A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases
Abstract
:1. Introduction
1.1. The Evidence for Modifier Genes
| HGNC Gene Symbol | HGNC-Approved Gene Name | Diagnosis | Gene Location | Assumed Inheritance Pattern | Oligogenic/Modifier Genes |
|---|---|---|---|---|---|
| Nephronophthisis | |||||
| NPHP1 [38,39] | Nephrocystin 1 | Nephronophthisis 1; Senior–Løken syndrome 1; Joubert syndrome 4; Bardet–Biedl syndrome. | 2q13 | AR | Homozygous NPHP1 is possibly modified by heterozygous NPHP4 with early-onset ESKD [40]; Heterozygous AHI1 variants are enriched in patients with homozygous NPHP1 and neurological symptoms [41]. |
| NPHP3 [42] | Nephrocystin 3 | Nephronophthisis 3; Senior–Løken syndrome 3; Meckel–Gruber syndrome 7; Renal-hepatic-pancreatic dysplasia 1; Situs inversus; Hepatic fibrosis. | 3q22.1 | AR | Possible digenic inheritance with INVS [40]; A heterozygous NPHP4 variant may modify compound heterozygous NPHP3 with early-onset ESKD and hepatic fibrosis [40,43]; In patients with syndromic nephronophthisis caused by several genes (including NPHP3, IQCB1, CEP290, and MKS1), an additional heterozygous variant in RPGRIP1L is associated with retinitis pigmentosa [44]. |
| CEP290 [45,46] | Centrosomal protein 290 | Nephronophthisis 6; Senior–Løken syndrome 6; Joubert syndrome; Bardet–Biedl syndrome 14; Hepatic fibrosis; Meckel–Gruber syndrome 4. | 12q21.32 | AR | Heterozygous pathogenic variants are present in several cases of homozygous NPHP1 but with no evidence of modifier effect on phenotype [41]; There is a more severe neurological disease in a patient with bi-allelic CEP290 variants and a heterozygous AHI1 [47]; Possible tri-allelic disease with heterozygous TMEM67 and homozygous CEP290 variants in BBS [48]; In patients with syndromic nephronophthisis caused by several genes (including NPHP3, IQCB1, CEP290, and MKS1), an additional heterozygous variant in RPGRIP1L is associated with retinitis pigmentosa [44]; A variant in barttin CLCNK-type accessory subunit beta (BSND) I, significantly associated with kidney disease severity in patients with CEP290 variants [49]. |
| RPGRIP1L [50] | Retinitis pigmentosa; GTPase regulator-interacting protein 1 like protein. | Nephronophthisis 8; Joubert syndrome 7; COACH syndrome; Hepatic fibrosis; Meckel–Gruber syndrome 5. | 16q12.2 | AR | In patients with syndromic nephronophthisis caused by several genes (including NPHP3, IQCB1, CEP290, and MKS1), an additional heterozygous variant in RPGRIP1L is associated with retinitis pigmentosa [44]. |
| TTC21B [51] | Tetratricopeptide repeat domain 21B | Nephronophthisis 12; Joubert syndrome 11; Short-rib thoracic dysplasia; Focal segmental glomerulosclerosis (FSGS). | 2q24.3 | AR, AD | Both causal and a possible modifier of multiple ciliopathy genes. including BBS1, BBS2, BBS4, MKKS, BBS7, BBS10, BBS12, NPHP4, CC2D2A, and TMEM216 [51]; TTC21B is a possible modifier in patients with FSGS and collagen type 4 gene variants (COL4A3 and COL4A5) [52]. |
| INVS [53] | Inversin | Nephronophthisis 2; Situs inversus; Hepatic fibrosis. | 9q31.1 | AR | Possible digenic inheritance with NPHP3 [40]. |
| NPHP4 [54,55] | Nephrocystin 4 | Nephronophthisis 4; Senior–Løken syndrome 4; Hepatic fibrosis. | 1p36.1 | AR | Homozygous NPHP1 possibly modified by heterozygous NPHP4 with early-onset ESKD [40]; Compound heterozygous NPHP3 modified by heterozygous NPHP4 variant with early-onset ESKD and hepatic fibrosis [40,43]; TTC21B contributes possible modifier alleles to NPHP4 [51]. |
| IQCB1 [56] | IQ motif-containing B1 | Nephronophthisis 5; Senior–Løken syndrome 5. | 3q13.33 | AR | Common variants are associated with elevated creatine in association studies [32]; In patients with syndromic nephronophthisis caused by several genes (including NPHP3, IQCB1, CEP290, and MKS1), an additional heterozygous variant in RPGRIP1L is associated with retinitis pigmentosa [44]. |
| GLIS2 [57] | GLIS family zinc finger 2 | Nephronophthisis 7 | 16p13.3 | AR | |
| NEK8 [58] | NIMA-related kinase 8 | Nephronophthisis 9; Renal-hepatic-pancreatic dysplasia 2; Hepatic fibrosis. | 17q11.2 | AR | |
| SDCCAG8 [59] | Serologically defined colon cancer antigen 8 | Nephronophthisis 10; Senior–Løken syndrome 7; Bardet–Biedl syndrome 16; Intellectual disability. | 1q43-q44 | AR | Common variants in SDCCAG8 are associated with elevated creatine in association studies [32]. |
| TMEM67 [60] | Transmembrane protein 67 | Nephronophthisis 11; Joubert syndrome 6; Meckel–Gruber syndrome 3; COACH syndrome; Hepatic fibrosis. | 8q22.1 | AR | Possible tri-allelic disease in BBS contributing heterozygous TMEM67 variants to homozygous truncating variants in CEP290 [48]; Possible tri-allelic disease contributing heterozygous TMEM67 and homozygous BBS9 variants in BBS [48]. |
| WDR19 [61] | WD repeat domain 19 | Nephronophthisis 13; Senior–Løken syndrome 8; Cranio-ectodermal dysplasia 4; Short-rib thoracic dysplasia 5; Hepatic fibrosis. | 4p14 | AR | |
| ZNF423 [62] | Zinc finger protein 423 | Nephronophthisis 14; Joubert syndrome 19; Situs inversus. | 16q12.1 | AR, AD | |
| CEP164 [62] | Centrosomal protein 164 | Nephronophthisis 15; Senior–Løken syndrome; Meckel–Gruber syndrome; Joubert syndrome; Hepatic fibrosis. | 11q23.3 | AR | |
| ANKS6 [63] | Ankyrin repeat and sterile alpha motif domain-containing 6 | Nephronophthisis 16; Situs inversus; Hepatic fibrosis. | 9q22.33 | AR | |
| IFT172 [64] | Intraflagellar transport 172 | Nephronophthisis 17; Bardet–Biedl syndrome; Short-rib thoracic dysplasia 10; Hepatic fibrosis. | 2p23.3 | AR | Common variants in IFT172 are associated with elevated creatine in association studies [32]. |
| CEP83 [65] | Centrosomal protein 83 | Nephronophthisis 18; Intellectual disability; Hepatic fibrosis. | 12q22 | AR | |
| DCDC2 [66] | Doublecortin domain-containing 2 | Nephronophthisis 19; Hepatic fibrosis; Non-syndromic recessive deafness. | 6q22.3 | AR | |
| MAPKBP1 [67] | Mitogen-activated protein kinase binding protein 1 | Nephronophthisis 20 | 15q15.1 | AR | |
| IFT81 [68] | Intraflagellar transport 81 | Nephronophthisis; Short-rib thoracic dysplasia 19. | 12q24.11 | AR | |
| TRAF3IP1 [69] | TRAF3-interacting protein 1 | Nephronophthisis; Senior–Løken syndrome 9; Intellectual disability. | 2q37.3 | AR | |
| ADAMTS9 [70] | ADAM metallopeptidase with thrombospondin type 1 motif 9 | Nephronophthisis | 3p14.1 | AR | |
| INPP5E [71] | Inositol polyphosphate-5-phosphatase E | Nephronophthisis; Joubert syndrome 1; Hepatic fibrosis; Intellectual disability. | 9q34.3 | AR | |
| TMEM216 [72] | Transmembrane protein 216 | Nephronophthisis; Joubert syndrome 2; Meckel–Gruber syndrome 2; Oro-facio-digital syndrome; Intellectual disability. | 11q13.1 | AR | TTC21B contributes possible modifier alleles [51]. |
| AHI1 [73] | Abelson helper-integration site 1 (Jouberin) | Nephronophthisis; Joubert syndrome 3; Intellectual disability. | 6q23.3 | AR | Heterozygous AHI1 variants are enriched in patients with homozygous NPHP1 and neurological symptoms [41]; More severe neurological disease in a patient with bi-allelic CEP290 variants and a heterozygous AHI1 [47]; Heterozygous AHI1 variants are associated with retinal disease irrespective of the underlying bi-allelic cause of nephronophthisis [74]. |
| CC2D2A [75] | Coiled-coil and C2 domain-containing 2A | Nephronophthisis (possible mild); Meckel–Gruber syndrome 6; Joubert syndrome 9; COACH syndrome 2; Hepatic fibrosis; Intellectual disability. | 4p15.32 | AR | TTC21B contributes possible modifier alleles [51]. |
| TMEM237 [76] | Transmembrane protein 237 | Nephronophthisis; Joubert syndrome 14; Meckel–Gruber syndrome. | 2q33.1 | AR | |
| TMEM138 [77] | Transmembrane protein 138 | Nephronophthisis (rare); Joubert syndrome 16; Oro-facio-digital syndrome. | 11q12.2 | AR | |
| TMEM231 [21] | Transmembrane protein 231 | Cystic kidneys; Joubert syndrome 20; Oro-facio-digital syndrome 3; Meckel-Gruber syndrome 11. | 16q23.1 | AR | |
| IFT122 [78] | Intraflagellar transport 122 | Nephronophthisis; Cranio-ectodermal dysplasia 1; Hepatic fibrosis. | 3q21.3-q22.1 | AR | |
| WDR35 [79] | WD repeat domain 35 | Nephronophthisis; Cranio-ectodermal dysplasia 2; Short-rib thoracic dysplasia 7; Hepatic fibrosis. | 2p24.1 | AR | |
| IFT43 [80] | Intraflagellar transport 43 | Nephronophthisis; Cranio-ectodermal dysplasia 3; Short-rib thoracic dysplasia 18; Hepatic fibrosis. | 14q24.3 | AR | |
| ALMS1 [81] | ALMS1 centrosome and basal-body-associated protein | Alström syndrome | 2p13.1 | AR | |
| Autosomal-Dominant Tubulointerstitial Kidney Disease (ADTKD) | |||||
| UMOD [17] | Uromodulin | ADTKD-UMOD | 16p12.3 | AD | Common promoter variants are associated with the risk of CKD and hypertension [31,82,83,84,85,86,87,88]; Bi-allelic variants are more severe [89]. |
| MUC1 [90] | Mucin 1, cell surface-associated | ADTKD-MUC1 | 1q22 | AD | Common splice-site variant increases the risk of CKD [30]. |
| HNF1B [91] | HNF1 homeobox B | ADTKD-HNF1B | 17q12 | AD | HNF1B has a role in transcriptional activation of UMOD, PKHD1, and PKD2 genes [92]. |
| REN [93] | Renin | ADTKD-REN; Renal tubular dysgenesis. | 1q32.1 | AD AR | Bi-allelic variants cause a more severe phenotype resulting in renal tubular dysgenesis [94]. |
| SEC61A1 [95] | SEC61 translocon alpha 1 subunit | ADTKD-SEC61A1 | 3q21.3 | AD | |
| DNAJB11 [18] | DnaJ heat-shock protein family (Hsp40) member B11 | ADTKD/ADPKD “hybrid”; Ivermark II syndrome–renal-hepatic-pancreatic dysplasia (RHPD) | 3q27.3 | AD AR | Bi-allelic variants cause a more severe phenotype with a foetal disease, including enlarged cystic kidneys, dilation and proliferation of pancreatic duct cells, and liver ductal plate malformation [96]. |
| Mitochondrial disorders | |||||
| MT-TF [13] | Mitochondrially encoded tRNA-Phe (UUU/C) | Mitochondrially inherited tubulointerstitial kidney disease (MITKD) | Mitochondria | Mitochondria | |
| MT-TL1 [97] | Mitochondrially encoded tRNA-Leu (UUA/G) 1 | Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS); Maternally inherited diabetes and deafness (MIDD) syndromes. | Mitochondria | Mitochondria | A possible modifying variant in tRNAlys in a family with m.3243A > G and MIDD rather than MELAS, the tRNAlys variant was absent in 75 controls [98]. |
| Renal tubular dysgenesis | |||||
| AGT [94] | Angiotensinogen | Renal tubular dysgenesis | 1q42.2 | AR | |
| AGTR1 [94] | Angiotensin II receptor type 1 | Renal tubular dysgenesis | 3q24 | AR | |
| ACE [94] | Angiotensin I converting enzyme | Renal tubular dysgenesis | 17q23.3 | AR | |
| Other | |||||
| XPNPEP3 [14] | X-prolyl aminopeptidase 3 | Nephronophthisis-like nephropathy 1 (NPHPL1) | 22q13.2 | AR | |
| GATM [15] | Glycine amidinotransferase | Fanconi syndrome and IFTA | 15q21.1 | AD | |
| SLC41A1 [99] | Solute carrier family 41 member 1 | Nephronophthisis-like nephropathy | 1q32.1 | AR | |
| FAN1 [100] | FANCD2- and FANCI-associated nuclease 1 | Karyomegalic interstitial nephritis | 15q13.3 | AR | |
1.1.1. NPHP1
1.1.2. NPHP3
1.1.3. CEP290
1.1.4. RGRIP1L
1.1.5. TTC21B
1.1.6. UMOD
1.1.7. MUC1
1.1.8. HNF1B
1.1.9. Mitochondrial Function
1.2. Evidence for Oligogenic Inheritance
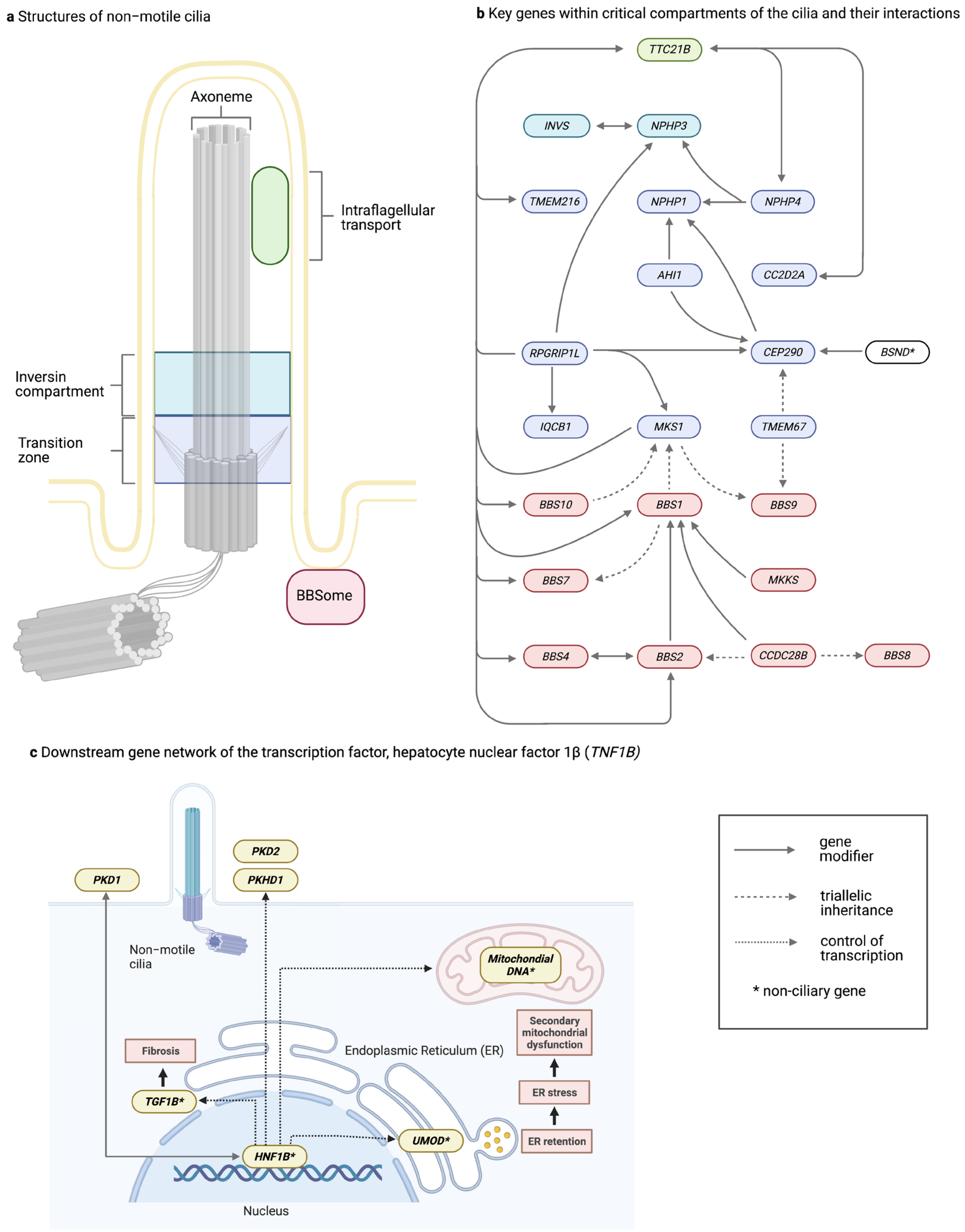
2. Discussion
3. Key Points
- Tubulointerstitial kidney disease may present with a severe syndromic phenotype traditionally diagnosed in childhood, yet most TKD patients manifest a subtle phenotype with little to differentiate them from other common forms of non-proteinuric chronic kidney disease.
- Phenotypes may vary even within families that share the same putative monogenic gene variant and shared environmental factors.
- Common variants in monogenic TKD genes are also associated with chronic kidney disease at the population level.
- There is growing evidence for tri-allelic inheritance as well as for rare modifiers of severe effect and common modifiers of moderate effect on patient phenotypes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleyer, A.J.; Kmoch, S.; Antignac, C.; Robins, V.; Kidd, K.; Kelsoe, J.R.; Hladik, G.; Klemmer, P.; Knohl, S.J.; Scheinman, S.J.; et al. Variable Clinical Presentation of an MUC1 Mutation Causing Medullary Cystic Kidney Disease Type 1. Clin. J. Am. Soc. Nephrol. 2014, 9, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollée, G.; Dahan, K.; Flamant, M.; Morinière, V.; Pawtowski, A.; Heidet, L.; Lacombe, D.; Devuyst, O.; Pirson, Y.; Antignac, C.; et al. Phenotype and Outcome in Hereditary Tubulointerstitial Nephritis Secondary to UMOD Mutations. Clin. J. Am. Soc. Nephrol. 2011, 6, 2429–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekici, A.B.; Hackenbeck, T.; Morinière, V.; Pannes, A.; Buettner, M.; Uebe, S.; Janka, R.; Wiesener, A.; Hermann, I.; Grupp, S.; et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int. 2014, 86, 589–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, H.; Zauner, I.; Strahm, B.; Bender, B.; Schollmeyer, P.; Blum, U.; Rohrbach, R.; Hildebrandt, F. Concise clinical report. Late occurrence of cysts in autosomal dominant medullary cystic kidney disease. Nephrol. Dial. Transplant. 1997, 12, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskowitz, J.L.; Piret, S.E.; Lhotta, K.; Kitzler, T.M.; Tashman, A.P.; Velez, E.; Thakker, R.V.; Kotanko, P. Association between Genotype and Phenotype in Uromodulin-Associated Kidney Disease. Clin. J. Am. Soc. Nephrol. 2013, 8, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Eckardt, K.-U.; Alper, S.L.; Antignac, C.; Bleyer, A.J.; Chauveau, D.; Dahan, K.; Deltas, C.; Hosking, A.; Kmoch, S.; Rampoldi, L.; et al. Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management—A KDIGO consensus report. Kidney Int. 2015, 88, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.T.F.; Hildebrandt, F. Nephronophthisis. Pediatr. Nephrol. 2011, 26, 181–194. [Google Scholar] [CrossRef]
- Wolf, M.T.F. Nephronophthisis and related syndromes. Curr. Opin. Pediatr. 2015, 27, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, F.; Zhou, W. Nephronophthisis-Associated Ciliopathies. J. Am. Soc. Nephrol. 2007, 18, 1855–1871. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Kranz, B.; König, S.; Schlingmann, K.P.; Titieni, A.; Tönshoff, B.; Habbig, S.; Pape, L.; Häffner, K.; Hansen, M.; et al. Phenotypic Spectrum of Children with Nephronophthisis and Related Ciliopathies. Clin. J. Am. Soc. Nephrol. 2017, 12, 1974–1983. [Google Scholar] [CrossRef] [Green Version]
- Alzarka, B.; Morizono, H.; Bollman, J.W.; Kim, D.; Guay-Woodford, L.M. Design and Implementation of the Hepatorenal Fibrocystic Disease Core Center Clinical Database: A Centralized Resource for Characterizing Autosomal Recessive Polycystic Kidney Disease and Other Hepatorenal Fibrocystic Diseases. Front. Pediatr. 2017, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Guay-Woodford, L. Autosomal recessive polycystic kidney disease: The prototype of the hepato-renal fibrocystic diseases. J. Pediatr. Genet. 2015, 3, 089–101. [Google Scholar] [CrossRef]
- Connor, T.M.; Hoer, S.; Mallett, A.; Gale, D.P.; Gomez-Duran, A.; Posse, V.; Antrobus, R.; Moreno, P.; Sciacovelli, M.; Frezza, C.; et al. Mutations in mitochondrial DNA causing tubulointerstitial kidney disease. PLOS Genet. 2017, 13, e1006620. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, J.F.; Liu, Y.; Davis, E.E.; Westlake, C.J.; Attanasio, M.; Otto, E.A.; Seelow, D.; Nurnberg, G.; Becker, C.; Nuutinen, M.; et al. Individuals with mutations in XPNPEP3, which encodes a mitochondrial protein, develop a nephronophthisis-like nephropathy. J. Clin. Investig. 2010, 120, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Reichold, M.; Klootwijk, E.D.; Reinders, J.; Otto, E.A.; Milani, M.; Broeker, C.; Laing, C.; Wiesner, J.; Devi, S.; Zhou, W.; et al. Glycine Amidinotransferase (GATM), Renal Fanconi Syndrome, and Kidney Failure. J. Am. Soc. Nephrol. 2018, 29, 1849–1858. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, F.; Attanasio, M.; Otto, E. Nephronophthisis: Disease Mechanisms of a Ciliopathy. J. Am. Soc. Nephrol. 2009, 20, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, T.C. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 2002, 39, 882–892. [Google Scholar] [CrossRef] [Green Version]
- Cornec-Le Gall, E.; Olson, R.J.; Besse, W.; Heyer, C.M.; Gainullin, V.G.; Smith, J.M.; Audrézet, M.-P.; Hopp, K.; Porath, B.; Shi, B.; et al. Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am. J. Hum. Genet. 2018, 102, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Srour, M.; Hamdan, F.F.; Schwartzentruber, J.A.; Patry, L.; Ospina, L.H.; Shevell, M.I.; Désilets, V.; Dobrzeniecka, S.; Mathonnet, G.; Lemyre, E.; et al. Mutations in TMEM231 cause Joubert syndrome in French Canadians. J. Med. Genet. 2012, 49, 636–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, R.; Ansari, S.; Mardawi, E.A.; Alshammari, M.J.; Alkuraya, F.S. Mutations in TMEM231 cause Meckel–Gruber syndrome. J. Med. Genet. 2013, 50, 160–162. [Google Scholar] [CrossRef] [Green Version]
- Roberson, E.C.; Dowdle, W.E.; Ozanturk, A.; Garcia-Gonzalo, F.R.; Li, C.; Halbritter, J.; Elkhartoufi, N.; Porath, J.D.; Cope, H.; Ashley-Koch, A.; et al. TMEM231, mutated in orofaciodigital and Meckel syndromes, organizes the ciliary transition zone. J. Cell Biol. 2015, 209, 129–142. [Google Scholar] [CrossRef]
- Havrilla, J.M.; Pedersen, B.S.; Layer, R.M.; Quinlan, A.R. A map of constrained coding regions in the human genome. Nat. Genet. 2019, 51, 88–95. [Google Scholar] [CrossRef]
- Walia, S. Discordant Phenotypes in Fraternal Twins Having an Identical Mutation in Exon ORF15 of the RPGR Gene. Arch. Ophthalmol. 2008, 126, 379. [Google Scholar] [CrossRef] [Green Version]
- Zaki, M.S.; Sattar, S.; Massoudi, R.A.; Gleeson, J.G. Co-occurrence of distinct ciliopathy diseases in single families suggests genetic modifiers. Am. J. Med. Genet. Part A 2011, 155, 3042–3049. [Google Scholar] [CrossRef]
- Maglic, D.; Stephen, J.; Malicdan, M.C.V.; Guo, J.; Fischer, R.; Konzman, D.; Program, N.C.S.; Mullikin, J.C.; Gahl, W.A.; Vilboux, T.; et al. TMEM231 Gene Conversion Associated with Joubert and Meckel-Gruber Syndromes in the Same Family: HUMAN MUTATION. Hum. Mutat. 2016, 37, 1144–1148. [Google Scholar] [CrossRef]
- Kidd, K.; Vylet’al, P.; Schaeffer, C.; Olinger, E.; Živná, M.; Hodaňová, K.; Robins, V.; Johnson, E.; Taylor, A.; Martin, L.; et al. Genetic and Clinical Predictors of Age of ESKD in Individuals With Autosomal Dominant Tubulointerstitial Kidney Disease Due to UMOD Mutations. Kidney Int. Rep. 2020, 5, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Hopp, K.; Cornec-Le Gall, E.; Senum, S.R.; Te Paske, I.B.A.W.; Raj, S.; Lavu, S.; Baheti, S.; Edwards, M.E.; Madsen, C.D.; Heyer, C.M.; et al. Detection and characterization of mosaicism in autosomal dominant polycystic kidney disease. Kidney Int. 2020, 97, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Wang, M.; Homburger, J.R.; Patel, A.P.; Bick, A.G.; Neben, C.L.; Lai, C.; Brockman, D.; Philippakis, A.; Ellinor, P.T.; et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat. Commun. 2020, 11, 3635. [Google Scholar] [CrossRef]
- Xu, X.; Eales, J.M.; Akbarov, A.; Guo, H.; Becker, L.; Talavera, D.; Ashraf, F.; Nawaz, J.; Pramanik, S.; Bowes, J.; et al. Molecular insights into genome-wide association studies of chronic kidney disease-defining traits. Nat. Commun. 2018, 9, 4800. [Google Scholar] [CrossRef]
- Trudu, M.; Janas, S.; Lanzani, C.; Debaix, H.; Schaeffer, C.; Ikehata, M.; Citterio, L.; Demaretz, S.; Trevisani, F.; Ristagno, G.; et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat. Med. 2013, 19, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Drivas, T.G.; Lucas, A.; Zhang, X.; Ritchie, M.D. Mendelian pathway analysis of laboratory traits reveals distinct roles for ciliary subcompartments in common disease pathogenesis. Am. J. Hum. Genet. 2021, 108, 482–501. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Solomonson, M.; Chao, K.R.; Goodrich, J.K.; Tiao, G.; Lu, W.; Riley-Gillis, B.M.; Tsai, E.A.; Kim, H.I.; Zheng, X.; et al. Systematic single-variant and gene-based association testing of thousands of phenotypes in 394,841 UK Biobank exomes. Cell Genom. 2022, 2, 100168. [Google Scholar] [CrossRef]
- Génin, E.; Feingold, J.; Clerget-Darpoux, F. Identifying modifier genes of monogenic disease: Strategies and difficulties. Hum. Genet. 2008, 124, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kousi, M.; Katsanis, N. Genetic Modifiers and Oligogenic Inheritance. Cold Spring Harb. Perspect. Med. 2015, 5, a017145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeau, J.H. Modifier genes in mice and humans. Nat. Rev. Genet. 2001, 2, 165–174. [Google Scholar] [CrossRef]
- Norman, C.S.; O’Gorman, L.; Gibson, J.; Pengelly, R.J.; Baralle, D.; Ratnayaka, J.A.; Griffiths, H.; Rose-Zerilli, M.; Ranger, M.; Bunyan, D.; et al. Identification of a functionally significant tri-allelic genotype in the Tyrosinase gene (TYR) causing hypomorphic oculocutaneous albinism (OCA1B). Sci. Rep. 2017, 7, 4415. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, F.; Otto, E.; Rensing, C.; Nothwang, H.G.; Vollmer, M.; Adolphs, J.; Hanusch, H.; Brandis, M. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat. Genet. 1997, 17, 149–153. [Google Scholar] [CrossRef]
- Saunier, S. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum. Mol. Genet. 1997, 6, 2317–2323. [Google Scholar] [CrossRef] [Green Version]
- Hoefele, J.; Wolf, M.T.F.; O’Toole, J.F.; Otto, E.A.; Schultheiss, U.; Deschenes, G.; Attanasio, M.; Utsch, B.; Antignac, C.; Hildebrandt, F. Evidence of Oligogenic Inheritance in Nephronophthisis. J. Am. Soc. Nephrol. 2007, 18, 2789–2795. [Google Scholar] [CrossRef] [Green Version]
- Tory, K.C.A.A.l.C.A.A.n.; Lacoste, T.; Burglen, L.; Moriniere, V.; Boddaert, N.; Macher, M.-A.; Llanas, B.; Nivet, H.; Bensman, A.; Niaudet, P.; et al. High NPHP1 and NPHP6 Mutation Rate in Patients with Joubert Syndrome and Nephronophthisis: Potential Epistatic Effect of NPHP6 and AHI1 Mutations in Patients with NPHP1 Mutations. J. Am. Soc. Nephrol. 2007, 18, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Olbrich, H.; Fliegauf, M.; Hoefele, J.; Kispert, A.; Otto, E.; Volz, A.; Wolf, M.T.; Sasmaz, G.; Trauer, U.; Reinhardt, R.; et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat. Genet. 2003, 34, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Penchev, V.; Boueva, A.; Kamenarova, K.; Roussinov, D.; Tzveova, R.; Ivanova, M.; Dimitrova, V.; Kremensky, I.; Mitev, V.; Kaneva, R.; et al. A familial case of severe infantile nephronophthisis explained by oligogenic inheritance. Eur. J. Med. Genet. 2017, 60, 321–325. [Google Scholar] [CrossRef]
- Khanna, H.; Davis, E.E.; Murga-Zamalloa, C.A.; Estrada-Cuzcano, A.; Lopez, I.; Den Hollander, A.I.; Zonneveld, M.N.; Othman, M.I.; Waseem, N.; Chakarova, C.F.; et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat. Genet. 2009, 41, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Sayer, J.A.; Otto, E.A.; O’Toole, J.F.; Nurnberg, G.; Kennedy, M.A.; Becker, C.; Hennies, H.C.; Helou, J.; Attanasio, M.; Fausett, B.V.; et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 2006, 38, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.M.; Silhavy, J.L.; Brancati, F.; Barrano, G.; Krishnaswami, S.R.; Castori, M.; Lancaster, M.A.; Boltshauser, E.; Boccone, L.; Al-Gazali, L.; et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 2006, 38, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, F.; Casteels, I.; Meire, F.; De Jaegere, S.; Hooghe, S.; Van Regemorter, N.; Van Esch, H.; Matulevičienė, A.; Nunes, L.; Meersschaut, V.; et al. Genetic screening of LCA in Belgium: Predominance of CEP290 and identification of potential modifier alleles in AHI1 of CEP290-related phenotypes. Hum. Mutat. 2010, 31, E1709–E1766. [Google Scholar] [CrossRef] [Green Version]
- Leitch, C.C.; Zaghloul, N.A.; Davis, E.E.; Stoetzel, C.; Diaz-Font, A.; Rix, S.; Alfadhel, M.; Lewis, R.A.; Eyaid, W.; Banin, E.; et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 2008, 40, 443–448. [Google Scholar] [CrossRef]
- Ramsbottom, S.A.; Thelwall, P.E.; Wood, K.M.; Clowry, G.J.; Devlin, L.A.; Silbermann, F.; Spiewak, H.L.; Shril, S.; Molinari, E.; Hildebrandt, F.; et al. Mouse genetics reveals Barttin as a genetic modifier of Joubert syndrome. Proc. Natl. Acad. Sci. 2020, 117, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delous, M.; Baala, L.; Salomon, R.; Laclef, C.; Vierkotten, J.; Tory, K.; Golzio, C.; Lacoste, T.; Besse, L.; Ozilou, C.; et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat. Genet. 2007, 39, 875–881. [Google Scholar] [CrossRef]
- Program, N.C.S.; Davis, E.E.; Zhang, Q.; Liu, Q.; Diplas, B.H.; Davey, L.M.; Hartley, J.; Stoetzel, C.; Szymanska, K.; Ramaswami, G.; et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 2011, 43, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Bullich, G.; Vargas, I.; Trujillano, D.; Mendizábal, S.; Piñero-Fernández, J.A.; Fraga, G.; García-Solano, J.; Ballarín, J.; Estivill, X.; Torra, R.; et al. Contribution of the TTC21B gene to glomerular and cystic kidney diseases. Nephrol. Dial. Transplant. 2016, 32, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, E.A.; Schermer, B.; Obara, T.; O’Toole, J.F.; Hiller, K.S.; Mueller, A.M.; Ruf, R.G.; Hoefele, J.; Beekmann, F.; Landau, D.; et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003, 34, 413–420. [Google Scholar] [CrossRef]
- Mollet, G.; Salomon, R.; Gribouval, O.; Silbermann, F.; Bacq, D.; Landthaler, G.; Milford, D.; Nayir, A.; Rizzoni, G.; Antignac, C.; et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat. Genet. 2002, 32, 300–305. [Google Scholar] [CrossRef]
- Otto, E.; Hoefele, J.; Ruf, R.; Mueller, A.M.; Hiller, K.S.; Wolf, M.T.F.; Schuermann, M.J.; Becker, A.; Birkenhäger, R.; Sudbrak, R.; et al. A Gene Mutated in Nephronophthisis and Retinitis Pigmentosa Encodes a Novel Protein, Nephroretinin, Conserved in Evolution. Am. J. Hum. Genet. 2002, 71, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, E.A.; Loeys, B.; Khanna, H.; Hellemans, J.; Sudbrak, R.; Fan, S.; Muerb, U.; O’Toole, J.F.; Helou, J.; Attanasio, M.; et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat. Genet. 2005, 37, 282–288. [Google Scholar] [CrossRef]
- Attanasio, M.; Uhlenhaut, N.H.; Sousa, V.H.; O’Toole, J.F.; Otto, E.; Anlag, K.; Klugmann, C.; Treier, A.-C.; Helou, J.; Sayer, J.A.; et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat. Genet. 2007, 39, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Otto, E.A.; Trapp, M.L.; Schultheiss, U.T.; Helou, J.; Quarmby, L.M.; Hildebrandt, F. NEK8 Mutations Affect Ciliary and Centrosomal Localization and May Cause Nephronophthisis. J. Am. Soc. Nephrol. 2008, 19, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Otto, E.A.; Hurd, T.W.; Airik, R.; Chaki, M.; Zhou, W.; Stoetzel, C.; Patil, S.B.; Levy, S.; Ghosh, A.K.; Murga-Zamalloa, C.A.; et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010, 42, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Otto, E.A.; Tory, K.; Attanasio, M.; Zhou, W.; Chaki, M.; Paruchuri, Y.; Wise, E.L.; Wolf, M.T.F.; Utsch, B.; Becker, C.; et al. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11). J. Med. Genet. 2009, 46, 663–670. [Google Scholar] [CrossRef]
- Bredrup, C.; Saunier, S.; Oud, M.M.; Fiskerstrand, T.; Hoischen, A.; Brackman, D.; Leh, S.M.; Midtbø, M.; Filhol, E.; Bole-Feysot, C.; et al. Ciliopathies with Skeletal Anomalies and Renal Insufficiency due to Mutations in the IFT-A Gene WDR19. Am. J. Hum. Genet. 2011, 89, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaki, M.; Airik, R.; Ghosh, A.K.; Giles, R.H.; Chen, R.; Slaats, G.G.; Wang, H.; Hurd, T.W.; Zhou, W.; Cluckey, A.; et al. Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling. Cell 2012, 150, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Hoff, S.; Halbritter, J.; Epting, D.; Frank, V.; Nguyen, T.-M.T.; Van Reeuwijk, J.; Boehlke, C.; Schell, C.; Yasunaga, T.; Helmstädter, M.; et al. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat. Genet. 2013, 45, 951–956. [Google Scholar] [CrossRef]
- Halbritter, J.; Bizet, A.A.; Schmidts, M.; Porath, J.D.; Braun, D.A.; Gee, H.Y.; McInerney-Leo, A.M.; Krug, P.; Filhol, E.; Davis, E.E.; et al. Defects in the IFT-B Component IFT172 Cause Jeune and Mainzer-Saldino Syndromes in Humans. Am. J. Hum. Genet. 2013, 93, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Failler, M.; Gee, H.Y.; Krug, P.; Joo, K.; Halbritter, J.; Belkacem, L.; Filhol, E.; Porath, J.D.; Braun, D.A.; Schueler, M.; et al. Mutations of CEP83 Cause Infantile Nephronophthisis and Intellectual Disability. Am. J. Hum. Genet. 2014, 94, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schueler, M.; Braun, D.A.; Chandrasekar, G.; Gee, H.Y.; Klasson, T.D.; Halbritter, J.; Bieder, A.; Porath, J.D.; Airik, R.; Zhou, W.; et al. DCDC2 Mutations Cause a Renal-Hepatic Ciliopathy by Disrupting Wnt Signaling. Am. J. Hum. Genet. 2015, 96, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Macia, M.S.; Halbritter, J.; Delous, M.; Bredrup, C.; Gutter, A.; Filhol, E.; Mellgren, A.E.C.; Leh, S.; Bizet, A.; Braun, D.A.; et al. Mutations in MAPKBP1 Cause Juvenile or Late-Onset Cilia-Independent Nephronophthisis. Am. J. Hum. Genet. 2017, 100, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Perrault, I.; Halbritter, J.; Porath, J.D.; Gérard, X.; Braun, D.A.; Gee, H.Y.; Fathy, H.M.; Saunier, S.; Cormier-Daire, V.; Thomas, S.; et al. IFT81, encoding an IFT-B core protein, as a very rare cause of a ciliopathy phenotype. J. Med. Genet. 2015, 52, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Bizet, A.A.; Becker-Heck, A.; Ryan, R.; Weber, K.; Filhol, E.; Krug, P.; Halbritter, J.; Delous, M.; Lasbennes, M.-C.; Linghu, B.; et al. Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat. Commun. 2015, 6, 8666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Halbritter, J.; Braun, D.A.; Schueler, M.; Schapiro, D.; Rim, J.H.; Nandadasa, S.; Choi, W.-i.; Widmeier, E.; Shril, S.; et al. Mutations of ADAMTS9 Cause Nephronophthisis-Related Ciliopathy. Am. J. Hum. Genet. 2019, 104, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Valente, E.M.; Logan, C.V.; Mougou-Zerelli, S.; Lee, J.H.; Silhavy, J.L.; Brancati, F.; Iannicelli, M.; Travaglini, L.; Romani, S.; Illi, B.; et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 2010, 42, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Ferland, R.J.; Eyaid, W.; Collura, R.V.; Tully, L.D.; Hill, R.S.; Al-Nouri, D.; Al-Rumayyan, A.; Topcu, M.; Gascon, G.; Bodell, A.; et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 2004, 36, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Louie, C.M.; Caridi, G.; Lopes, V.S.; Brancati, F.; Kispert, A.; Lancaster, M.A.; Schlossman, A.M.; Otto, E.A.; Leitges, M.; Gröne, H.-J.; et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat. Genet. 2010, 42, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gorden, N.T.; Arts, H.H.; Parisi, M.A.; Coene, K.L.M.; Letteboer, S.J.F.; Van Beersum, S.E.C.; Mans, D.A.; Hikida, A.; Eckert, M.; Knutzen, D.; et al. CC2D2A Is Mutated in Joubert Syndrome and Interacts with the Ciliopathy-Associated Basal Body Protein CEP290. Am. J. Hum. Genet. 2008, 83, 559–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Szymanska, K.; Jensen, V.L.; Janecke, A.R.; Innes, A.M.; Davis, E.E.; Frosk, P.; Li, C.; Willer, J.R.; Chodirker, B.N.; et al. TMEM237 Is Mutated in Individuals with a Joubert Syndrome Related Disorder and Expands the Role of the TMEM Family at the Ciliary Transition Zone. Am. J. Hum. Genet. 2011, 89, 713–730. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Silhavy, J.L.; Lee, J.E.; Al-Gazali, L.; Thomas, S.; Davis, E.E.; Bielas, S.L.; Hill, K.J.; Iannicelli, M.; Brancati, F.; et al. Evolutionarily Assembled cis-Regulatory Module at a Human Ciliopathy Locus. Science 2012, 335, 966–969. [Google Scholar] [CrossRef] [Green Version]
- Alazami, A.M.; Seidahmed, M.Z.; Alzahrani, F.; Mohammed, A.O.; Alkuraya, F.S. Novel IFT122 mutation associated with impaired ciliogenesis and cranioectodermal dysplasia. Mol. Genet. Genom. Med. 2014, 2, 103–106. [Google Scholar] [CrossRef]
- Gilissen, C.; Arts, H.H.; Hoischen, A.; Spruijt, L.; Mans, D.A.; Arts, P.; Van Lier, B.; Steehouwer, M.; Van Reeuwijk, J.; Kant, S.G.; et al. Exome Sequencing Identifies WDR35 Variants Involved in Sensenbrenner Syndrome. Am. J. Hum. Genet. 2010, 87, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Arts, H.H.; Bongers, E.M.H.F.; Mans, D.A.; Van Beersum, S.E.C.; Oud, M.M.; Bolat, E.; Spruijt, L.; Cornelissen, E.A.M.; Schuurs-Hoeijmakers, J.H.M.; De Leeuw, N.; et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J. Med. Genet. 2011, 48, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Hearn, T.; Renforth, G.L.; Spalluto, C.; Hanley, N.A.; Piper, K.; Brickwood, S.; White, C.; Connolly, V.; Taylor, J.F.N.; Russell-Eggitt, I.; et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat. Genet. 2002, 31, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.-J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B.; et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böger, C.A.; Gorski, M.; Li, M.; Hoffmann, M.M.; Huang, C.; Yang, Q.; Teumer, A.; Krane, V.; O’Seaghdha, C.M.; Kutalik, Z.; et al. Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD. PLoS Genet. 2011, 7, e1002292. [Google Scholar] [CrossRef] [Green Version]
- Gudbjartsson, D.F.; Holm, H.; Indridason, O.S.; Thorleifsson, G.; Edvardsson, V.; Sulem, P.; De Vegt, F.; d’Ancona, F.C.H.; Den Heijer, M.; Franzson, L.; et al. Association of Variants at UMOD with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases. PLoS Genet. 2010, 6, e1001039. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Melander, O.; Johnson, T.; Di Blasio, A.M.; Lee, W.K.; Gentilini, D.; Hastie, C.E.; Menni, C.; Monti, M.C.; Delles, C.; et al. Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near UMOD Associated with Hypertension. PLoS Genet. 2010, 6, e1001177. [Google Scholar] [CrossRef] [PubMed]
- Ghirotto, S.; Tassi, F.; Barbujani, G.; Pattini, L.; Hayward, C.; Vollenweider, P.; Bochud, M.; Rampoldi, L.; Devuyst, O. The Uromodulin Gene Locus Shows Evidence of Pathogen Adaptation through Human Evolution. J. Am. Soc. Nephrol. 2016, 27, 2983–2996. [Google Scholar] [CrossRef]
- Köttgen, A.; Hwang, S.-J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.H.L.; Harris, T.B.; et al. Uromodulin Levels Associate with a Common UMOD Variant and Risk for Incident CKD. J. Am. Soc. Nephrol. 2010, 21, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Devuyst, O.; Pattaro, C. The UMOD Locus: Insights into the Pathogenesis and Prognosis of Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Rezende-Lima, W.; Parreira, K.S.; García-González, M.; Riveira, E.; Banet, J.F.; Lens, X.M. Homozygosity for uromodulin disorders: FJHN and MCKD-type 2. Kidney Int. 2004, 66, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Kirby, A.; Gnirke, A.; Jaffe, D.B.; Barešová, V.; Pochet, N.; Blumenstiel, B.; Ye, C.; Aird, D.; Stevens, C.; Robinson, J.T.; et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat. Genet. 2013, 45, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Lindner, T. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum. Mol. Genet. 1999, 8, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Gresh, L.; Fischer, E.; Reimann, A.; Tanguy, M.; Garbay, S.; Shao, X.; Hiesberger, T.; Fiette, L.; Igarashi, P.; Yaniv, M.; et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004, 23, 1657–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stibůrková, B.; Majewski, J.; Hodaňová, K.; Ondrová, L.; Jeřábková, M.; Zikánová, M.; Vylet’al, P.; Šebesta, I.; Marinaki, A.; Simmonds, A.; et al. Familial juvenile hyperuricaemic nephropathy (FJHN): Linkage analysis in 15 families, physical and transcriptional characterisation of the FJHN critical region on chromosome 16p11.2 and the analysis of seven candidate genes. Eur. J. Hum. Genet. 2003, 11, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Gribouval, O.; Gonzales, M.; Neuhaus, T.; Aziza, J.; Bieth, E.; Laurent, N.; Bouton, J.M.; Feuillet, F.; Makni, S.; Amar, H.B.; et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat. Genet. 2005, 37, 964–968. [Google Scholar] [CrossRef]
- Bolar, N.A.; Golzio, C.; Živná, M.; Hayot, G.; Van Hemelrijk, C.; Schepers, D.; Vandeweyer, G.; Hoischen, A.; Huyghe, J.R.; Raes, A.; et al. Heterozygous Loss-of-Function SEC61A1 Mutations Cause Autosomal-Dominant Tubulo-Interstitial and Glomerulocystic Kidney Disease with Anemia. Am. J. Hum. Genet. 2016, 99, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Jordan, P.; Arrondel, C.; Bessières, B.; Tessier, A.; Attié-Bitach, T.; Guterman, S.; Morinière, V.; Antignac, C.; Saunier, S.; Gubler, M.-C.; et al. Bi-allelic pathogenic variations in DNAJB11 cause Ivemark II syndrome, a renal-hepatic-pancreatic dysplasia. Kidney Int. 2021, 99, 405–409. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, T.; Suzuki, T.; Ueda, T.; Ohta, S.; Watanabe, K. Modification Defect at Anticodon Wobble Nucleotide of Mitochondrial tRNAsLeu(UUR) with Pathogenic Mutations of Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis, and Stroke-like Episodes. J. Biol. Chem. 2000, 275, 4251–4257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Ouweland, J.M.W.; Lemkes, H.H.P.J.; Ruitenbeek, W.; Sandkuijl, L.A.; De Vijlder, M.F.; Struyvenberg, P.A.A.; Van De Kamp, J.J.P.; Maassen, J.A. Mutation in mitochondrial tRNALeu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat. Genet. 1992, 1, 368–371. [Google Scholar] [CrossRef]
- Hurd, T.W.; Otto, E.A.; Mishima, E.; Gee, H.Y.; Inoue, H.; Inazu, M.; Yamada, H.; Halbritter, J.; Seki, G.; Konishi, M.; et al. Mutation of the Mg2+ Transporter SLC41A1 Results in a Nephronophthisis-Like Phenotype. J. Am. Soc. Nephrol. 2013, 24, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Otto, E.A.; Cluckey, A.; Airik, R.; Hurd, T.W.; Chaki, M.; Diaz, K.; Lach, F.P.; Bennett, G.R.; Gee, H.Y.; et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012, 44, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028191. [Google Scholar] [CrossRef] [PubMed]
- Saunier, S.; Calado, J.; Benessy, F.; Silbermann, F.; Heilig, R.; Weissenbach, J.; Antignac, C. Characterization of the NPHP1 Locus: Mutational Mechanism Involved in Deletions in Familial Juvenile Nephronophthisis. Am. J. Hum. Genet. 2000, 66, 778–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snoek, R.; Van Setten, J.; Keating, B.J.; Israni, A.K.; Jacobson, P.A.; Oetting, W.S.; Matas, A.J.; Mannon, R.B.; Zhang, Z.; Zhang, W.; et al. NPHP1 (Nephrocystin-1) Gene Deletions Cause Adult-Onset ESRD. J. Am. Soc. Nephrol. 2018, 29, 1772–1779. [Google Scholar] [CrossRef] [Green Version]
- Caridi, G.; Murer, L.; Bellantuono, R.; Sorino, P.; Caringella, D.; Gusmano, R.; Ghiggeri, G. Renal-retinal syndromes: Association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am. J. Kidney Dis. 1998, 32, 1059–1062. [Google Scholar] [CrossRef]
- Parisi, M.A.; Bennett, C.L.; Eckert, M.L.; Dobyns, W.B.; Gleeson, J.G.; Shaw, D.W.W.; McDonald, R.; Eddy, A.; Chance, P.F.; Glass, I.A. The NPHP1 Gene Deletion Associated with Juvenile Nephronophthisis Is Present in a Subset of Individuals with Joubert Syndrome. Am. J. Hum. Genet. 2004, 75, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Lindstrand, A.; Davis, E.E.; Carvalho, C.M.B.; Pehlivan, D.; Willer, J.R.; Tsai, I.-C.; Ramanathan, S.; Zuppan, C.; Sabo, A.; Muzny, D.; et al. Recurrent CNVs and SNVs at the NPHP1 Locus Contribute Pathogenic Alleles to Bardet-Biedl Syndrome. Am. J. Hum. Genet. 2014, 94, 745–754. [Google Scholar] [CrossRef]
- Tory, K.; Rousset-Rouvière, C.; Gubler, M.-C.; Morinière, V.; Pawtowski, A.; Becker, C.; Guyot, C.; Gié, S.; Frishberg, Y.; Nivet, H.; et al. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int. 2009, 75, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hollander, A.I.; Koenekoop, R.K.; Yzer, S.; Lopez, I.; Arends, M.L.; Voesenek, K.E.J.; Zonneveld, M.N.; Strom, T.M.; Meitinger, T.; Brunner, H.G.; et al. Mutations in the CEP290 (NPHP6) Gene Are a Frequent Cause of Leber Congenital Amaurosis. Am. J. Hum. Genet. 2006, 79, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Baala, L.; Romano, S.; Khaddour, R.; Saunier, S.; Smith, U.M.; Audollent, S.; Ozilou, C.; Faivre, L.; Laurent, N.; Foliguet, B.; et al. The Meckel-Gruber Syndrome Gene, MKS3, Is Mutated in Joubert Syndrome. Am. J. Hum. Genet. 2007, 80, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Helou, J.; Otto, E.A.; Attanasio, M.; Allen, S.J.; Parisi, M.A.; Glass, I.; Utsch, B.; Hashmi, S.; Fazzi, E.; Omran, H.; et al. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior Loken syndrome. J. Med. Genet. 2007, 44, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Frank, V.; Den Hollander, A.I.; Brüchle, N.O.; Zonneveld, M.N.; Nürnberg, G.; Becker, C.; Du Bois, G.; Kendziorra, H.; Roosing, S.; Senderek, J.; et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum. Mutat. 2008, 29, 45–52. [Google Scholar] [CrossRef]
- Wheway, G.; Lord, J.; Baralle, D. Splicing in the pathogenesis, diagnosis and treatment of ciliopathies. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2019, 1862, 194433. [Google Scholar] [CrossRef]
- Cummings, B.B.; Karczewski, K.J.; Kosmicki, J.A.; Seaby, E.G.; Watts, N.A.; Singer-Berk, M.; Mudge, J.M.; Karjalainen, J.; Satterstrom, F.K.; O’Donnell-Luria, A.H.; et al. Transcript expression-aware annotation improves rare variant interpretation. Nature 2020, 581, 452–458. [Google Scholar] [CrossRef]
- Vidal-Petiot, E.; Cheval, L.; Faugeroux, J.; Malard, T.; Doucet, A.; Jeunemaitre, X.; Hadchouel, J. A New Methodology for Quantification of Alternatively Spliced Exons Reveals a Highly Tissue-Specific Expression Pattern of WNK1 Isoforms. PLoS ONE 2012, 7, e37751. [Google Scholar] [CrossRef]
- Schäfer, T.; Pütz, M.; Lienkamp, S.; Ganner, A.; Bergbreiter, A.; Ramachandran, H.; Gieloff, V.; Gerner, M.; Mattonet, C.; Czarnecki, P.G.; et al. Genetic and physical interaction between the NPHP5 and NPHP6 gene products. Hum. Mol. Genet. 2008, 17, 3655–3662. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Seo, S.; Bhattarai, S.; Bugge, K.; Searby, C.C.; Zhang, Q.; Drack, A.V.; Stone, E.M.; Sheffield, V.C. BBS mutations modify phenotypic expression of CEP290-related ciliopathies. Hum. Mol. Genet. 2014, 23, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Arts, H.H.; Doherty, D.; Van Beersum, S.E.C.; Parisi, M.A.; Letteboer, S.J.F.; Gorden, N.T.; Peters, T.A.; Märker, T.; Voesenek, K.; Kartono, A.; et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat. Genet. 2007, 39, 882–888. [Google Scholar] [CrossRef]
- Wolf, M.T.F.; Saunier, S.; O’Toole, J.F.; Wanner, N.; Groshong, T.; Attanasio, M.; Salomon, R.; Stallmach, T.; Sayer, J.A.; Waldherr, R.; et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007, 72, 1520–1526. [Google Scholar] [CrossRef] [Green Version]
- Otto, E.A.; Ramaswami, G.; Janssen, S.; Chaki, M.; Allen, S.J.; Zhou, W.; Airik, R.; Hurd, T.W.; Ghosh, A.K.; Wolf, M.T.; et al. Mutation analysis of 18 nephronophthisis associated ciliopathy disease genes using a DNA pooling and next generation sequencing strategy. J. Med. Genet. 2011, 48, 105–116. [Google Scholar] [CrossRef]
- Brancati, F.; Travaglini, L.; Zablocka, D.; Boltshauser, E.; Accorsi, P.; Montagna, G.; Silhavy, J.; Barrano, G.; Bertini, E.; Emma, F.; et al. RPGRIP1L mutations are mainly associated with the cerebello-renal phenotype of Joubert syndrome-related disorders. Clin. Genet. 2008, 74, 164–170. [Google Scholar] [CrossRef]
- Kroes, H.Y.; Monroe, G.R.; Van Der Zwaag, B.; Duran, K.J.; De Kovel, C.G.; Van Roosmalen, M.J.; Harakalova, M.; Nijman, I.J.; Kloosterman, W.P.; Giles, R.H.; et al. Joubert syndrome: Genotyping a Northern European patient cohort. Eur. J. Hum. Genet. 2016, 24, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.; Fritsch, J.; Lehmann-Che, J.; Bali, M.; Arous, N.; Goossens, M.; Edelman, A.; Fanen, P. Two Mild Cystic Fibrosis-associated Mutations Result in Severe Cystic Fibrosis When Combined in Cis and Reveal a Residue Important for Cystic Fibrosis Transmembrane Conductance Regulator Processing and Function. J. Biol. Chem. 2001, 276, 9045–9049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, N.; Olinger, E.; Adam, J.; Kelly, M.; Schiano, G.; Ramsbottom, S.A.; Sandford, R.; Devuyst, O.; Sayer, J.A. A novel homozygous UMOD mutation reveals gene dosage effects on uromodulin processing and urinary excretion. Nephrol. Dial. Transplant. 2017, 32, 1994–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edghill, E.L. Mutations in hepatocyte nuclear factor-1 and their related phenotypes. J. Med. Genet. 2005, 43, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-z.; Gao, Q.; Zhao, X.-z.; Chen, Y.-z.; Craig, L.B.; Xiong, X.-s.; Mei, C.-l.; Shi, Y.-q.; Chen, X.-m. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin. Med. J. 2010, 123, 3326–3333. [Google Scholar]
- Heidet, L.; Decramer, S.; Pawtowski, A.; Morinière, V.; Bandin, F.; Knebelmann, B.; Lebre, A.-S.; Faguer, S.; Guigonis, V.; Antignac, C.; et al. Spectrum of HNF1B Mutations in a Large Cohort of Patients Who Harbor Renal Diseases. Clin. J. Am. Soc. Nephrol. 2010, 5, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Clissold, R.L.; Ashfield, B.; Burrage, J.; Hannon, E.; Bingham, C.; Mill, J.; Hattersley, A.; Dempster, E.L. Genome-wide methylomic analysis in individuals with HNF1B intragenic mutation and 17q12 microdeletion. Clin. Epigenetics 2018, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Clissold, R.L.; Shaw-Smith, C.; Turnpenny, P.; Bunce, B.; Bockenhauer, D.; Kerecuk, L.; Waller, S.; Bowman, P.; Ford, T.; Ellard, S.; et al. Chromosome 17q12 microdeletions but not intragenic HNF1B mutations link developmental kidney disease and psychiatric disorder. Kidney Int. 2016, 90, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, C.; Von Bothmer, J.; Ortiz Brüchle, N.; Venghaus, A.; Frank, V.; Fehrenbach, H.; Hampel, T.; Pape, L.; Buske, A.; Jonsson, J.; et al. Mutations in Multiple PKD Genes May Explain Early and Severe Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 2047–2056. [Google Scholar] [CrossRef] [Green Version]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Ren. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Guéry, B.; Choukroun, G.; Noël, L.-H.; Clavel, P.; Rötig, A.; Lebon, S.; Rustin, P.; Bellané-Chantelot, C.; Mougenot, B.; Grünfeld, J.-P.; et al. The Spectrum of Systemic Involvement in Adults Presenting with Renal Lesion and Mitochondrial tRNA(Leu) Gene Mutation. J. Am. Soc. Nephrol. 2003, 14, 2099–2108. [Google Scholar] [CrossRef] [Green Version]
- Kemter, E.; Fröhlich, T.; Arnold, G.J.; Wolf, E.; Wanke, R. Mitochondrial Dysregulation Secondary to Endoplasmic Reticulum Stress in Autosomal Dominant Tubulointerstitial Kidney Disease – UMOD (ADTKD-UMOD). Sci. Rep. 2017, 7, 42970. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, E.; Sparks, K.; Best, S.; Borrows, S.; Hoskins, B.; Sabir, A.; Barrett, T.; Williams, D.; Mohammed, S.; Goldsmith, D.; et al. Risk Factors for Severe Renal Disease in Bardet–Biedl Syndrome. J. Am. Soc. Nephrol. 2017, 28, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Katsanis, N.; Ansley, S.J.; Badano, J.L.; Eichers, E.R.; Lewis, R.A.; Hoskins, B.E.; Scambler, P.J.; Davidson, W.S.; Beales, P.L.; Lupski, J.R. Triallelic Inheritance in Bardet-Biedl Syndrome, a Mendelian Recessive Disorder. Science 2001, 293, 2256–2259. [Google Scholar] [CrossRef] [Green Version]
- Beales, P.L.; Badano, J.L.; Ross, A.J.; Ansley, S.J.; Hoskins, B.E.; Kirsten, B.; Mein, C.A.; Froguel, P.; Scambler, P.J.; Lewis, R.A.; et al. Genetic Interaction of BBS1 Mutations with Alleles at Other BBS Loci Can Result in Non-Mendelian Bardet-Biedl Syndrome. Am. J. Hum. Genet. 2003, 72, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Badano, J.L.; Leitch, C.C.; Ansley, S.J.; May-Simera, H.; Lawson, S.; Lewis, R.A.; Beales, P.L.; Dietz, H.C.; Fisher, S.; Katsanis, N. Dissection of epistasis in oligogenic Bardet–Biedl syndrome. Nature 2006, 439, 326–330. [Google Scholar] [CrossRef]
- Katsanis, N. The oligogenic properties of Bardet-Biedl syndrome. Hum. Mol. Genet. 2004, 13, 65R–71R. [Google Scholar] [CrossRef] [Green Version]
- Mykytyn, K.; Nishimura, D.Y.; Searby, C.C.; Shastri, M.; Yen, H.-j.; Beck, J.S.; Braun, T.; Streb, L.M.; Cornier, A.S.; Cox, G.F.; et al. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat. Genet. 2002, 31, 435–438. [Google Scholar] [CrossRef]
- Mykytyn, K.; Nishimura, D.Y.; Searby, C.C.; Beck, G.; Bugge, K.; Haines, H.L.; Cornier, A.S.; Cox, G.F.; Fulton, A.B.; Carmi, R.; et al. Evaluation of Complex Inheritance Involving the Most Common Bardet-Biedl Syndrome Locus (BBS1). Am. J. Hum. Genet. 2003, 72, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Abu-Safieh, L.; Al-Anazi, S.; Al-Abdi, L.; Hashem, M.; Alkuraya, H.; Alamr, M.; Sirelkhatim, M.O.; Al-Hassnan, Z.; Alkuraya, B.; Mohamed, J.Y.; et al. In search of triallelism in Bardet–Biedl syndrome. Eur. J. Hum. Genet. 2012, 20, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Hichri, H.; Stoetzel, C.; Laurier, V.; Caron, S.; Sigaudy, S.; Sarda, P.; Hamel, C.; Martin-Coignard, D.; Gilles, M.; Leheup, B.; et al. Testing for triallelism: Analysis of six BBS genes in a Bardet–Biedl syndrome family cohort. Eur. J. Hum. Genet. 2005, 13, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badano, J.L. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum. Mol. Genet. 2003, 12, 1651–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanis, N.; Eichers, E.R.; Ansley, S.J.; Lewis, R.A.; Kayserili, H.; Hoskins, B.E.; Scambler, P.J.; Beales, P.L.; Lupski, J.R. BBS4 Is a Minor Contributor to Bardet-Biedl Syndrome and May Also Participate in Triallelic Inheritance. Am. J. Hum. Genet. 2002, 71, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Kousi, M.; Söylemez, O.; Ozanturk, A.; Mourtzi, N.; Akle, S.; Jungreis, I.; Muller, J.; Cassa, C.A.; Brand, H.; Mokry, J.A.; et al. Evidence for secondary-variant genetic burden and non-random distribution across biological modules in a recessive ciliopathy. Nat. Genet. 2020, 52, 1145–1150. [Google Scholar] [CrossRef]
- Beales, P.L.; Elcioglu, N.; Woolf, A.S.; Parker, D.; Flinter, F.A. New criteria for improved diagnosis of Bardet-Biedl syndrome: Results of a population survey. J. Med. Genet. 1999, 36, 437–446. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Bingham, C.; Ellard, S.; Van’T Hoff, W.G.; Simmonds, H.A.; Marinaki, A.M.; Badman, M.K.; Winocour, P.H.; Stride, A.; Lockwood, C.R.; Nicholls, A.J.; et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1β gene mutation. Kidney Int. 2003, 63, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.C.; Zhang, Y.; Shao, A.; Avdulov, S.; Herrera, J.; Aboudehen, K.; Pontoglio, M.; Igarashi, P. Mechanism of Fibrosis in HNF1B-Related Autosomal Dominant Tubulointerstitial Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2493–2509. [Google Scholar] [CrossRef] [Green Version]
- Casemayou, A.; Fournel, A.; Bagattin, A.; Schanstra, J.; Belliere, J.; Decramer, S.; Marsal, D.; Gillet, M.; Chassaing, N.; Huart, A.; et al. Hepatocyte Nuclear Factor-1β Controls Mitochondrial Respiration in Renal Tubular Cells. J. Am. Soc. Nephrol. 2017, 28, 3205–3217. [Google Scholar] [CrossRef] [Green Version]
- Fauser, S. Further support for digenic inheritance in Bardet-Biedl syndrome. J. Med. Genet. 2003, 40, e104. [Google Scholar] [CrossRef]
- Badano, J.L.; Ansley, S.J.; Leitch, C.C.; Lewis, R.A.; Lupski, J.R.; Katsanis, N. Identification of a Novel Bardet-Biedl Syndrome Protein, BBS7, That Shares Structural Features with BBS1 and BBS2. Am. J. Hum. Genet. 2003, 72, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Massa, F.; Garbay, S.; Bouvier, R.; Sugitani, Y.; Noda, T.; Gubler, M.-C.; Heidet, L.; Pontoglio, M.; Fischer, E. Hepatocyte nuclear factor 1β controls nephron tubular development. Development 2013, 140, 886–896. [Google Scholar] [CrossRef] [Green Version]
- Slaats, G.G.; Lilien, M.R.; Giles, R.H. Nephronophthisis: Should we target cysts or fibrosis? Pediatr. Nephrol. 2016, 31, 545–554. [Google Scholar] [CrossRef]
- Bolignano, D.; Zoccali, C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol. Dial. Transplant. 2017, 32, ii194–ii199. [Google Scholar] [CrossRef] [Green Version]
- Živná, M.; Hůlková, H.; Matignon, M.; Hodaňová, K.; Vylet’al, P.; Kalbáčová, M.; Barešová, V.; Sikora, J.; Blažková, H.; Živný, J.; et al. Dominant Renin Gene Mutations Associated with Early-Onset Hyperuricemia, Anemia, and Chronic Kidney Failure. Am. J. Hum. Genet. 2009, 85, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Riedhammer, K.M.; Braunisch, M.C.; Günthner, R.; Wagner, M.; Hemmer, C.; Strom, T.M.; Schmaderer, C.; Renders, L.; Tasic, V.; Gucev, Z.; et al. Exome Sequencing and Identification of Phenocopies in Patients With Clinically Presumed Hereditary Nephropathies. Am. J. Kidney Dis. 2020, 76, 460–470. [Google Scholar] [CrossRef]
- Van Der Ven, A.T.; Connaughton, D.M.; Ityel, H.; Mann, N.; Nakayama, M.; Chen, J.; Vivante, A.; Hwang, D.-y.; Schulz, J.; Braun, D.A.; et al. Whole-Exome Sequencing Identifies Causative Mutations in Families with Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. 2018, 29, 2348–2361. [Google Scholar] [CrossRef]
- Sheng, X.; Guan, Y.; Ma, Z.; Wu, J.; Liu, H.; Qiu, C.; Vitale, S.; Miao, Z.; Seasock, M.J.; Palmer, M.; et al. Mapping the genetic architecture of human traits to cell types in the kidney identifies mechanisms of disease and potential treatments. Nat. Genet. 2021, 53, 1322–1333. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
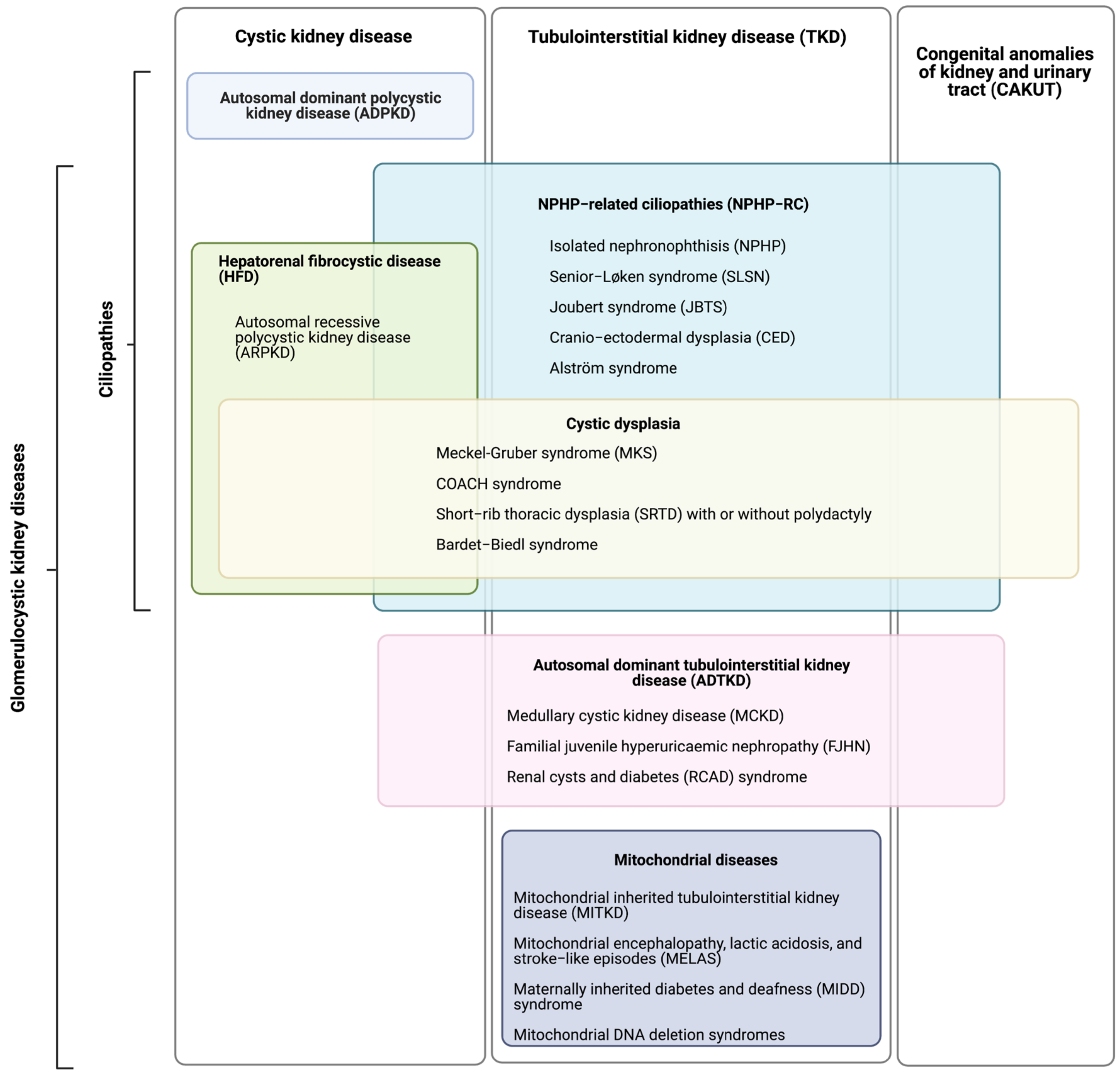

| Disease | Phenotype | Genes (Alias Symbols in Parentheses) |
|---|---|---|
| Autosomal-dominant tubulointerstitial kidney disease (ADTKD-UMOD) Also known as: uromodulin-associated kidney disease (UAKD), familial juvenile hyperuricaemic nephropathy (FJHN), medullary cystic kidney disease type 2 (MCKD2) | Variably progressive CKD with IF/TA and minimal to no proteinuria; Early-onset hyperuricaemia/gout. | UMOD |
| Autosomal -dominant tubulointerstitial kidney disease (ADTKD-MUC1) Also known as: mucin-1 kidney disease (MKD), medullary cystic kidney disease type 1 (MCKD1) | Variably progressive CKD with IF/TA and minimal to no proteinuria. | MUC1 |
| Autosomal-dominant tubulointerstitial kidney Disease (ADTKD-REN) Also known as: familial juvenile hyperuricaemic nephropathy type 2 (FJHN2) | Variably progressive CKD with IF/TA and minimal to no proteinuria; Childhood/adolescent onset: anaemia, hyperkalaemia, acidosis, progressive CKD, and development of gout; Adult-onset: slowly progressive CKD from the third decade, with or without gout. | REN |
| Autosomal-dominant tubulointerstitial kidney disease (ADTKD-HNF1B) | Associated features are variable and include: Variably progressive CKD with IF/TA and minimal to no proteinuria; Congenital anomalies of kidney and urinary tract (CAKUT); RCAD (Renal Cyst and Diabetes Syndrome); Pancreatic hypoplasia; MODY5 (Maturity-Onset Diabetes mellitus of the Young type 5); Urogenital malformations; Hypomagnesaemia; Cognitive impairment/autism spectrum disorder (associated with 17q12 deletion). | HNF1B |
| Autosomal-dominant tubulointerstitial kidney disease (ADTKD-SEC61A1) | Variably progressive CKD with IF/TA and minimal to no proteinuria; Small dysplastic kidneys; Congenital anaemia and neutropenia (with recurrent cutaneous abscesses); Growth retardation. | SEC61A1 |
| Autosomal-dominant tubulointerstitial kidney disease/autosomal-dominant polycystic kidney disease (ADTKD/ADPKD) overlap | Variably progressive CKD and hypertension; Non-enlarged cystic kidneys with interstitial fibrosis progressing to renal atrophy; Gout. | DNAJB11 |
| Nephronophthisis (NPHP) | Impaired urinary concentrating ability and sodium reabsorption (polyuria, polydipsia); Normal or slightly small kidneys with increased echogenicity; Variably progressive CKD with IF/TA and minimal to no proteinuria; (Hepatic fibrosis, Situs inversus); Sometimes categorised by median age of onset:
| NPHP1, INVS † (NPHP2), NPHP3 †, NPHP4 †, IQCB1 (NPHP5), CEP290 † (NPHP6), GLIS2 (NPHP7), RGRIP1L † (NPHP8), NEK8 (NPHP9), SDCCAG8 (NPHP10), TMEM67 † (NPHP11), TTC21B † (NPHP12), WDR19 (NPHP13), ZNF423 (NPHP14), CEP164 (NPHP15), ANKS6 (NPHP16), IFT172 (NPHP17), CEP83 (NPHP18), DCDC2 (NPHP19), MAPKBP1 (NPHP20), IFT81, TRAF3IP1, ADMATS9, INPP5E, TMEM216, AHI1 †, CC2D2A, TMEM237, TMEM138, IFT122, WDR35, IFT43. |
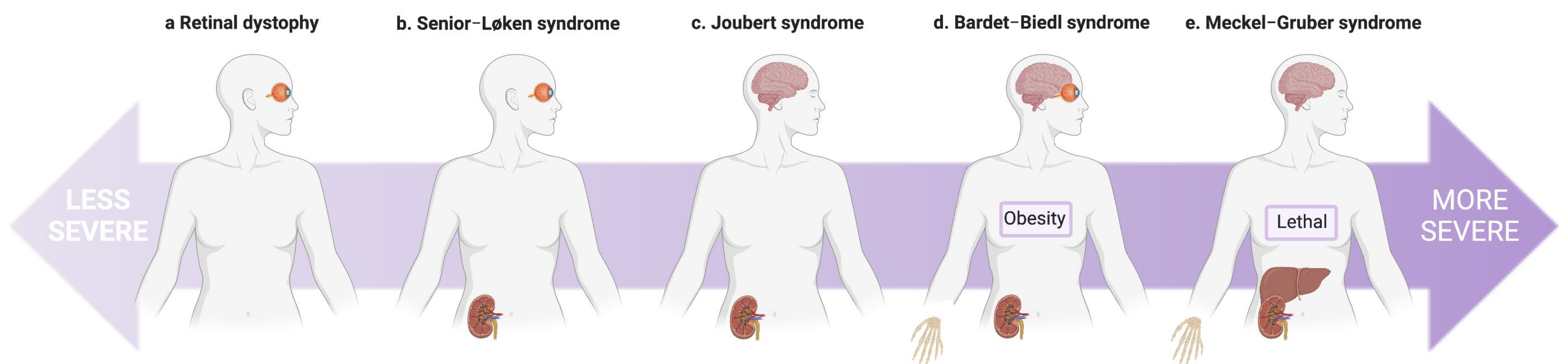
| Senior–Løken syndrome (SLSN) | Nephronophthisis; Retinitis pigmentosa, Leber congenital amaurosis (LCA); (Hepatic fibrosis, situs inversus). | NPHP1 (SLSN1), NPHP3 † (SLSN3), NPHP4 † (SLSN4), IQCB1 (SLSN5, NPHP5), CEP290 † (SLSN6), SDCCAG8 (SLSN7), WDR19 (SLSN8), CEP164, TRAF3IP1 (SLSN9). |
| Joubert syndrome (JBTS) | Nephronophthisis; Renal cystic dysplasia; Cerebellar vermis hypoplasia (characteristic “molar tooth” sign on MRI brain); Ataxia, hypotonia; Hepatic fibrosis; Situs inversus; Polydactyly; Intellectual disability. | INPP5E (JBTS1), TMEM216 (JBTS2), AHI1 † (JBTS3), NPHP1 (JBTS4), CEP290 † (JBTS5), TMEM67 † (JBTS6), RPGRIP1L † (JBTS7), ARL13B * (JBTS8), CC2D2A (JBTS9), OFD1 (JBTS10), TTC21B † (JBTS11/NPHP12), KIF7 * (JBTS12), TCTN1 * (JBTS13), TMEM237 (JBTS14), CEP41 * (JBTS15), TMEM138 (JBTS16), CPLANE1 * (JBTS17), TCTN3 (JBTS18), ZNF423 (JBTS19), TMEM231 (JBTS20), CSPP1 (JBTS21), PDE6D (JBTS22), KIAA0586 * (JBTS23), TCTN2 * (JBTS24), CEP104 * (JBTS25), KIAA0556 * (JBTS26), B9D1 (JBTS27), MKS1 (JBTS28), TMEM107 (JBTS29), ARMC9 * (JBTS30), CEP120 (JBTS31), SUFU (JBTS32), PIBF1 (JBTS33), B9D2 (JBTS34), ARL3 (JBTS35), BSND ≠. |
| Meckel–Gruber syndrome (MKS) | Enlarged dysplastic cystic kidneys; Occipital encephalocele; Cleft palate; Hepatic fibrosis; Variable: polydactyly, skeletal dysplasia, and situs inversus. | MKS1, TMEM216 (MKS2), TMEM67 † (MKS3), CEP290 † (MKS4), RPGRIP1L † (MKS5), CC2D2A (MKS6), NPHP3 † (MKS7), TCTN2 * (MKS8), B9D1 (MKS9), B9D2 (MKS10), TMEM231 (MKS11), KIF14 (MKS12), TMEM107 (MKS13), CSPP1, TXNDC15, TMEM237, CPLANE1 *, CEP55. |
| COACH syndrome | Nephronophthisis; Renal cystic dysplasia; Cerebellar vermis hypoplasia, oligophrenia, ataxia, coloboma, and hepatic fibrosis. | TMEM67 †, CC2D2A, RPGRIP1L †. |
| Short-rib thoracic dysplasia (SRTD) with or without polydactyly Also known as: asphyxiating thoracic dystrophy; Jeune syndrome | Nephronophthisis; Renal cystic dysplasia; Constricted thoracic cage; Short ribs; Shortened tubular bones; Variable: multiorgan involvement, polydactyly, hepatic fibrosis, and intellectual disability. | CEP120, CSPP1, DYNC2H1, DYNC2LI1, IFT140, IFT172, IFT43, IFT52, IFT80, IFT81, INTU, KIAA0586 *, NEK1, TCTEX1D2, TTC21B †, WDR19, WDR34, WDR35, WDR60. |
| Cranio-ectodermal dysplasia (CED) | Nephronophthisis; Skeletal abnormalities; Craniosynostosis (premature closure of cranial sutures); Ectodermal abnormalities. | IFT122 (CED1), WDR35 (CED2), IFT43 (CED3), WDR19 (NPHP13, CED4). |
| Bardet–Biedl Syndrome (BBS) | Nephronophthisis; Renal cystic dysplasia; Focal segmental glomerulosclerosis (FSGS); Rod-cone dystrophy; Polydactyly; Obesity; Genital malformations; Intellectual disability. | BBS1, BBS2, ARL6 (BBS3), BBS4, BBS5, MKKS (BBS6), BBS7, TTC8 (BBS8), BBS9, BBS10, TRIM32 (BBS11), BBS12, MKS1 † (BBS13), CEP290 † (BBS14), WDPCP † (BBS15), SDCCAG8 (BBS16), LZTFL1 (BBS17), BBIP1 (BBS18), IFT27 (BBS19), IFT74 (BBS20), C8ORF37 (BBS21), IFT172, NPHP1, CCDC28B ≠, TMEM67 †. |
| Alström syndrome | Progressive CKD with IF/TA; Cone-rod dystrophy; Obesity; Progressive sensorineural hearing loss; Cardiomyopathy; Type 2 diabetes. | ALMS1 |
| Karyomegalic Interstitial Nephritis (KIN) | Variably progressive CKD; IF/TA with enlarged and atypical tubular epithelial cell nuclei. | FAN1 |
| Renal tubular dysgenesis | Foetal anuria and perinatal death from pulmonary hypoplasia and oligohydramnios (Potter syndrome). | REN, AGT, AGTR1, ACE. |
| Mitochondrial Inherited Tubulointerstitial Kidney Disease (MITKD) | Isolated variably progressive CKD with bland urinalysis and IF/TA and no Fanconi syndrome or extra-renal manifestations; Tubulopathy, including Fanconi syndrome; FSGS, glomerulocystic kidney disease; A multisystem disease of muscles and neurological system but can include other organ systems: Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS); Maternally inherited diabetes and deafness (MIDD) syndrome; Mitochondrial DNA deletion syndromes. | MTTF (mt-tRNAPhe), ML-TL1 (mt-tRNALeu), mitochondrial DNA deletions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leggatt, G.P.; Seaby, E.G.; Veighey, K.; Gast, C.; Gilbert, R.D.; Ennis, S. A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases. Genes 2023, 14, 1582. https://doi.org/10.3390/genes14081582
Leggatt GP, Seaby EG, Veighey K, Gast C, Gilbert RD, Ennis S. A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases. Genes. 2023; 14(8):1582. https://doi.org/10.3390/genes14081582
Chicago/Turabian StyleLeggatt, Gary P., Eleanor G. Seaby, Kristin Veighey, Christine Gast, Rodney D. Gilbert, and Sarah Ennis. 2023. "A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases" Genes 14, no. 8: 1582. https://doi.org/10.3390/genes14081582
APA StyleLeggatt, G. P., Seaby, E. G., Veighey, K., Gast, C., Gilbert, R. D., & Ennis, S. (2023). A Role for Genetic Modifiers in Tubulointerstitial Kidney Diseases. Genes, 14(8), 1582. https://doi.org/10.3390/genes14081582







