Genome-Wide Analysis of the BAHD Family in Welsh Onion and CER2-LIKEs Involved in Wax Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Identification of the AfBAHDs Family in Welsh Onion Genome
2.3. Phylogenetic, Protein Properties, and Sequence Analyses
2.4. Chromosome Location of the BAHDs in Welsh Onion
2.5. cis-Acting Element Analysis of the BAHDs Genes in Welsh Onion
2.6. RNA Extraction and RT-qPCR Analysis
2.7. RNA-Seq and Differential Expression Genes Analysis
3. Results
3.1. Identification of BAHD Family Genes Welsh Onion
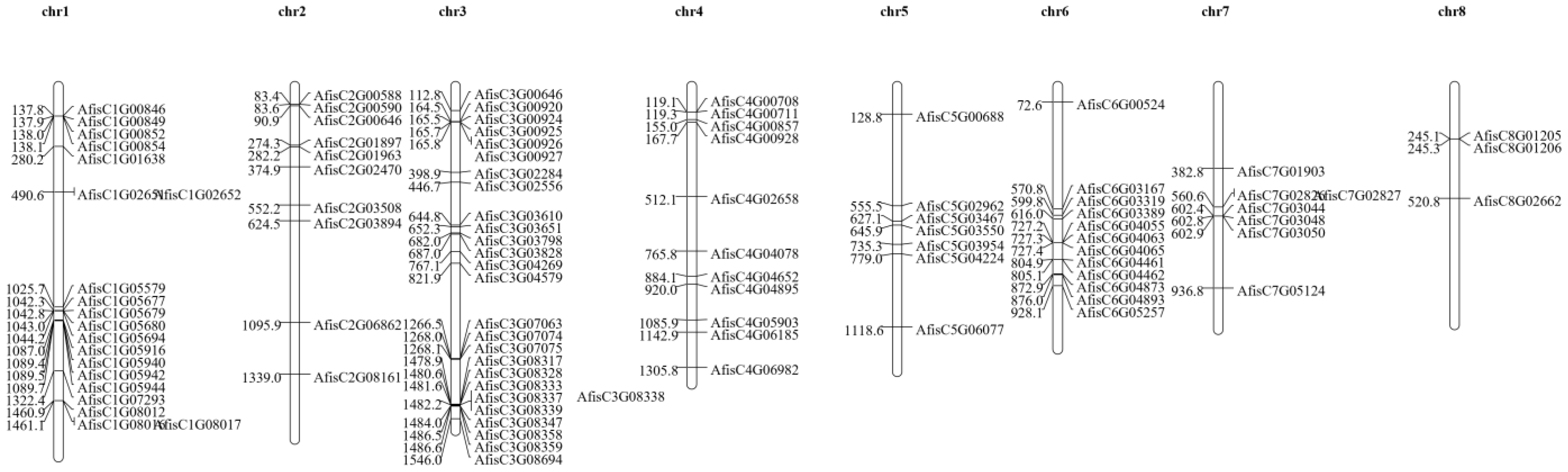
3.2. Location of BAHDs Family Genes in Welsh Onion Chromosomes
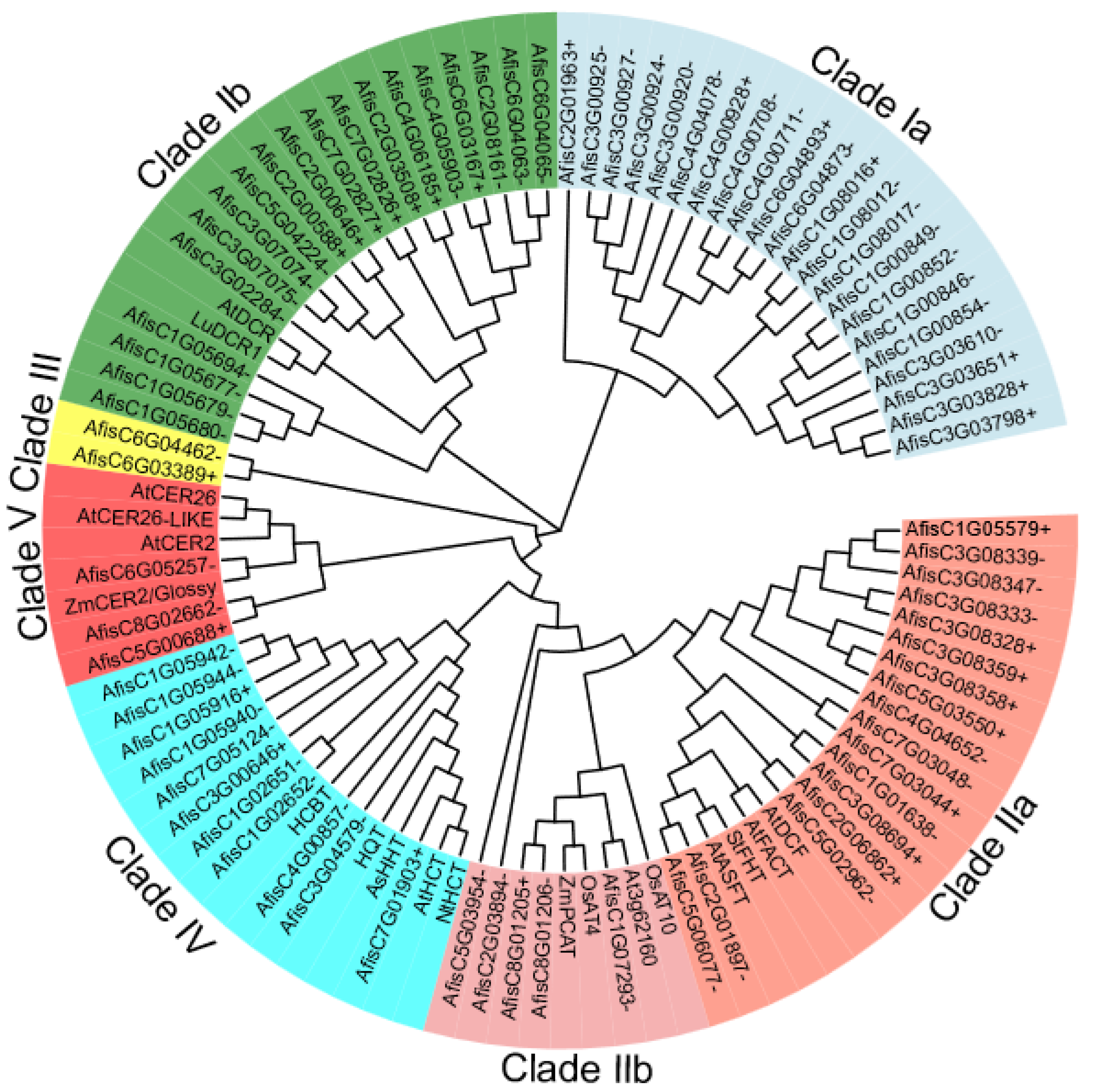
3.3. Phylogenetic Analysis of the BAHD Family Genes in Welsh Onion
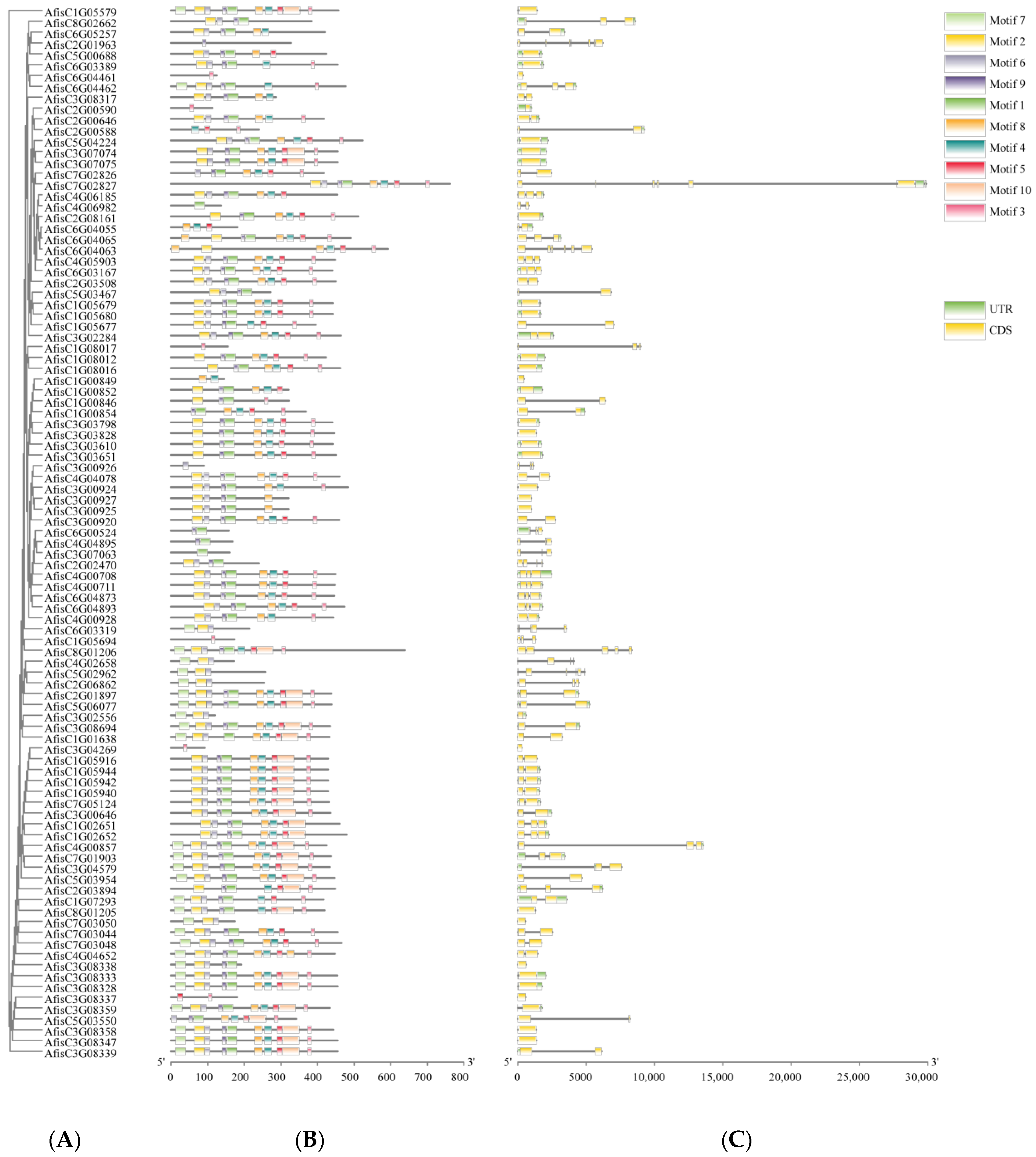
3.4. Gene Structures and Function Domains of BAHD Family Genes in Welsh Onion
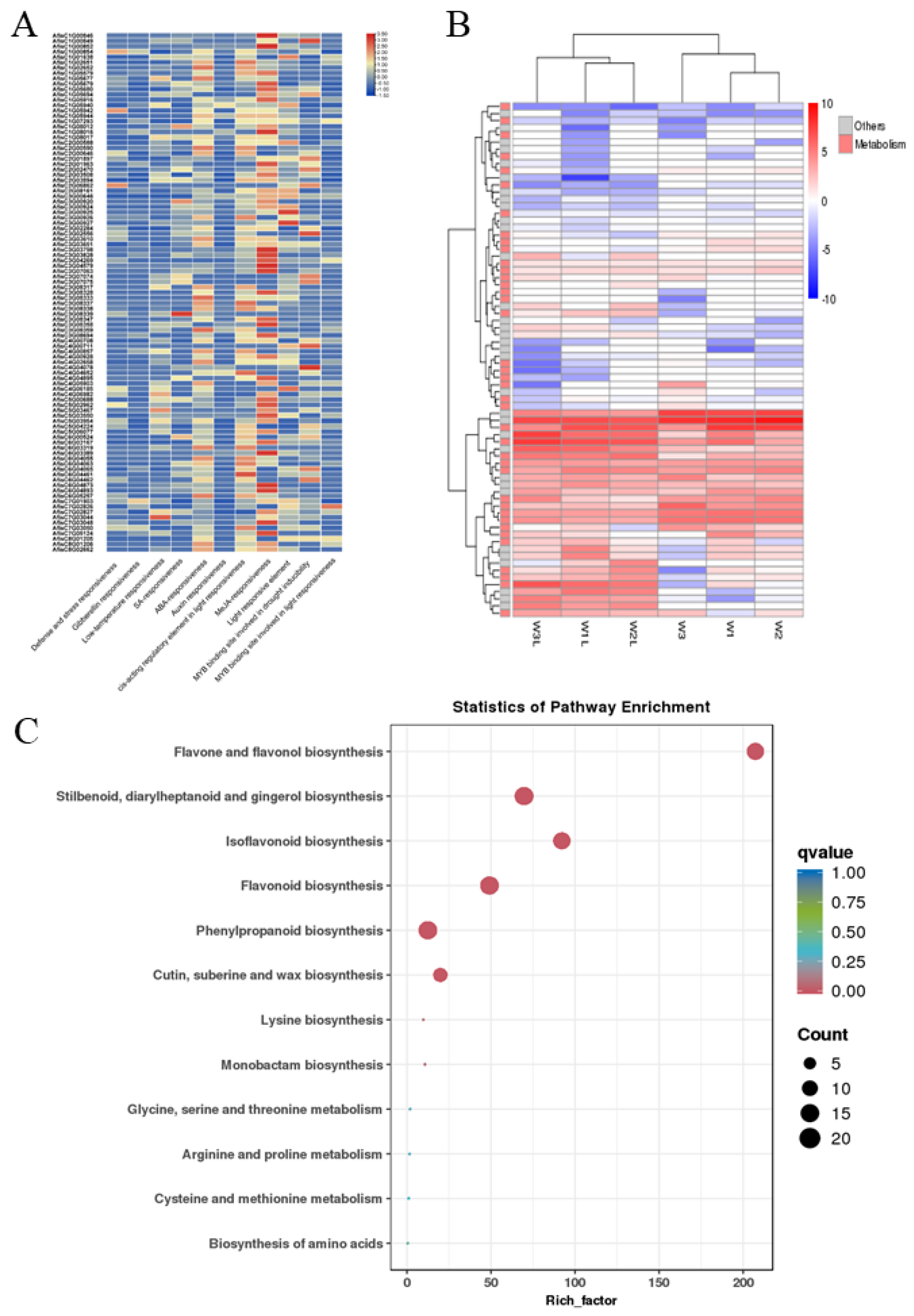
3.5. Analysis of cis-Acting Element and Gene Expression in the BAHD Family Genes of Welsh Onion
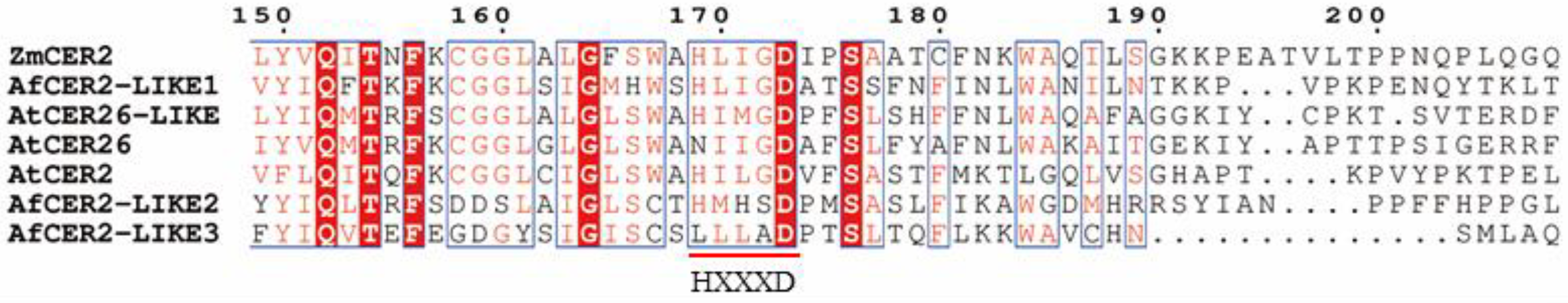
3.6. Sequence Analysis of CER2-LIKE Genes in Welsh Onion
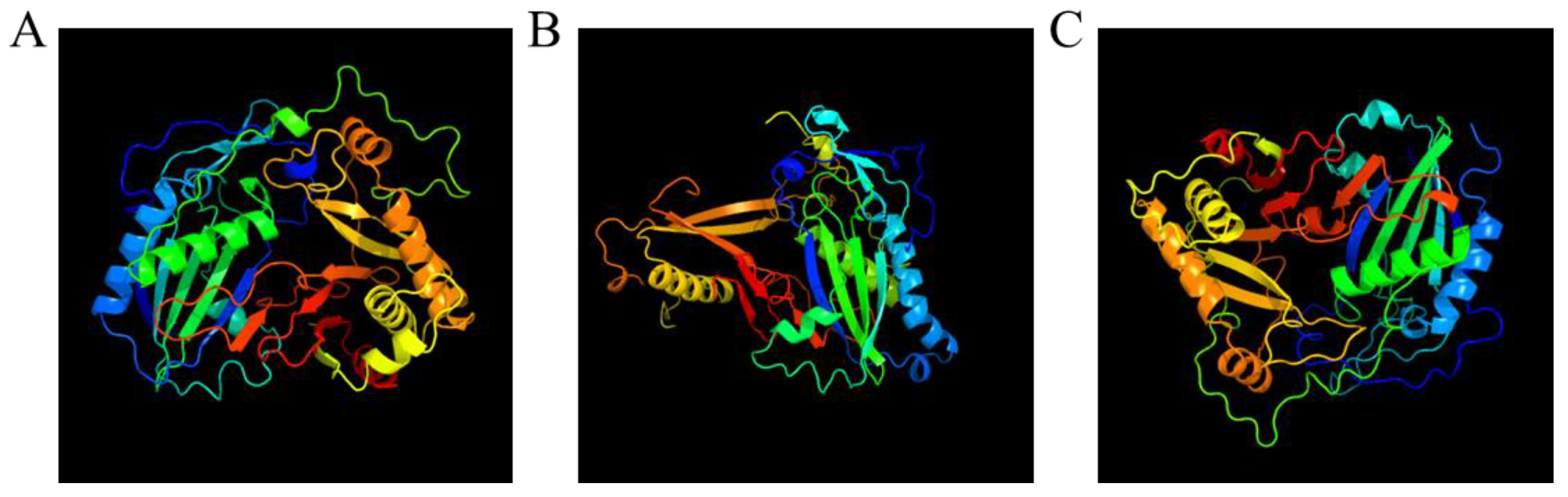
3.7. 3D Modeling of AfCER2-LIKEs
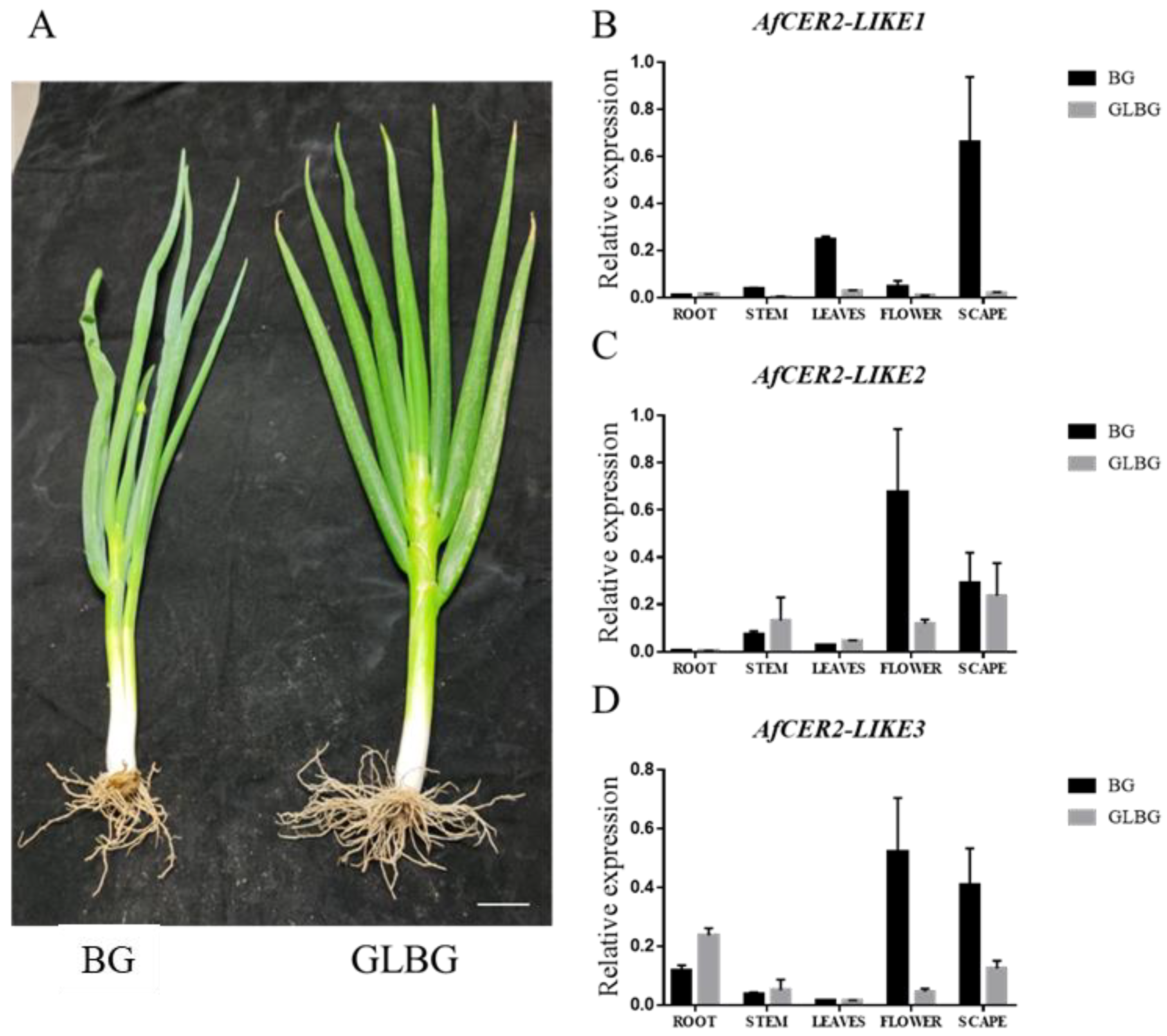
3.8. Expression of AfCER2-LIKE Genes in Wildtype Welsh Onion and Wax-Deficient Welsh Onion
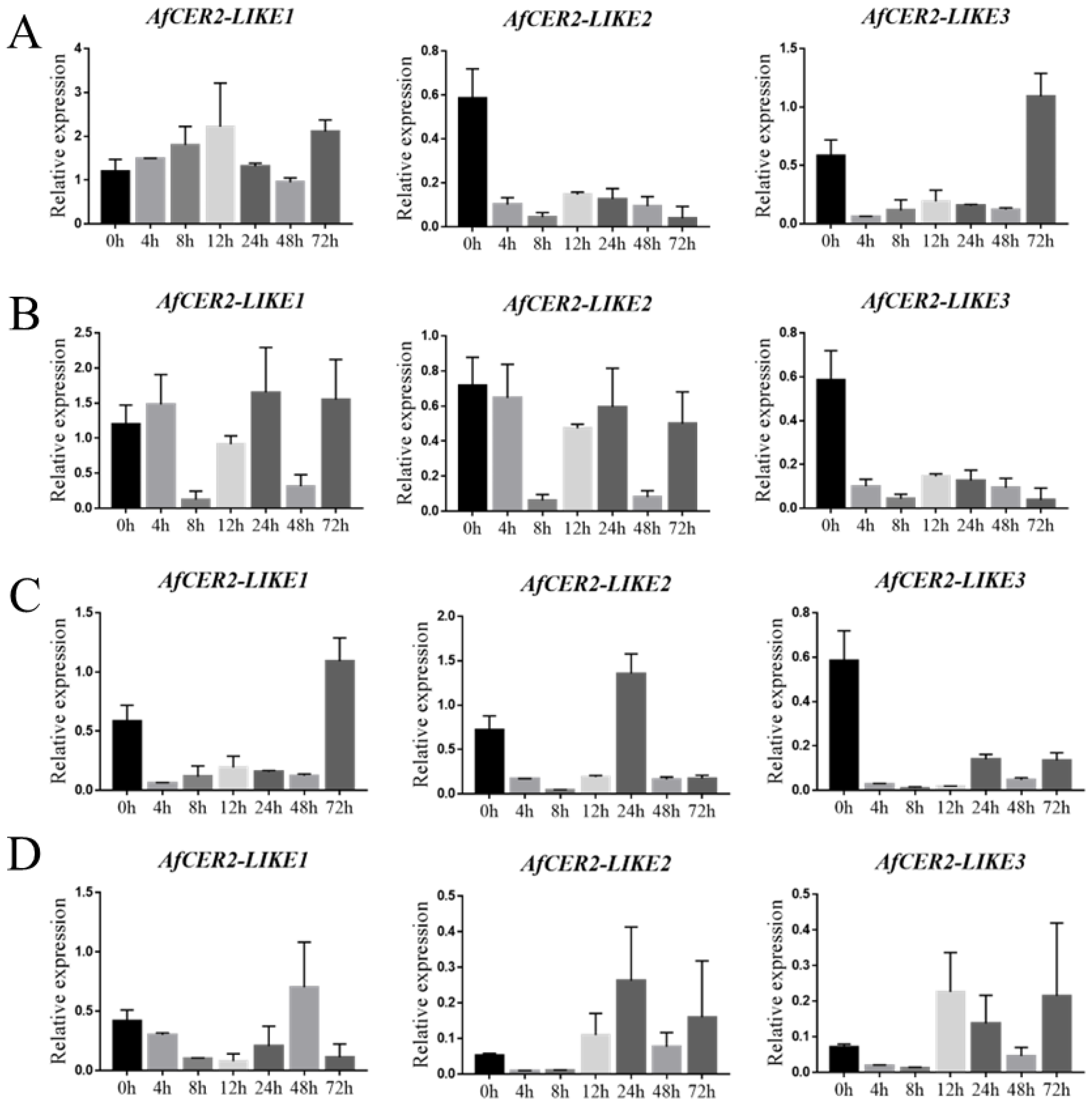
3.9. Expression of AfCER2s Genes under Abiotic Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghe, G.; Kruse, L.H.; Petersen, M.; Scossa, F.; Fernie, A.R.; Gaquerel, E.; D’Auria, J.C. BAHD Company: The Ever-Expanding Roles of the BAHD Acyltransferase Gene Family in Plants. Annu. Rev. Plant Biol. 2023, 74, 165–194. [Google Scholar] [CrossRef]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis DCR Encoding a Soluble BAHD Acyltransferase Is Required for Cutin Polyester Formation and Seed Hydration Properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar] [CrossRef] [PubMed]
- Lashbrooke, J.; Cohen, H.; Levy-Samocha, D.; Tzfadia, O.; Panizel, I.; Zeisler, V.; Massalha, H.; Stern, A.; Trainotti, L.; Schreiber, L.; et al. MYB107 and MYB9 Homologs Regulate Suberin Deposition in Angiosperms. Plant Cell. 2016, 28, 2097–2116. [Google Scholar] [CrossRef]
- Nadakuduti, S.S.; Uebler, J.B.; Liu, X.; Jones, A.D.; Barry, C.S. Characterization of Trichome-Expressed BAHD Acyltransferases in Petunia Axillaris Reveals Distinct Acylsugar Assembly Mechanisms within the Solanaceae. Plant Physiol. 2017, 175, 36–50. [Google Scholar] [CrossRef]
- St-Pierre, B.; De Luca, V. Evolution of Acyltransferase Genes: Origin and Diversification of the BAHD Superfamily of Acyltransferases Involved in Secondary Metabolism. Recent Adv. Phytochem. 2000, 34, 285–315. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in Plants: A Good Time to Be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tie, W.; Yan, Y.; Xu, B.; Liu, J.; Li, M.; Yang, J.; Zeng, J.; Hu, W.; Jin, Z. Identification and Expression of the BAHD Family during Development, Ripening, and Stress Response in Banana. Mol. Biol. Rep. 2021, 48, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, X.; Cui, J.; Zhang, F.; Wan, X.; Liu, Q.; Zhong, Y.; Lin, T. Physiological and Transcriptomic Analysis of Yellow Leaf Coloration in Populus Deltoides Marsh. PLoS ONE 2019, 14, e0216879. [Google Scholar] [CrossRef]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid Metabolism: Critical Roles in Male Fertility and Other Aspects of Reproductive Development in Plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef]
- Shi, H.; Liu, G.; Wei, Y.; Chan, Z. The Zinc-Finger Transcription Factor ZAT6 Is Essential for Hydrogen Peroxide Induction of Anthocyanin Synthesis in Arabidopsis. Plant Mol. Biol. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Ji, J.; Cao, W.; Tong, L.; Fang, Z.; Zhang, Y.; Zhuang, M.; Wang, Y.; Yang, L.; Lv, H. Identification and Validation of an ECERIFERUM2- LIKE Gene Controlling Cuticular Wax Biosynthesis in Cabbage (Brassica Oleracea L. Var. Capitata L.). Theor. Appl. Genet. 2021, 134, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.E.; Okazaki, Y.; Schelling, M.A.; Davis, A.; Zheng, X.; Rizhsky, L.; Yandeau-Nelson, M.D.; Saito, K.; Nikolau, B.J. Maize Glossy2 and Glossy2-like Genes Have Overlapping and Distinct Functions in Cuticular Lipid Deposition. Plant Physiol. 2020, 183, 840–853. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Y.; Zhang, D.; Dong, X.; Tian, L.; Qu, L.Q. A β-Ketoacyl-CoA Synthase Is Involved in Rice Leaf Cuticular Wax Synthesis and Requires a CER2-LIKE Protein as a Cofactor. Plant Physiol. 2017, 173, 944–955. [Google Scholar] [CrossRef]
- Haslam, T.M.; Gerelle, W.K.; Graham, S.W.; Kunst, L. The Unique Role of the ECERIFERUM2-LIKE Clade of the Bahd Acyltransferase Superfamily in Cuticularwax Metabolism. Plants 2017, 6, 23. [Google Scholar] [CrossRef]
- Negruk, V.; Yang, P.; Subramanian, M.; McNevin, J.P.; Lemieux, B. Molecular Cloning and Characterization of the CER2 Gene of Arabidopsis Thaliana. Plant J. 1996, 9, 137–145. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant Cuticles Shine: Advances in Wax Biosynthesis and Export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, J.H.; Lee, S.B.; Go, Y.S.; Kim, H.J.; Cahoon, R.; Markham, J.E.; Cahoon, E.B.; Suh, M.C. Arabidopsis 3-Ketoacyl-Coenzyme a Synthase9 Is Involved in the Synthesis of Tetracosanoic Acids as Precursors of Cuticular Waxes, Suberins, Sphingolipids, and Phospholipids. Plant Physiol. 2013, 162, 567–580. [Google Scholar] [CrossRef]
- Gou, J.Y.; Yu, X.H.; Liu, C.J. A Hydroxycinnamoyltransferase Responsible for Synthesizing Suberin Aromatics in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 18855–18860. [Google Scholar] [CrossRef] [PubMed]
- Kosma, D.K.; Molina, I.; Ohlrogge, J.B.; Pollard, M. Identification of an Arabidopsis Fatty Alcohol: Caffeoyl-Coenzyme a Acyltransferase Required for the Synthesis of Alkyl Hydroxycinnamates in Root Waxes. Plant Physiol. 2012, 160, 237–248. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of Plant Cuticular Waxes. In Annual Plant Reviews; E-Publishing Inc.: Chicago, CA, USA, 2007; Volume 23. [Google Scholar]
- Cheng, A.X.; Gou, J.Y.; Yu, X.H.; Yang, H.; Fang, X.; Chen, X.Y.; Liu, C.J. Characterization and Ectopic Expression of a Populus Hydroxyacid Hydroxycinnamoyltransferase. Mol. Plant 2013, 6, 1889–1903. [Google Scholar] [CrossRef]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritzenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Silencing of Hydroxycinnamoyl-Coenzyme A Shikimate/Quinate Hydroxycinnamoyltransferase Affects Phenylpropanoid Biosynthesis. Plant Cell. 2004, 16, 1446–1465. [Google Scholar] [CrossRef]
- Xu, D.; Shi, J.; Rautengarten, C.; Yang, L.; Qian, X.; Uzair, M.; Zhu, L.; Luo, Q.; An, G.; Waßmann, F.; et al. Defective Pollen Wall 2 (Dpw2) Encodes an Acyl Transferase Required for Rice Pollen Development. Plant Physiol. 2017, 173, 240–255. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.L.; Zhou, W.T.; Zhang, C.; Zhang, Z.Y.; Lou, Y.; Xiong, S.X.; Yao, X.Z.; Fan, J.J.; Zhu, J.; et al. The Regulation of Sporopollenin Biosynthesis Genes for Rapid Pollen Wall Formation. Plant Physiol. 2018, 178, 283–294. [Google Scholar] [CrossRef]
- Grienenberger, E.; Quilichini, T.D. The Toughest Material in the Plant Kingdom: An Update on Sporopollenin. Front. Plant Sci. 2021, 12, 703864. [Google Scholar] [CrossRef]
- Roumani, M.; Besseau, S.; Gagneul, D.; Robin, C.; Larbat, R. Phenolamides in Plants: An Update on Their Function, Regulation, and Origin of Their Biosynthetic Enzymes. J. Exp. Bot. 2021, 72, 2334–2355. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.E.; Esfeld, K.; Jäggi, L.; Mandel, T.; Cannarozzi, G.M.; Kuhlemeier, C. Complex Evolution of Novel Red Floral Color in Petunia. Plant Cell. 2021, 33, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y. AfCHIL, a Type IV Chalcone Isomerase, Enhances the Biosynthesis of Naringenin in Metabolic Engineering. Front. Plant Sci. 2022, 13, 891066. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Saito, K. S-Alk(En)Ylcysteine Sulfoxides in the Genus Allium: Proposed Biosynthesis, Chemical Conversion, and Bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef]
- Liu, Q.; Lan, Y.; Wen, C.; Zhao, H.; Wang, J.; Wang, Y. Transcriptome Sequencing Analyses between the Cytoplasmic Male Sterile Line and Its Maintainer Line in Welsh Onion (Allium Fistulosum L.). Int. J. Mol. Sci. 2016, 17, 1058. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Vigil, E.; vonLoessl, M.E.; Chen, J.Y.; Li, S.; Haslam, T.M.; Kunst, L.; Mansfield, S.D. Understanding the Role of Populus ECERIFERUM2-Likes in the Biosynthesis of Very-Long-Chain Fatty Acids for Cuticular Waxes. Plant Cell. Physiol. 2021, 62, 827–838. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Wang, Y.; Liu, L. Identification and Characterization of a Glossy Mutant in Welsh Onion (Allium Fistulosum L.). Sci. Hortic. (Amsterdam). 2017, 225, 122–127. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Miao, J.; Hu, X.; Lyu, X.; Fang, H.; Zhou, Y.M.; Mahmoud, A.; Deng, G.; Meng, Y.Q.; et al. Chromosome-Level Genome Assembly of Bunching Onion Illuminates Genome Evolution and Flavor Formation in Allium Crops. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, L.K.; Johnson, V.E.; Tsai, C.J. Differential Phylogenetic Expansions in BAHD Acyltransferases across Five Angiosperm Taxa and Evidence of Divergent Expression among Populus Paralogues. BMC Genom. 2011, 12, 236. [Google Scholar] [CrossRef]
- Peng, M.; Gao, Y.; Chen, W.; Wang, W.; Shen, S.; Shi, J.; Wang, C.; Zhang, Y.; Zou, L.; Wang, S.; et al. Evolutionarily Distinct BAHD N-Acyltransferases Are Responsible for Natural Variation of Aromatic Amine Conjugates in Rice. Plant Cell. 2016, 28, 1533–1550. [Google Scholar] [CrossRef] [PubMed]
- Joubès, J.; Raffaele, S.; Bourdenx, B.; Garcia, C.; Laroche-Traineau, J.; Moreau, P.; Domergue, F.; Lessire, R. The VLCFA Elongase Gene Family in Arabidopsis Thaliana: Phylogenetic Analysis, 3D Modelling and Expression Profiling. Plant Mol. Biol. 2008, 67, 547–566. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Xu, H.; Zhang, W.; Xing, J.; Zhu, M.; Zhang, Y.; Wang, Y. Genome-Wide Analysis of the BAHD Family in Welsh Onion and CER2-LIKEs Involved in Wax Metabolism. Genes 2023, 14, 1286. https://doi.org/10.3390/genes14061286
Liu L, Xu H, Zhang W, Xing J, Zhu M, Zhang Y, Wang Y. Genome-Wide Analysis of the BAHD Family in Welsh Onion and CER2-LIKEs Involved in Wax Metabolism. Genes. 2023; 14(6):1286. https://doi.org/10.3390/genes14061286
Chicago/Turabian StyleLiu, Lecheng, Huanhuan Xu, Wanyue Zhang, Jiayi Xing, Mingzhao Zhu, Yuchen Zhang, and Yongqin Wang. 2023. "Genome-Wide Analysis of the BAHD Family in Welsh Onion and CER2-LIKEs Involved in Wax Metabolism" Genes 14, no. 6: 1286. https://doi.org/10.3390/genes14061286
APA StyleLiu, L., Xu, H., Zhang, W., Xing, J., Zhu, M., Zhang, Y., & Wang, Y. (2023). Genome-Wide Analysis of the BAHD Family in Welsh Onion and CER2-LIKEs Involved in Wax Metabolism. Genes, 14(6), 1286. https://doi.org/10.3390/genes14061286




