regulates Cardinal Pathological Features of Psoriasis. Clin. Epigenetics 2018, 10, 108. [Google Scholar] [CrossRef]
- Gao, L.; Lu, Q. The Critical Importance of Epigenetics in Autoimmune-Related Skin Diseases. Front. Med. 2023, 17, 43–57. [Google Scholar] [CrossRef]
- Nobeyama, Y.; Umezawa, Y.; Nakagawa, H. Less-Invasive Analysis of DNA Methylation Using Psoriatic Scales. J. Dermatol. Sci. 2016, 83, 70–73. [Google Scholar] [CrossRef]
- Gallais Sérézal, I.; Cheuk, S.; Martini, E.; Eidsmo, L. Cellular Scars and Local Crosstalk in Relapsing Psoriasis: An Example of a Skin Sticking Disease. Scand. J. Immunol. 2020, 92, e12953. [Google Scholar] [CrossRef]
- Ghaffarinia, A.; Ayaydin, F.; Póliska, S.; Manczinger, M.; Bolla, B.S.; Flink, L.B.; Balogh, F.; Veréb, Z.; Bozó, R.; Szabó, K.; et al. Psoriatic Resolved Skin Epidermal Keratinocytes Retain Disease-Residual Transcriptomic and Epigenomic Profiles. Int. J. Mol. Sci. 2023, 24, 4556. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Ekman, A.K.; Bivik Eding, C.; Enerbäck, C. Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis. J. Investig. Dermatol. 2018, 138, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Su, Y.; Chen, H.; Zhao, M.; Lu, Q. Abnormal DNA Methylation in Skin Lesions and PBMCs of Patients with Psoriasis Vulgaris. J. Dermatol. Sci. 2010, 60, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Yin, G.; An, P.; Wang, C.; Liu, R.; Yang, Y.; Yan, X.; Li, J.; Li, X.; Zhang, K. DNA Methylation of Dermal MSCs in Psoriasis: Identification of Epigenetically Dysregulated Genes. J. Dermatol. Sci. 2013, 72, 103–109. [Google Scholar] [CrossRef]
- Charras, A.; Garau, J.; Hofmann, S.R.; Carlsson, E.; Cereda, C.; Russ, S.; Abraham, S.; Hedrich, C.M. DNA Methylation Patterns in CD8+ T Cells Discern Psoriasis From Psoriatic Arthritis and Correlate With Cutaneous Disease Activity. Front. Cell Dev. Biol. 2021, 9, 746145. [Google Scholar] [CrossRef]
- Deng, M.; Su, Y.; Wu, R.; Li, S.; Zhu, Y.; Tang, G.; Shi, X.; Zhou, T.; Zhao, M.; Lu, Q. DNA Methylation Markers in Peripheral Blood for Psoriatic Arthritis. J. Dermatol. Sci. 2022, 108, 39–47. [Google Scholar] [CrossRef]
- Vecellio, M.; Rodolfi, S.; Selmi, C. Advanced Genomics and Clinical Phenotypes in Psoriatic Arthritis. Semin. Immunol. 2021, 58, 101665. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.; Mariscal, A.G.; Wu, X.; Hülse, J.; Pedersen, E.; Helin, K.; Waisman, A.; Vinkel, C.; Thomsen, S.F.; et al. Epigenetic Control of IL-23 Expression in Keratinocytes Is Important for Chronic Skin Inflammation. Nat. Commun. 2018, 9, 1420. [Google Scholar] [CrossRef]
- Ovejero-Benito, M.C.; Reolid, A.; Sánchez-Jiménez, P.; Saiz-Rodríguez, M.; Muñoz-Aceituno, E.; Llamas-Velasco, M.; Martín-Vilchez, S.; Cabaleiro, T.; Román, M.; Ochoa, D.; et al. Histone Modifications Associated with Biological Drug Response in Moderate-to-Severe Psoriasis. Exp. Dermatol. 2018, 27, 1361–1371. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, W.; Xu, L.; Chen, X.; Zhan, Y.; Yang, Q.; Liu, S.; Chen, P.; Jiang, Y.; Sun, X.; et al. The Histone H3 Lysine-27 Demethylase Jmjd3 Plays a Critical Role in Specific Regulation of Th17 Cell Differentiation. J. Mol. Cell Biol. 2015, 7, 505–516. [Google Scholar] [CrossRef]
- Tovar-Castillo, L.E.; Cancino-Díaz, J.C.; García-Vázquez, F.; Cancino-Gómez, F.G.; León-Dorantes, G.; Blancas-González, F.; Jiménez-Zamudio, L.; García-Latorre, E.; Cancino-Díaz, M.E. Under-Expression of VHL and over-Expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in Affected Skin Biopsies of Patients with Psoriasis. Int. J. Dermatol. 2007, 46, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cao, G.; Sun, G.; Zhu, L.; Tian, Y.; Song, Y.; Guo, C.; Wang, X.; Zhong, J.; Zhou, W.; et al. GLS1-Mediated Glutaminolysis Unbridled by MALT1 Protease Promotes Psoriasis Pathogenesis. J. Clin. Investig. 2020, 130, 5180–5196. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, R.; Tang, D.; Kang, R. The BET Family in Immunity and Disease. Signal Transduct. Target Ther. 2021, 6, 5180–5196. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Al-Harbi, N.O.; Al-Harbi, M.M.; El-Sherbeeny, A.M.; Ahmad, S.F.; Siddiqui, N.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; Al-Hosaini, K.A.; et al. Imiquimod-Induced Psoriasis-like Skin Inflammation Is Suppressed by BET Bromodomain Inhibitor in Mice through RORC/IL-17A Pathway Modulation. Pharmacol. Res. 2015, 99, 248–257. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Polygenic Risk Scores: From Research Tools to Clinical Instruments. Genome Med. 2020, 12, 44. [Google Scholar] [CrossRef]
- Gao, M.; Si, X. Rapamycin Ameliorates Psoriasis by Regulating the Expression and Methylation Levels of Tropomyosin via ERK1/2 and MTOR Pathways in Vitro and in Vivo. Exp. Dermatol. 2018, 27, 1112–1119. [Google Scholar] [CrossRef]
- Thatikonda, S.; Pooladanda, V.; Sigalapalli, D.K.; Godugu, C. Piperlongumine Regulates Epigenetic Modulation and Alleviates Psoriasis-like Skin Inflammation via Inhibition of Hyperproliferation and Inflammation. Cell Death Dis. 2020, 11, 21. [Google Scholar] [CrossRef]
- Feng, H.; Wu, R.; Zhang, S.; Kong, Y.; Liu, Z.; Wu, H.; Wang, H.; Su, Y.; Zhao, M.; Lu, Q. Topical Administration of Nanocarrier MiRNA-210 Antisense Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis in Mice. J. Dermatol. 2020, 47, 147–154. [Google Scholar] [CrossRef]
| Pathway | Gene | Function |
|---|---|---|
| Antigen presentation | HLA-C*0602 | Antigen presentation |
| ERAP1 | Modification of MHC-I-binding peptides | |
| Th1 Signaling Pathway | IL12B | p40 subunit of IL12 |
| TYK2 | Downstream molecule of IL12 receptor | |
| ZC3H12C | Macrophage activation | |
| STAT5A/B | Signaling pathway of IL2 familiy cytokines | |
| ILF3 | IL2 expression in T-cells | |
| Th17 Signaling Pathway | TYK2 | Downstream molecule of IL23 receptor |
| JAK2 | Downstream molecule of IL23 receptor | |
| STAT3 | Downstream molecule of IL23 receptor | |
| SOCS1 | Th17 differentiation | |
| ETS1 | Th17 differentiation | |
| IL17RD | IL17 receptor | |
| IL22 | Differentiation and proliferation of keratinocytes | |
| TRAF3IP2 | Signaling pathway of IL17A/F | |
| KLF4 | Regulation of IL17A production | |
| Innate immunity | C-REL | NF-kB pathway activation |
| TRAF3IP2 | NF-kB pathway activation | |
| CARD14 | NF-kB pathway activation | |
| MICA | NK, NKT and T-cells activation | |
| TNFAIP3 | NF-kB pathway downregulation | |
| TNIP1 | NF-kB pathway downregulation | |
| NFKBIA | NF-kB pathway downregulation | |
| DDX58 | INF pathway and antiviral response | |
| IFIHI | INF pathway and antiviral response | |
| Skin barrier function | DEFB4 | Secretion of β-defensins |
| LCE3B/C | Epidermis differentiation and hyperproliferation | |
| GJB2 | Connexin 26, epidermal gap junction |
| Epigenetic Change | Function | |
|---|---|---|
| miRNA downregulation | miRNA125b | Keratinocyte proliferation and differentiation |
| miR-203 | Keratinocyte proliferation | |
| mir-383 | Keratinocyte apoptosis and inflammation | |
| 214-3p | Cell cycle check-points and keratinocyte proliferation | |
| miR-125a-5p | Keratinocyte proliferation | |
| miRNA upregulation | miR-378a | Psoriatic inflammation |
| Mir-31 | Keratinocyte proliferation | |
| mir-210 | Inflammation | |
| miR-200c | Associated with PASI | |
| miR-155 | Psoriatic inflammation | |
| lncRNA upregulation | lncRNA-RP6- 65G23.1 | Immune response, keratinocyte proliferation, apoptosis suppression |
| MIR31HG | Keratinocyte proliferation | |
| MSX2P1 | Keratinocyte proliferation | |
| XIST | Keratinocyte proliferation | |
| FABP5P3 | Keratinocyte proliferation and inflammation | |
| KLDHC7B-DT | Keratinocyte proliferation and inflammation | |
| SPRR2C | Keratinocyte proliferation and apoptosis | |
| lncRNA downregulation | MEG3 | Keratinocyte proliferation and apoptosis |
| GAS5 | Related to psoriasis severity | |
| PRINS | Keratinocyte proliferation and inflammation | |
| NEAT1 | Keratinocyte proliferation | |
| circRNA downregulation | circRAB3B | Keratinocyte proliferation |
| circRNA upregulation | circOAS3 | Keratinocyte proliferation and apoptosis |
| circEIF5 | Keratinocyte proliferation | |
| circ_0060531 | Keratinocyte proliferation, migration &inflammation | |
| hsa_circ_0003738 | Treg modulation | |
| hsa_circ_0061012 | Keratinocyte proliferation and migration | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateu-Arrom, L.; Puig, L. Genetic and Epigenetic Mechanisms of Psoriasis. Genes 2023, 14, 1619. https://doi.org/10.3390/genes14081619
Mateu-Arrom L, Puig L. Genetic and Epigenetic Mechanisms of Psoriasis. Genes. 2023; 14(8):1619. https://doi.org/10.3390/genes14081619
Chicago/Turabian StyleMateu-Arrom, Laura, and Lluis Puig. 2023. "Genetic and Epigenetic Mechanisms of Psoriasis" Genes 14, no. 8: 1619. https://doi.org/10.3390/genes14081619
APA StyleMateu-Arrom, L., & Puig, L. (2023). Genetic and Epigenetic Mechanisms of Psoriasis. Genes, 14(8), 1619. https://doi.org/10.3390/genes14081619





