Next-Generation Sequencing for Screening Analysis of Cystic Fibrosis: Spectrum and Novel Variants in a South–Central Italian Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Extraction and Next-Generation Sequencing
2.3. NGS Data Analysis and Interpretation
3. Results
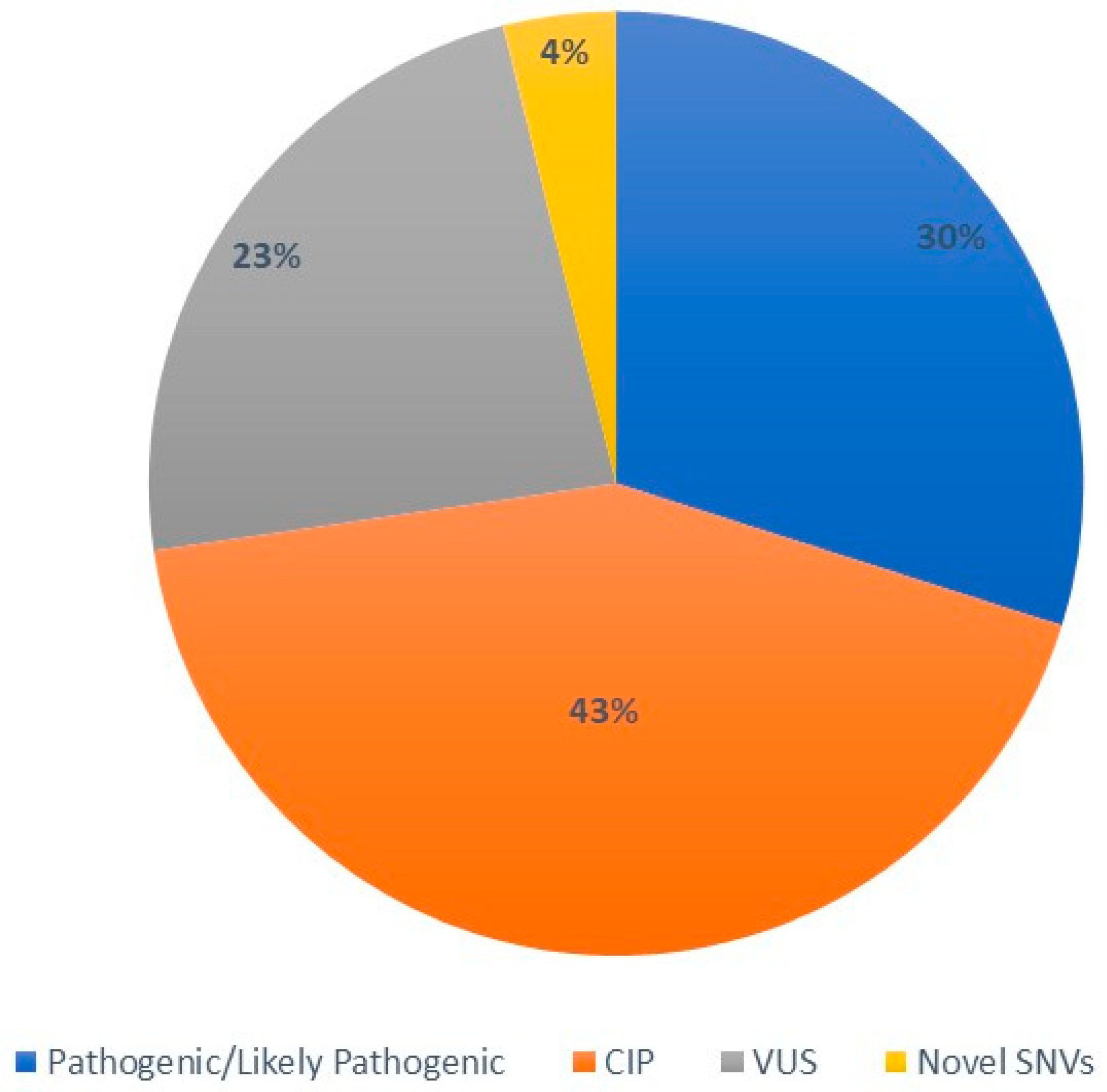
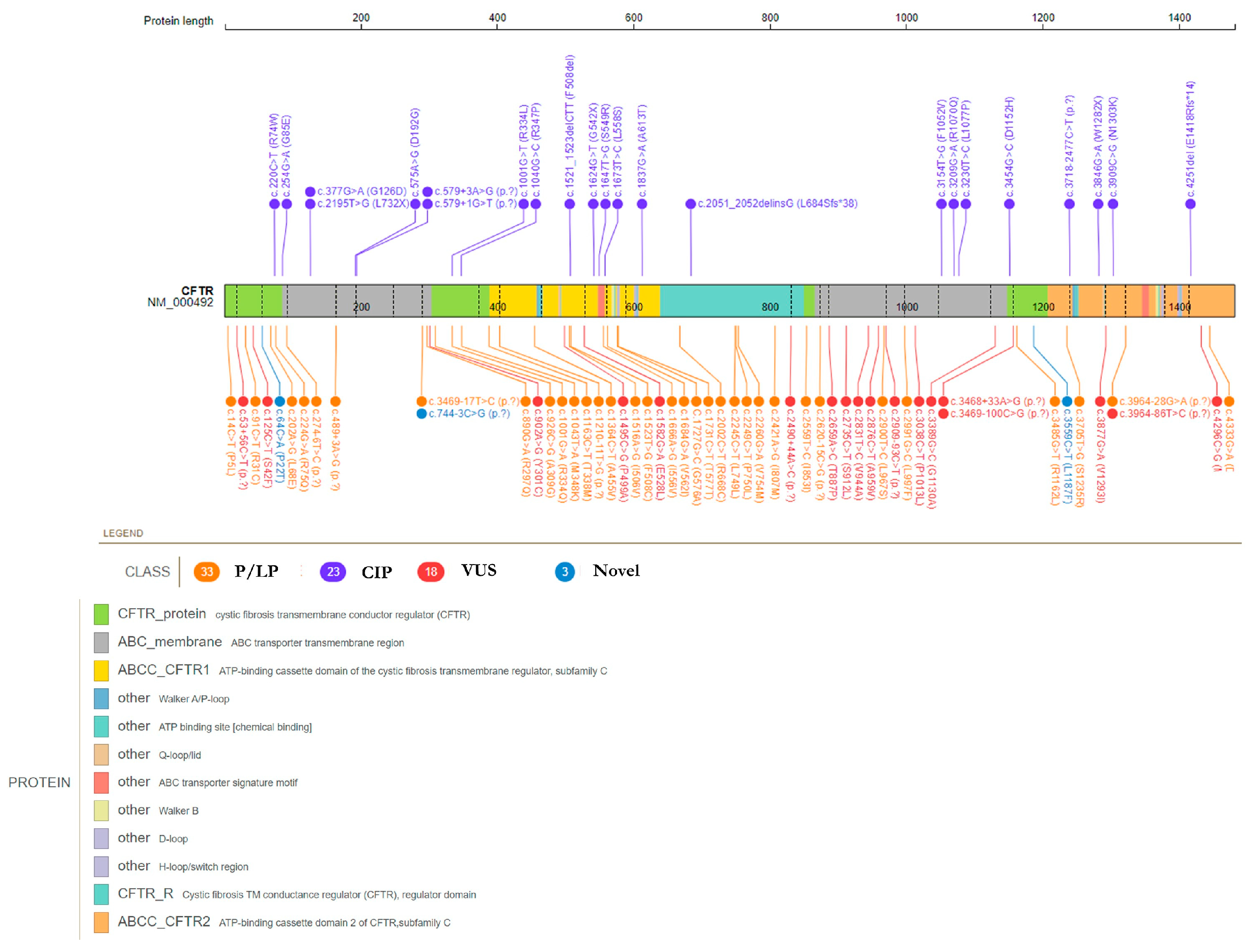
3.1. Overall Description of CFTR Mutational Spectrum
3.2. CFTR Pathogenic/Likely Pathogenic Variants
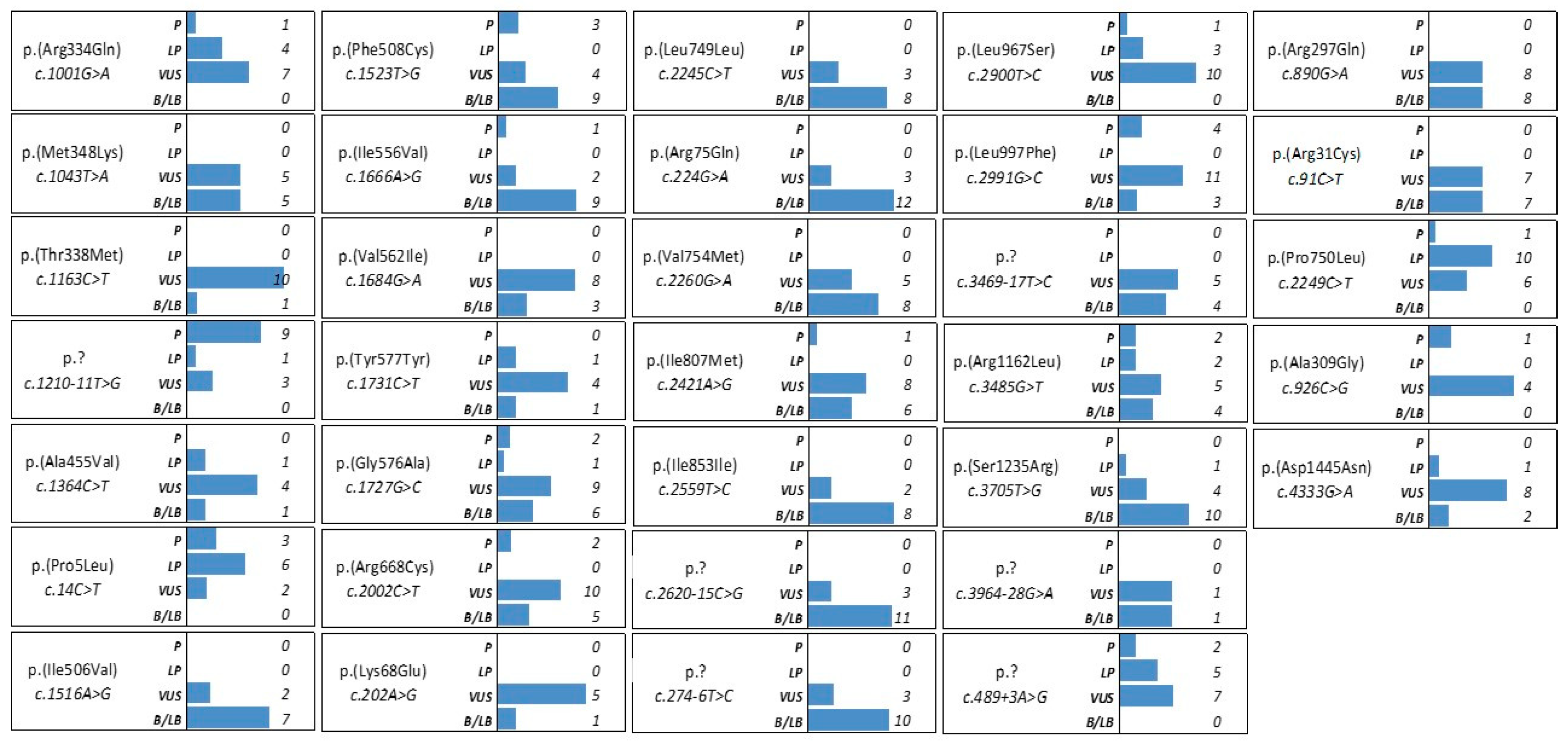
3.3. CFTR Variants with Conflicting Interpretation of Pathogenicity and Variants of Uncertain Significance
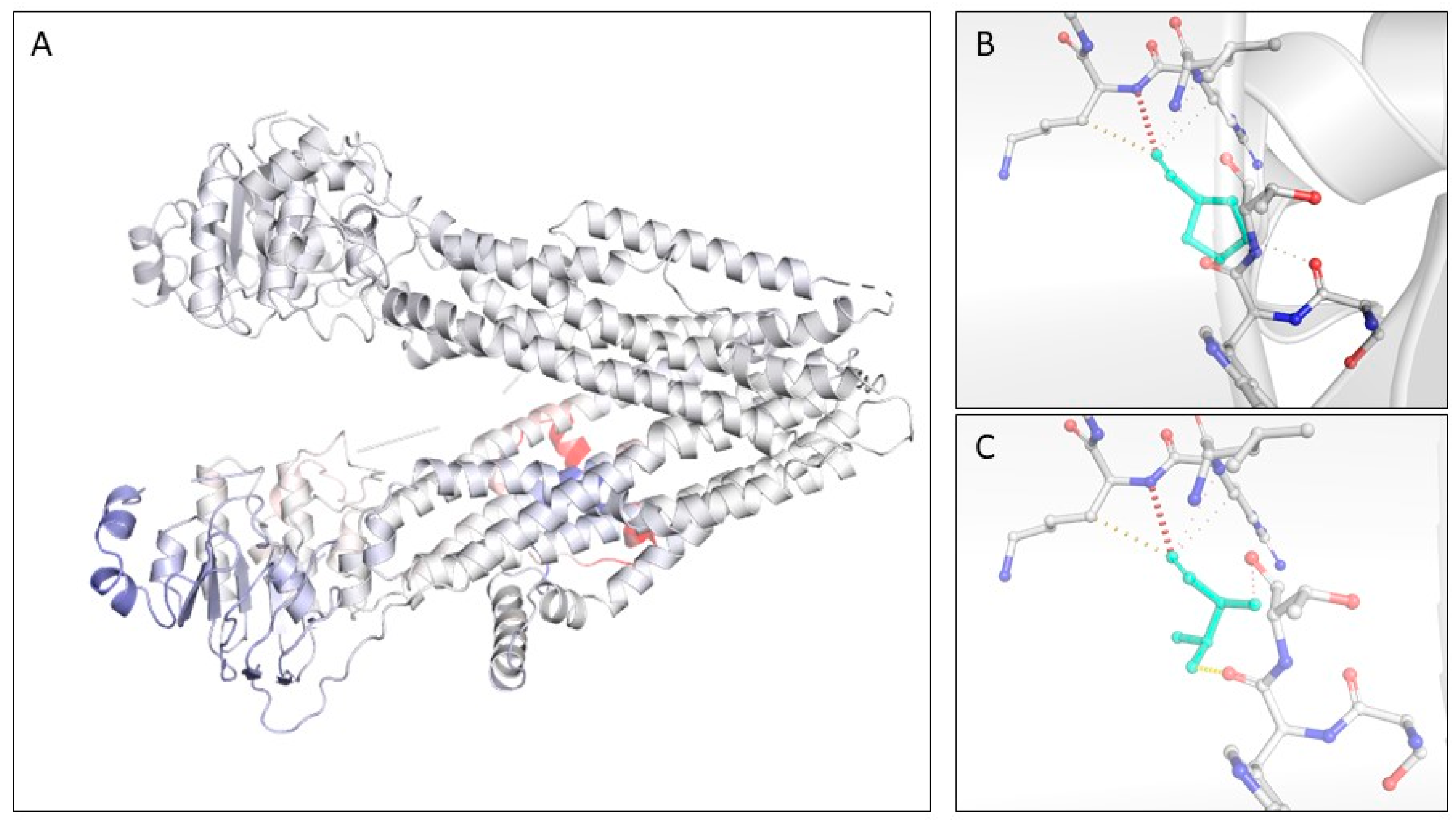
3.4. CFTR Novel Variants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savant, A.; Lyman, B.; Bojanowski, C.; Upadia, J.; Adam, M.P.; Mirzaa, G.M.; Geddes, D. Cystic Fibrosis; Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; GeneReviews®. University of Washington: Seattle, WA, USA, 2001. [Google Scholar]
- Kerem, B.S.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef]
- Hanssens, L.S.; Duchateau, J.; Casimir, G.J. CFTR Protein: Not Just a Chloride Channel? Cells 2021, 10, 2844. [Google Scholar] [CrossRef]
- Trezise, A.E.O. Exquisite and Multilevel Regulation of CFTR Expression; Bush, A., Alton, E.W.F.W., Davies, J.C., Griesenbach, U., Jaffe, A., Eds.; Cystic Fibrosis in the 21st Century, Karger: Basel, Switzerland, 2006. [Google Scholar]
- Vukovic, V.; Agodi, A.; Assael, B.; Calabro’, G.E.; Campanella, P.; Castellani, C.; Coviello, D.; Di Pietro, M.L.; Favaretti, C.; Gurrieri, F.; et al. VP103 Health Technology Assessment Of Genetic Tests For Cystic Fibrosis Carrier Screening In Italy. Int. J. Technol. Assess. Health Care 2017, 33, 197. [Google Scholar] [CrossRef]
- Castellani, C.; Picci, L.; Tridello, G.; Casati, E.; Tamanini, A.; Bartoloni, L.; Scarpa, M.; Assael, B.M. Cystic fibrosis carrier screening effects on birth prevalence and newborn screening. Genet. Med. 2016, 18, 145–151. [Google Scholar] [CrossRef]
- Radhakrishnan, M.; van Gool, K.; Hall, J.; Delatycki, M.; Massie, J. Economic evaluation of cystic fibrosis screening: A review of the literature. Health Policy 2008, 85, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Picci, L.; Cameran, M.; Marangon, O.; Marzenta, D.; Ferrari, S.; Frigo, A.C.; Scarpa, M. A 10-year large-scale cystic fibrosis carrier screening in the Italian population. J. Cyst. Fibros. 2010, 9, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Grody, W.W.; Cutting, G.R.; Watson, M.S. The Cystic Fibrosis mutation “arms race”: When less is more. Genet. Med. 2007, 9, 739–744. [Google Scholar] [CrossRef]
- Norman, R.; van Gool, K.; Hall, J.; Delatycki, M.; Massie, J. Cost-effectiveness of carrier screening for cystic fibrosis in Australia. J. Cyst. Fibros. 2012, 11, 281–287. [Google Scholar] [CrossRef]
- Castellani, C.; Picci, L.; Tamanini, A.; Girardi, P.; Rizzotti, P.; Assael, B.M. Association between carrier screening and incidence of cystic fibrosis. JAMA 2009, 302, 2573–2579. [Google Scholar] [CrossRef]
- Doherty, R.A. National Institutes of Health consensus development conference statement on genetic testing for cystic fibrosis. J. Med. Screen. 1997, 4, 179–180. [Google Scholar] [CrossRef]
- Castellani, C.; Massie, J. Newborn screening and carrier screening for cystic fibrosis: Alternative or complementary? Eur. Respir. J. 2014, 43, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, A.; Taulan-Cadars, M.; Claustres, M.; Raynal, C. Current and future molecular approaches in the diagnosis of cystic fibrosis. Expert Rev. Respir. Med. 2018, 12, 415–426. [Google Scholar] [CrossRef] [PubMed]
- ClinVar, National Cencer for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 15 June 2023).
- Cystic Fibrosis Mutation Database. Available online: http://www.genet.sickkids.on.ca/ (accessed on 15 June 2023).
- CFTR-France Database. Available online: https://cftr.iurc.montp.inserm.fr/cftr/ (accessed on 15 June 2023).
- CFTR 2. Available online: https://cftr2.org/ (accessed on 15 June 2023).
- LOVD v.3.0-Leiden Open Variation Database. Available online: https://databases.lovd.nl/shared/genes/CFTR (accessed on 15 June 2023).
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Kai, W. InterVar: Clinical interpretation of genetic variants by ACMG-AMP 2015 guideline. Am. J. Hum. Genet. 2017, 100, 267–280. [Google Scholar]
- Cystic Fibrosis Missense Analysis. Available online: https://cysma.iurc.montp.inserm.fr/cysma/ (accessed on 15 June 2023).
- Sasorith, S.; Baux, D.; Bergougnoux, A. The CYSMA web server: An example of integrative tool for in silico analysis of missense variants identified in Mendelian disorders. Hum. Mutat. 2020, 41, 375–386. [Google Scholar] [CrossRef] [PubMed]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 15 June 2023).
- SWISS-MODEL. Available online: https://wissmodel.expasy.org/ (accessed on 15 June 2023).
- DynaMut 2. Available online: https://biosig.lab.uq.edu.au/dynamut2/ (accessed on 15 June 2023).
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, B.P. DynaMut2: Assessing changes in stability and flexibility upon single and multiple point missense mutations. Protein Sci. 2021, 30, 60–69. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Poly-Phen2. Available online: http://genetics.bwh.harvard.edu/pph2/dokuwiki/about (accessed on 15 June 2023).
- SIFT. Available online: https://sift.bii.a-star.edu.sg/ (accessed on 15 June 2023).
- Human Splicing Finder. Available online: https://www.genomnis.com/access-hsf (accessed on 15 June 2023).
- MobyDetails. Available online: https://mobidetails.iurc.montp.inserm.fr/MD/ (accessed on 15 June 2023).
- Chamayou, S.; Sicali, M.; Lombardo, D.; Maglia, E.; Liprino, A.; Cardea, C.; Guglielmino, A. The true panel of cystic fibrosis mutations in the Sicilian population. BMC Med. Genet. 2020, 21, 89. [Google Scholar] [CrossRef]
- Bareil, C.; Bergougnoux, A. CFTR gene variants, epidemiology and molecular pathology. Arch. Pediatr. 2020, 27 (Suppl. S1), eS8–eS12. [Google Scholar] [CrossRef]
- Registro Italiano Fibrosi Cistica. Available online: https://www.registroitalianofibrosicistica.it/servizi-36-rapporti_e_pubblicazioni (accessed on 15 June 2023).
- Dell’Edera, D.; Benedetto, M.; Gadaleta, G.; Carone, D.; Salvatore, D.; Angione, A.; Epifania, A.A. Analysis of cystic fibrosis gene mutations in children with cystic fibrosis and in 964 infertile couples within the region of Basilicata, Italy: A research study. J Med. Case. Rep. 2014, 8, 39. [Google Scholar] [CrossRef]
- WHO Human Genetics Programme. The Molecular Genetic Epidemiology of Cystic Fibrosis: Report of a Joint Meeting of WHO/IECFTN/ICF(M)A/ECFS, Genoa, Italy, 19 June 2002. World Health Organization (2004). Available online: https://apps.who.int/iris/handle/10665/68702 (accessed on 15 June 2023).
- Società Italiana per lo Studio della Fibrosi Cistica. Raccomandazioni sul Test del Portatore di Mutazioni del Gene CFTR. Available online: https://www.sifc.it/documento/raccomandazioni-sul-test-del-portatore-di-mutazioni-del-gene-ctfr/ (accessed on 15 June 2023).
- Çolak, Y.; Nordestgaard, B.G.; Afzal, S. Morbidity and mortality in carriers of the cystic fibrosis mutation CFTR Phe508del in the general population. Eur. Respir. J. 2020, 56, 2000558. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.C.; Comellas, A.P.; Hornick, D.B.; Stoltz, D.A.; Cavanaugh, J.E.; Gerke, A.K.; Polgreen, P.M. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc. Natl. Acad. Sci. USA 2020, 117, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Burgel, P.R. Carriers of a single CFTR mutation are asymptomatic: An evolving dogma? Eur. Respir. J. 2020, 56, 2002645. [Google Scholar] [CrossRef] [PubMed]
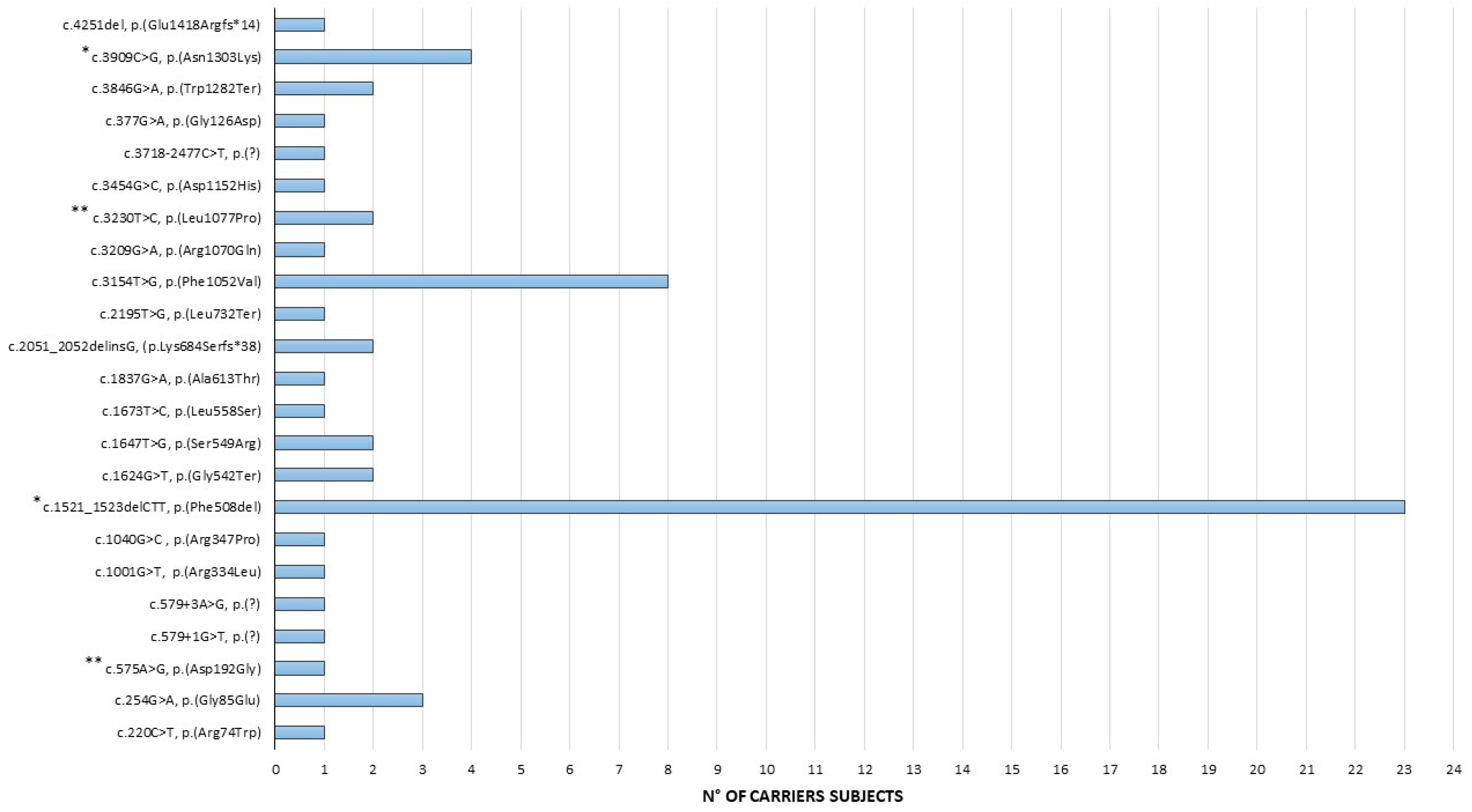
| HGVS cDNA Change | Protein Change | dbSNP |
|---|---|---|
| c.220C>T | p.(Arg74Trp) | rs115545701 |
| c.254G>A | p.(Gly85Glu) | rs75961395 |
| c.377G>A | p.(Gly126Asp) | rs397508609 |
| c.575A>G | p.(Asp192Gly) | rs397508758 |
| c.579+1G>T | p.(?) | rs77188391 |
| c.579+3A>G | p.(?) | rs397508761 |
| c.1001G>T | p.(Arg334Leu) | rs397508137 |
| c.1040G>C | p.(Arg347Pro) | rs77932196 |
| c.1521_1523delCTT | p.(Phe508del) | rs113993960 |
| c.1624G>T | p.(Gly542Ter) | rs113993959 |
| c.1647T>G | p.(Ser549Arg) | rs121909005 |
| c.1673T>C | p.(Leu558Ser) | rs193922504 |
| c.1837G>A | p.(Ala613Thr) | rs201978662 |
| c.2051_2052delinsG | p.(Lys684Serfs*38) | rs121908799 |
| c.2195T>G | p.(Leu732Ter) | rs397508609 |
| c.3154T>G | p.(Phe1052Val) | rs150212784 |
| c.3209G>A | p.(Arg1070Gln) | rs78769542 |
| c.3230T>C | p.(Leu1077Pro) | rs139304906 |
| c.3454G>C | p.(Asp1152His) | rs75541969 |
| c.3718-2477C>T | p.(?) | rs75039782 |
| c.3846G>A | p.(Trp1282Ter) | rs77010898 |
| c.3909C>G | p.(Asn1303Lys) | rs80034486 |
| c.4251del | p.(Glu1418Argfs*14) | rs397508706 |
| HGVS cDNA Change | Protein Change | dbSNP |
|---|---|---|
| c.14C>T | p.(Pro5Leu) | rs193922501 |
| c.91C>T | p.(Arg31Cys) | rs1800073 |
| c.202A>G | p.(Lys68Glu) | rs397508332 |
| c.224G>A | p.(Arg75Gln) | rs1800076 |
| c.274-6T>C | p.(?) | rs371315549 |
| c.489+3A>G | p.(?) | rs377729736 |
| c.890G>A | p.(Arg297Gln) | rs143486492 |
| c.926C>G | p.(Ala309Gly) | rs397508818 |
| c.1001G>A | p.(Arg334Gln) | rs397508137 |
| c.1043T>A | p.(Met348Lys) | rs142920240 |
| c.1163C>T | p.(Thr338Met) | rs143860237 |
| c.1210-11T>G | p.(?) | rs73715573 |
| c.1364C>T | p.(Ala455Val) | rs74551128 |
| c.1516A>G | p.(Ile506Val) | rs1800091 |
| c.1523T>G | p.(Phe508Cys) | rs74571530 |
| c.1666A>G | p.(Ile556Val) | rs75789129 |
| c.1684G>A | p.(Val562Ile) | rs1800097 |
| c.1731C>T | p.(Tyr577=) | rs55928397 |
| c.1727G>C | p.(Gly576Ala) | rs1800098 |
| c.2002C>T | p.(Arg668Cys) | rs1800100 |
| c.2245C>T | p.(Leu749Leu) | rs151235408 |
| c.2249C>T | p.(Pro750Leu) | rs140455771 |
| c.2260G>A | p.(Val754Met) | rs150157202 |
| c.2421A>G | p.(Ile807Met) | rs1800103 |
| c.2559T>C | p.(Ile853Ile) | rs1800104 |
| c.2620-15C>G | p.(?) | rs139379077 |
| c.2900T>C | p.(Leu967Ser) | rs1800110 |
| c.2991G>C | p.(Leu997Phe) | rs1800111 |
| c.3469-17T>C | p.(?) | rs79718042 |
| c.3485G>T | p.(Arg1162Leu) | rs1800120 |
| c.3705T>G | p.(Ser1235Arg) | rs34911792 |
| c.3964-28G>A | p.(?) | rs397508651 |
| c.4333G>A | p.(Asp1445Asn) | rs148783445 |
| HGVS cDNA Change | Protein Change | N° of Carriers | db SNP | CFTR-France a | CFTR2 b | LOVD c | InterVar d | Varsome e |
|---|---|---|---|---|---|---|---|---|
| c.125C>T | p.(Ser42Phe) | 3 | rs143456784 | VUS | n/a | P/VUS | VUS | VUS |
| c.902A>G | p.(Tyr301Cys) | 1 | rs150691494 | VUS | n/a | VUS | VUS | VUS |
| c.1495C>G | p.(Pro499Ala) | 1 | rs397508219 | n/a | n/a | n/a | VUS | LP |
| c.1582G>A | p.(Glu528Lys) | 1 | rs773018372 | n/a | n/a | n/a | VUS | VUS |
| c.2659A>C | p.(Thr887Pro) | 1 | rs770359007 | n/a | n/a | n/a | LB | VUS |
| c.2735C>T | p.(Ser912Leu) | 1 | rs121909034 | VUS | VUS | VUS | B | LB |
| c.2831T>C | p.(Val944Ala) | 1 | rs141747560 | n/a | n/a | n/a | VUS | LP |
| c.2876C>T | p.(Ala959Val) | 1 | rs397508448 | VUS | n/a | n/a | VUS | LP |
| c.3038C>T | p.(Pro1013Leu) | 1 | rs193922516 | VUS | n/a | VUS | VUS | LP |
| c.3389G>C | p.(Gly1130Ala) | 1 | rs397508550 | n/a | n/a | n/a | VUS | LP |
| c.3468+33A>G | p.(?) | 1 | rs1792459342 | n/a | n/a | n/a | n/a | VUS |
| c.3877G>A | p.(Val1293Ile) | 1 | rs769931559 | n/a | n/a | n/a | VUS | LP |
| c.4296C>G | p.(Asn1432Lys) | 1 | rs761669740 | n/a | n/a | n/a | LB | LP |
| c.53+56C>T (IVS1+56C>T) | p.(?) | 1 | rs140393487 | n/a | n/a | n/a | n/a | LB |
| c.2490+44A>C (IVS14+44A>C) | p.(?) | 1 | rs375692108 | n/a | n/a | n/a | n/a | LB |
| c.2909-93C>T (IVS17-93C>T) | p.(?) | 3 | rs144455881 | n/a | n/a | n/a | n/a | LB |
| c.3469-100C>G (IVS21-100C>G) | p.(?) | 2 | rs946757675 | n/a | n/a | n/a | n/a | LB |
| c.3964-86T>C (IVS24-86T>C) | p.(?) | 1 | rs1340773814 | n/a | n/a | n/a | n/a | LB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paolis, E.; Tilocca, B.; Lombardi, C.; De Bonis, M.; Concolino, P.; Onori, M.E.; Ricciardi Tenore, C.; Perrucci, A.; Roncada, P.; Capoluongo, E.; et al. Next-Generation Sequencing for Screening Analysis of Cystic Fibrosis: Spectrum and Novel Variants in a South–Central Italian Cohort. Genes 2023, 14, 1608. https://doi.org/10.3390/genes14081608
De Paolis E, Tilocca B, Lombardi C, De Bonis M, Concolino P, Onori ME, Ricciardi Tenore C, Perrucci A, Roncada P, Capoluongo E, et al. Next-Generation Sequencing for Screening Analysis of Cystic Fibrosis: Spectrum and Novel Variants in a South–Central Italian Cohort. Genes. 2023; 14(8):1608. https://doi.org/10.3390/genes14081608
Chicago/Turabian StyleDe Paolis, Elisa, Bruno Tilocca, Carla Lombardi, Maria De Bonis, Paola Concolino, Maria Elisabetta Onori, Claudio Ricciardi Tenore, Alessia Perrucci, Paola Roncada, Ettore Capoluongo, and et al. 2023. "Next-Generation Sequencing for Screening Analysis of Cystic Fibrosis: Spectrum and Novel Variants in a South–Central Italian Cohort" Genes 14, no. 8: 1608. https://doi.org/10.3390/genes14081608
APA StyleDe Paolis, E., Tilocca, B., Lombardi, C., De Bonis, M., Concolino, P., Onori, M. E., Ricciardi Tenore, C., Perrucci, A., Roncada, P., Capoluongo, E., Urbani, A., Minucci, A., & Santonocito, C. (2023). Next-Generation Sequencing for Screening Analysis of Cystic Fibrosis: Spectrum and Novel Variants in a South–Central Italian Cohort. Genes, 14(8), 1608. https://doi.org/10.3390/genes14081608







