A Genome-Wide Analysis of a Sudden Cardiac Death Cohort: Identifying Novel Target Variants in the Era of Molecular Autopsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Controls
- Normal heart, when no macroscopic and microscopic alterations were found;
- Atherosclerotic coronary artery disease (CAD), when an acute coronary occlusion or severe atherosclerotic plaque with coronary luminal stenosis of >75%, in the absence of other acute diseases, was found;
- Other “highly probable” CoDs such as cardiomyopathies, myocarditis, congenital coronary artery anomalies, and channelopathies, etc.
2.2. Post-Mortem Examination
2.3. Genotyping and Data Quality Control
- Retention of autosomal markers only;
- Removal of duplicate variants;
- Retention of variants with a missingness rate lower than 5% (--geno 0.05);
- Retention of individuals with a missingness rate lower than 5% (--mind 0.05);
- Retention of variants with values of probability for Hardy–Weinberg equilibrium test below the threshold of α = 0.01/number of markers, considering the Bonferroni correction for multiple testing/(--hwe α);
- Removal of variants with a minor allele frequency (MAF) lower than 0.01 (--maf 0.01).
2.4. Bio-Geographical Ancestry
- Removal of variants with a minor allele frequency (MAF) lower than 0.01 (--maf 0.01);
- Removal of variants in linkage disequilibrium (LD), by computing pairwise linkage disequilibrium among markers in a sliding window of 50 single nucleotide variants, with a step of 5 variants and LD threshold of 0.1 (--indep-pairwise 50 5 0.1);
- Retention of individuals with a values of identity-by-descent (IBD) coefficient lower than 0.125 (--genome).
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Post-Mortem Data Collection
3.2. Analysis of Data
| Variant | Variant Type | Gene | Association |
|---|---|---|---|
| rs11220463 | Intron variant | ST3GAL4 | Total cholesterol and LDL levels, carotid intima-media thickness [30,31,32] |
| rs6693954 | Intron variant | REN | Blood pressure in type 2 diabetes patients [33] |
| rs17222723 | Missense variant | ABCC2 | Doxorubicin-induced cardiotoxicity [34] |
| rs7905784 | Missense variant | MCM10 | Myocardial infarction risk [35] |
| rs3813867 | 2KB upstream variant | CYP2E1 | Ischemic stroke, alcoholic liver cirrhosis [36,37] |
| rs9332119 | Intron variant | CYP2C9 | Warfarin dosage [38] |
| rs310831 | Missense variant | E2F7 | Venous thromboembolism [39] |
| rs1087 | 3′ UTR variant | CPB2 | Fibrinolysis inhibition level [40] |
| rs938886 | Missense variant | TEP1 | Cardiac frequency increase in gastric cancer patients [41] |
| rs2985684 | Missense variant | DNAAF2 | Carotid intima-media thickness [42] |
| rs4775041 | Intergenic variant | LIPC | Levels of triglycerides, HDL and total cholesterol [31,43] |
| rs1126464 | Missense variant | DPEP1 | Hypertension, homocysteine levels [44,45] |
| rs12986742 | Intron variant | LINC01122 | HDL levels [46] |
| rs2061347 | Intergenic variant | - | Serum linoleic acid concentration in metabolic syndrome [47] |
| rs2228314 | Missense variant | SREBF2 | Hypercholesterolemia, atherosclerosis, sudden cardiac death [29,48,49,50] |
| rs3738000 | Missense variant | NEK11 | Carotid intima-media thickness [42] |
| rs1053239 | 3′ UTR variant | CIDEC | Hypertension, response to antihypertensive drugs [51] |
| rs1870377 | Missense variant | KDR | Atherosclerosis, ischemic stroke [52] |
| rs9991328 | Intron variant | FAM13A | Triglycerides and HDL levels [31] |
| rs619203 | Missense variant | ROS1 | Atherothrombotic ischemic stroke [53] |
| rs4148821 | Intron variant | ABCB4 | Alanine aminotransferase levels [54] |
| rs42524 | Missense variant | COL1A2 | Risk of sporadic intracranial aneurysm [55] |
| rs6472155 | Intron variant | CYP7B1 | Coronary artery disease risk [56] |
| rs4149264 | Intron variant | ABCA1 | Influence on statins effectiveness [57] |
| rs7853989 | Missense variant | ABO | Risk of venous thrombosis, reduced clearance of coagulation factor VIII [58,59] |
3.3. Pathway Enrichment Analysis
3.4. Association with Autopsy Findings
4. Discussion
4.1. Case Control Study
4.2. Association with the Cause of Death (CoD)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castiglione, V.; Modena, M.; Aimo, A.; Chiti, E.; Botto, N.; Vittorini, S.; Guidi, B.; Vergaro, G.; Barison, A.; Rossi, A.; et al. Molecular Autopsy of Sudden Cardiac Death in the Genomics Era. Diagnostics 2021, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Wellens, H.J.J. Sudden Cardiac Death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- Eckart, R.E.; Shry, E.A.; Burke, A.P.; McNear, J.A.; Appel, D.A.; Castillo-Rojas, L.M.; Avedissian, L.; Pearse, L.A.; Potter, R.N.; Tremaine, L.; et al. Sudden Death in Young Adults: An Autopsy-Based Series of a Population Undergoing Active Surveillance. J. Am. Coll. Cardiol. 2011, 58, 1254–1261. [Google Scholar] [CrossRef]
- Isbister, J.; Semsarian, C. Sudden Cardiac Death: An Update. Intern. Med. J. 2019, 49, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.; Sobrino, B.; Martinez, M.; Blanco-Verea, A.; Carracedo, A. Massive Parallel Sequencing Applied to the Molecular Autopsy in Sudden Cardiac Death in the Young. Forensic Sci. Int. Genet. 2015, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.; Blanco-Verea, A.; Sobrino, B.; Santori, M.; Gil, R.; Ramos-Luis, E.; Martinez, M.; Amigo, J.; Carracedo, A. Next Generation Sequencing Challenges in the Analysis of Cardiac Sudden Death Due to Arrhythmogenic Disorders. Electrophoresis 2014, 35, 3111–3116. [Google Scholar] [CrossRef]
- Arking, D.E.; Junttila, M.J.; Goyette, P.; Huertas-Vazquez, A.; Eijgelsheim, M.; Blom, M.T.; Newton-Cheh, C.; Reinier, K.; Teodorescu, C.; Uy-Evanado, A.; et al. Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals. PLoS Genet. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Das, K.J.; Duflou, J.; Semsarian, C. Exome Analysis–Based Molecular Autopsy in Cases of Sudden Unexplained Death in the Young. Heart Rhythm 2014, 11, 655–662. [Google Scholar] [CrossRef]
- Bagnall, R.D.; Weintraub, R.G.; Ingles, J.; Duflou, J.; Yeates, L.; Lam, L.; Davis, A.M.; Thompson, T.; Connell, V.; Wallace, J.; et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N. Engl. J. Med. 2016, 374, 2441–2452. [Google Scholar] [CrossRef]
- Huertas-Vazquez, A.; Nelson, C.P.; Guo, X.; Reinier, K.; Uy-Evanado, A.; Teodorescu, C.; Ayala, J.; Jerger, K.; Chugh, H.; WTCCC+; et al. Novel Loci Associated with Increased Risk of Sudden Cardiac Death in the Context of Coronary Artery Disease. PLoS ONE 2013, 8, e59905. [Google Scholar] [CrossRef]
- Khera, A.V.; Mason-Suares, H.; Brockman, D.; Wang, M.; VanDenburgh, M.J.; Senol-Cosar, O.; Patterson, C.; Newton-Cheh, C.; Zekavat, S.M.; Pester, J.; et al. Rare Genetic Variants Associated with Sudden Cardiac Death in Adults. J. Am. Coll. Cardiol. 2019, 74, 2623–2634. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.; Castiglione, V.; Aretini, P.; Mazzanti, C.M.; Chiti, E.; Giannoni, A.; Emdin, M.; Di Paolo, M. Unveiling a Sudden Unexplained Death Case by Whole Exome Sequencing and Bioinformatic Analysis. Mol. Genet. Genom. Med. 2020, 8, e1182. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for Autopsy Investigation of Sudden Cardiac Death: 2017 Update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; McCarthy, S.A.; Hui, R.; Almarri, M.A.; Ayub, Q.; Danecek, P.; Chen, Y.; Felkel, S.; Hallast, P.; Kamm, J.; et al. Insights into Human Genetic Variation and Population History from 929 Diverse Genomes. Science 2020, 367, eaay5012. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B. Harmonisation of Medico-Legal Autopsy Rules. Committee of Ministers. Council of Europe. Int. J. Leg. Med. 1999, 113, 1–14. [Google Scholar] [CrossRef]
- Leone, O.; Agostini, V.; Graziosi, M.; Rossi, C.; Pelletti, G.; Foà, A.; Guadagnini, G.; Riefolo, M.; Ziacchi, M.; Fais, P.; et al. La morte improvvisa giovanile e in età adulta: Cause e concause. L’esperienza della rete multidisciplinare in Emilia-Romagna. G. Ital. Cardiol. 2022, 23, 200–210. [Google Scholar]
- Pelletti, G.; Rossi, F.; Garagnani, M.; Barone, R.; Roffi, R.; Fais, P.; Pelotti, S. Optimization of Cloned Enzyme Donor Immunoassay Cut-Offs for Drugs of Abuse in Post-Mortem Whole Blood. Forensic Sci. Int. 2020, 312, 110291. [Google Scholar] [CrossRef]
- Pelletti, G.; Barone, R.; Giorgetti, A.; Garagnani, M.; Rossi, F.; Fais, P.; Pelotti, S. “Light Cannabis” Consumption in a Sample of Young Adults: Preliminary Pharmacokinetic Data and Psychomotor Impairment Evaluation. Forensic Sci. Int. 2021, 323, 110822. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Giorgetti, A.; Cardella, R.; Rossi, F.; Garagnani, M.; Pascali, J.P.; Mohamed, S.; Fais, P.; Pelletti, G. Development and Validation of a Fast UPLC-MS/MS Screening Method for the Detection of 68 Psychoactive Drugs and Metabolites in Whole Blood and Application to Post-Mortem Cases. J. Pharm. Biomed. Anal. 2023, 228, 115315. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Sazzini, M.; Gnecchi Ruscone, G.A.; Giuliani, C.; Sarno, S.; Quagliariello, A.; De Fanti, S.; Boattini, A.; Gentilini, D.; Fiorito, G.; Catanoso, M.; et al. Complex Interplay between Neutral and Adaptive Evolution Shaped Differential Genomic Background and Disease Susceptibility along the Italian Peninsula. Sci. Rep. 2016, 6, 32513. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Patterson, N.; Price, A.L.; Reich, D. Population Structure and Eigenanalysis. PLoS Genet. 2006, 2, e190. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Beccacece, L.; Abondio, P.; Bini, C.; Pelotti, S.; Luiselli, D. The Link between Prostanoids and Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4193. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.J.; Adler, A.; Green, S.; Al-Zoughool, F.; Doroshenko, P.; Orr, N.; Uppal, S.; Healey, J.S.; Birnie, D.; Sanatani, S.; et al. Evaluation of Genes Encoding for the Transient Outward Current (Ito) Identifies the KCND2 Gene as a Cause of J-Wave Syndrome Associated with Sudden Cardiac Death. Circ. Cardiovasc. Genet. 2014, 7, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Sarma, D.; Sternick, E.B. Human Γ2-AMPK Mutations. Methods Mol. Biol. 2018, 1732, 581–619. [Google Scholar] [CrossRef]
- Fan, Y.-M.; Karhunen, P.J.; Levula, M.; Ilveskoski, E.; Mikkelsson, J.; Kajander, O.A.; Järvinen, O.; Oksala, N.; Thusberg, J.; Vihinen, M.; et al. Expression of Sterol Regulatory Element-Binding Transcription Factor (SREBF) 2 and SREBF Cleavage-Activating Protein (SCAP) in Human Atheroma and the Association of Their Allelic Variants with Sudden Cardiac Death. Thromb. J. 2008, 6, 17. [Google Scholar] [CrossRef]
- Ligthart, S.; Vaez, A.; Hsu, Y.-H.; Inflammation Working Group of the CHARGE Consortium; PMI-WG-XCP; LifeLines Cohort Study; Stolk, R.; Uitterlinden, A.G.; Hofman, A.; Alizadeh, B.Z.; et al. Bivariate Genome-Wide Association Study Identifies Novel Pleiotropic Loci for Lipids and Inflammation. BMC Genom. 2016, 17, 443. [Google Scholar] [CrossRef]
- Hoffmann, T.J.; Theusch, E.; Haldar, T.; Ranatunga, D.K.; Jorgenson, E.; Medina, M.W.; Kvale, M.N.; Kwok, P.-Y.; Schaefer, C.; Krauss, R.M.; et al. A Large Electronic-Health-Record-Based Genome-Wide Study of Serum Lipids. Nat. Genet. 2018, 50, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Lazarenko, V.; Churilin, M.; Azarova, I.; Klyosova, E.; Bykanova, M.; Ob’edkova, N.; Churnosov, M.; Bushueva, O.; Mal, G.; Povetkin, S.; et al. Comprehensive Statistical and Bioinformatics Analysis in the Deciphering of Putative Mechanisms by Which Lipid-Associated GWAS Loci Contribute to Coronary Artery Disease. Biomedicines 2022, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, L.U.; Östgren, C.J.; Nyström, F.H.; Länne, T. Associations of Genetic Polymorphisms in the Renin-Angiotensin System with Central Aortic and Ambulatory Blood Pressure in Type 2 Diabetic Patients. J. Renin-Angiotensin-Aldosterone Syst. 2014, 15, 61–68. [Google Scholar] [CrossRef]
- Wojnowski, L.; Kulle, B.; Schirmer, M.; Schlüter, G.; Schmidt, A.; Rosenberger, A.; Vonhof, S.; Bickeböller, H.; Toliat, M.R.; Suk, E.-K.; et al. NAD(P)H Oxidase and Multidrug Resistance Protein Genetic Polymorphisms Are Associated with Doxorubicin-Induced Cardiotoxicity. Circulation 2005, 112, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, D.; O’Meara, E.S.; Bare, L.A.; Rowland, C.M.; Louie, J.Z.; Arellano, A.R.; Lumley, T.; Rice, K.; Iakoubova, O.; Luke, M.M.; et al. Association of Gene Variants with Incident Myocardial Infarction in the Cardiovascular Health Study. Arter. Thromb. Vasc. Biol. 2008, 28, 173–179. [Google Scholar] [CrossRef]
- Kim, S.K.; Yim, S.-V.; Lee, B.-C. Association between Cytochrome P450 Promoter Polymorphisms and Ischemic Stroke. Exp. Ther. Med. 2012, 3, 261–268. [Google Scholar] [CrossRef]
- Khan, A.J.; Ruwali, M.; Choudhuri, G.; Mathur, N.; Husain, Q.; Parmar, D. Polymorphism in Cytochrome P450 2E1 and Interaction with Other Genetic Risk Factors and Susceptibility to Alcoholic Liver Cirrhosis. Mutat. Res. 2009, 664, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, D.; Ciccacci, C.; Rufini, S.; Forte, V.; Novelli, G.; Borgiani, P. Resequencing of VKORC1, CYP2C9 and CYP4F2 Genes in Italian Patients Requiring Extreme Low and High Warfarin Doses. Thromb. Res. 2013, 132, 123–126. [Google Scholar] [CrossRef]
- Deguchi, H.; Shukla, M.; Hayat, M.; Torkamani, A.; Elias, D.J.; Griffin, J.H. Novel Exomic Rare Variants Associated with Venous Thrombosis. Br. J. Haematol. 2020, 190, 783–786. [Google Scholar] [CrossRef]
- Stanne, T.M.; Olsson, M.; Lorentzen, E.; Pedersen, A.; Gummesson, A.; Gils, A.; Jood, K.; Engström, G.; Melander, O.; Declerck, P.J.; et al. A Genome-Wide Study of Common and Rare Genetic Variants Associated with Circulating Thrombin Activatable Fibrinolysis Inhibitor. Thromb. Haemost. 2018, 118, 298–308. [Google Scholar] [CrossRef]
- Yuan, Y.; Yao, S.; Luo, G.-H.; Zhang, X.-Y. Impact of Metabolism-Related Mutations on the Heart Rate of Gastric Cancer Patients after Peritoneal Lavage. World J. Clin. Cases 2021, 9, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Myint, P.K.; Voora, D.; Laskowitz, D.T.; Shi, P.; Ren, F.; Wang, H.; Yang, Y.; Huo, Y.; Gao, W.; et al. Genome-Wide Association Study on Progression of Carotid Artery Intima Media Thickness over 10 Years in a Chinese Cohort. Atherosclerosis 2015, 243, 30–37. [Google Scholar] [CrossRef]
- Willer, C.J.; Sanna, S.; Jackson, A.U.; Scuteri, A.; Bonnycastle, L.L.; Clarke, R.; Heath, S.C.; Timpson, N.J.; Najjar, S.S.; Stringham, H.M.; et al. Newly Identified Loci That Influence Lipid Concentrations and Risk of Coronary Artery Disease. Nat. Genet. 2008, 40, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-Ancestry Meta-Analyses Identify Rare and Common Variants Associated with Blood Pressure and Hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Parker, A.N.; Zee, R.R.Y.; Mälarstig, A.; Seedorf, U.; Collins, R.; Watkins, H.; Hamsten, A.; Miletich, J.P.; et al. Novel Associations of CPS1, MUT, NOX4, and DPEP1 with Plasma Homocysteine in a Healthy Population: A Genome-Wide Evaluation of 13 974 Participants in the Women’s Genome Health Study. Circ. Cardiovasc. Genet. 2009, 2, 142–150. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the Relationship between Circulating Lipoprotein Lipids and Apolipoproteins with Risk of Coronary Heart Disease: A Multivariable Mendelian Randomisation Analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Barragán, R.; González, J.I.; Giménez-Alba, I.M.; Zanón-Moreno, V.; Estruch, R.; Ramírez-Sabio, J.B.; Pascual, E.C.; et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients 2020, 12, 310. [Google Scholar] [CrossRef]
- Miserez, A.R.; Muller, P.Y.; Barella, L.; Barella, S.; Staehelin, H.B.; Leitersdorf, E.; Kark, J.D.; Friedlander, Y. Sterol-Regulatory Element-Binding Protein (SREBP)-2 Contributes to Polygenic Hypercholesterolaemia. Atherosclerosis 2002, 164, 15–26. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Li, Y.; Zhang, Z.; Zhao, Y.; Dao, J.; Xiao, Y. The Effect of Sterol Regulatory Element-Binding Protein 2 Polymorphism on the Serum Lipid in Northern Chinese Subjects. J. Lipid Res. 2005, 46, 252–257. [Google Scholar] [CrossRef]
- Robinet, P.; Védie, B.; Chironi, G.; Gariépy, J.; Simon, A.; Moatti, N.; Paul, J.-L. Characterization of Polymorphic Structure of SREBP-2 Gene: Role in Atherosclerosis. Atherosclerosis 2003, 168, 381–387. [Google Scholar] [CrossRef]
- Wang, H.; Ti, Y.; Zhang, J.-B.; Peng, J.; Zhou, H.-M.; Zhong, M.; Xing, Y.-Q.; Zhang, Y.; Zhang, W.; Wang, Z.-H. Single Nucleotide Polymorphisms in CIDEC Gene Are Associated with Metabolic Syndrome Components Risks and Antihypertensive Drug Efficacy. Oncotarget 2017, 8, 27481–27488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ge, H.; Peng, L.; Wang, B. A Meta-Analysis of the Relationship between VEGFR2 Polymorphisms and Atherosclerotic Cardiovascular Diseases. Clin. Cardiol. 2019, 42, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Metoki, N.; Yoshida, H.; Satoh, K.; Kato, K.; Hibino, T.; Yokoi, K.; Watanabe, S.; Ichihara, S.; Aoyagi, Y.; et al. Genetic Factors for Ischemic and Hemorrhagic Stroke in Japanese Individuals. Stroke 2008, 39, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Rom, O.; Surakka, I.; Graham, S.E.; Zhou, W.; Roychowdhury, T.; Fritsche, L.G.; Gagliano Taliun, S.A.; Sidore, C.; Liu, Y.; et al. Loss-of-Function Genomic Variants Highlight Potential Therapeutic Targets for Cardiovascular Disease. Nat. Commun. 2020, 11, 6417. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Ge, M.; Xu, S.; Zhao, G.; Wang, H.; Qian, H.; Zhu, N.; Pang, Q. Polymorphism Rs42524 of COL1A2 and Sporadic Intracranial Aneurysms in the Chinese Population. J. Neurosurg. 2008, 109, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, X.; Liang, A.; Wu, H.; Wang, Q.; He, J.; Long, M.; Jin, T. The Effect of CYP7B1 Polymorphisms on the Risk of Coronary Heart Disease in Hainan Han Population. BMC Med. Genom. 2021, 14, 220. [Google Scholar] [CrossRef]
- Peters, B.J.M.; Pett, H.; Klungel, O.H.; Stricker, B.H.C.; Psaty, B.M.; Glazer, N.L.; Wiggins, K.L.; Bis, J.C.; de Boer, A.; Maitland-van der Zee, A.-H. Genetic Variability within the Cholesterol Lowering Pathway and the Effectiveness of Statins in Reducing the Risk of MI. Atherosclerosis 2011, 217, 458–464. [Google Scholar] [CrossRef]
- Soria, J.M.; Morange, P.-E.; Vila, J.; Souto, J.C.; Moyano, M.; Trégouët, D.-A.; Mateo, J.; Saut, N.; Salas, E.; Elosua, R. Multilocus Genetic Risk Scores for Venous Thromboembolism Risk Assessment. J. Am. Heart Assoc. 2014, 3, e001060. [Google Scholar] [CrossRef]
- Garcia-Martínez, I.; Borràs, N.; Martorell, M.; Parra, R.; Altisent, C.; Ramírez, L.; Álvarez-Román, M.T.; Nuñez, R.; Megias-Vericat, J.E.; Corrales, I.; et al. Common Genetic Variants in ABO and CLEC4M Modulate the Pharmacokinetics of Recombinant FVIII in Severe Hemophilia A Patients. Thromb. Haemost. 2020, 120, 1395–1406. [Google Scholar] [CrossRef]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and Lipids in Cardiovascular Disease: From Mechanistic Insights to Therapeutic Targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Chung, M.K.; Campbell, K.B.; Hammadah, M.; Joglar, J.A.; Leclerc, J.; Rajagopalan, B.; On behalf of the American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Drug-Induced Arrhythmias: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e214–e233. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Amemiya-Kudo, M.; Shimano, H.; Hasty, A.H.; Yahagi, N.; Yoshikawa, T.; Matsuzaka, T.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Transcriptional Activities of Nuclear SREBP-1a, -1c, and -2 to Different Target Promoters of Lipogenic and Cholesterogenic Genes. J. Lipid Res. 2002, 43, 1220–1235. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A Review on Coronary Artery Disease, Its Risk Factors, and Therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Sara, J.D.; Eleid, M.F.; Gulati, R.; Holmes, D.R. Sudden Cardiac Death from the Perspective of Coronary Artery Disease. Mayo Clin. Proc. 2014, 89, 1685–1698. [Google Scholar] [CrossRef]
- Oh, S.-H.; Kim, Y.-H.; Park, S.-M.; Cho, S.-H.; Park, J.-S.; Jang, A.-S.; Park, S.-W.; Uh, S.-T.; Lee, Y.-M.; Kim, M.-K.; et al. Association Analysis of Thromboxane A Synthase 1 Gene Polymorphisms with Aspirin Intolerance in Asthmatic Patients. Pharmacogenomics 2011, 12, 351–363. [Google Scholar] [CrossRef]
- de Jong, J.S.S.G.; Dekker, L.R.C. Platelets and Cardiac Arrhythmia. Front. Physiol. 2010, 1, 166. [Google Scholar] [CrossRef]
- Wenzel-Seifert, K.; Wittmann, M.; Haen, E. QTc Prolongation by Psychotropic Drugs and the Risk of Torsade de Pointes. Dtsch. Ärzteblatt Int. 2011, 108, 687–693. [Google Scholar] [CrossRef]
- Paik, H.; Lee, E.; Park, I.; Kim, J.; Lee, D. Prediction of Cancer Prognosis with the Genetic Basis of Transcriptional Variations. Genomics 2011, 97, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. ABC Family Transporters. In Drug Transporters in Drug Disposition, Effects and Toxicity; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1141, pp. 13–100. [Google Scholar] [CrossRef]
- Niemeijer, M.N.; van den Berg, M.E.; Deckers, J.W.; Aarnoudse, A.L.H.J.; Hofman, A.; Franco, O.H.; Uitterlinden, A.G.; Rijnbeek, P.R.; Eijgelsheim, M.; Stricker, B.H. ABCB1 Gene Variants, Digoxin and Risk of Sudden Cardiac Death in a General Population. Heart 2015, 101, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Peck, G.R.; Blachon, S.; Lienhard, G.E. A Potential Link between Insulin Signaling and GLUT4 Translocation: Association of Rab10-GTP with the Exocyst Subunit Exoc6/6b. Biochem. Biophys. Res. Commun. 2015, 465, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.; Yaseen Hachim, M.; Khalique, A.; Mohammed, A.K.; Al Heialy, S.; Taneera, J. EXOC6 (Exocyst Complex Component 6) Is Associated with the Risk of Type 2 Diabetes and Pancreatic β-Cell Dysfunction. Biology 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Papatheodorou, E.; Mellor, G.; Raju, H.; Bastiaenen, R.; Wijeyeratne, Y.; Wasim, S.; Ensam, B.; Finocchiaro, G.; Gray, B.; et al. The Diagnostic Yield of Brugada Syndrome After Sudden Death with Normal Autopsy. J. Am. Coll. Cardiol. 2018, 71, 1204–1214. [Google Scholar] [CrossRef]
- Dampney, R.A.L. Central Neural Control of the Cardiovascular System: Current Perspectives. Adv. Physiol. Educ. 2016, 40, 283–296. [Google Scholar] [CrossRef]
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What Gets on the Nerves of Cardiac Patients? Pathophysiological Changes in Cardiac Innervation. J. Physiol. 2022, 600, 451–461. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT Mutations, Tauopathy, and Mechanisms of Neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Elia, A.; Fossati, S. Autonomic Nervous System and Cardiac Neuro-Signaling Pathway Modulation in Cardiovascular Disorders and Alzheimer’s Disease. Front. Physiol. 2023, 14, 1060666. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Machaliński, B. BDNF—A Key Player in Cardiovascular System. J. Mol. Cell. Cardiol. 2017, 110, 54–60. [Google Scholar] [CrossRef]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-Analysis of Genome-Wide Association Studies for Body Fat Distribution in 694 649 Individuals of European Ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The Relationship between Obesity and Cognitive Health and Decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
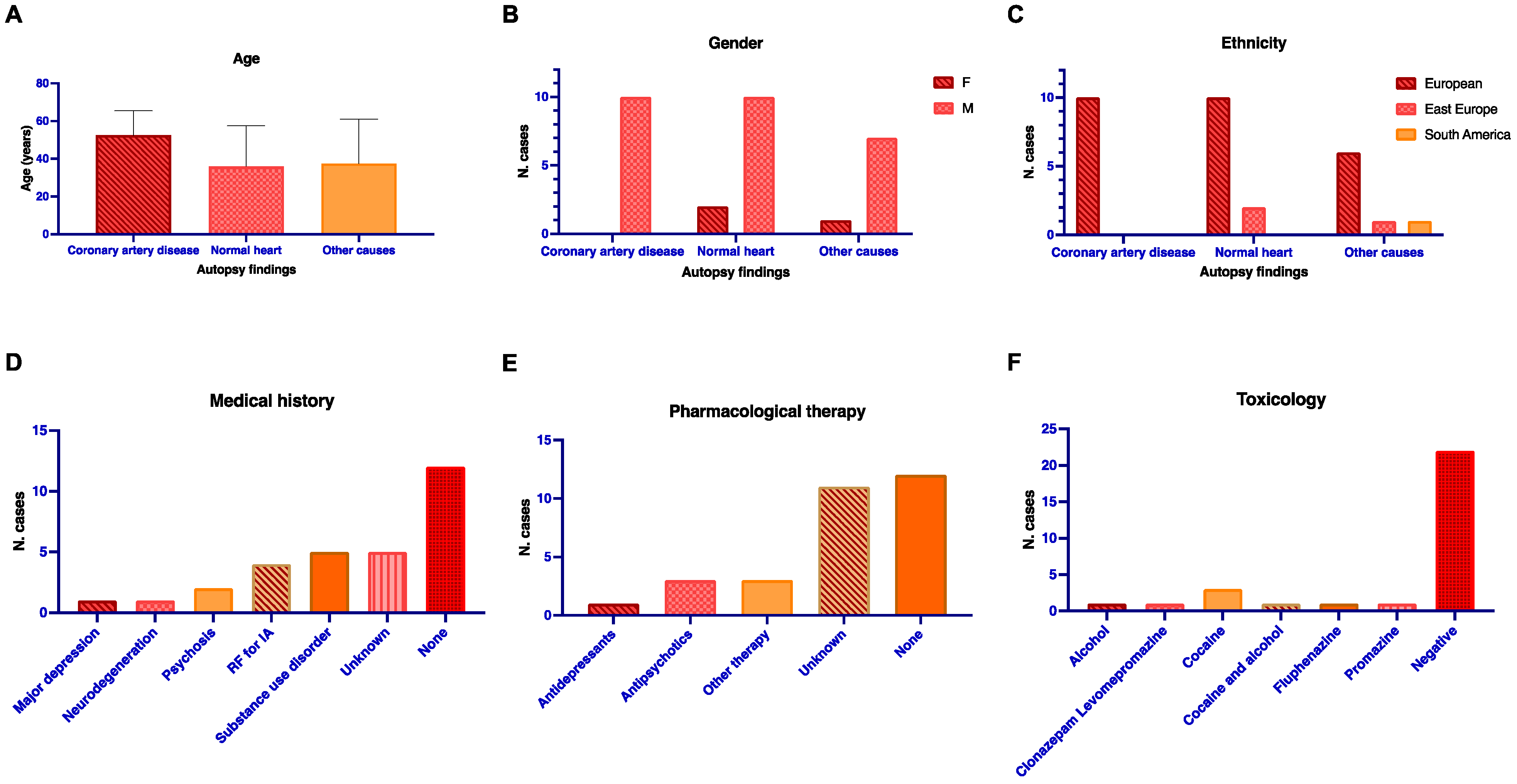

| Description | Normal Heart (n = 12) | Coronary Artery Disease (n = 10) | Other Known CoD (n = 8) | Total (n = 30) | p-Value | |
|---|---|---|---|---|---|---|
| Ancestry | European | 10 | 10 | 6 | 26 | 0.455 |
| Other | 2 | 0 | 2 | 4 | ||
| Age (mean, SD) | 38.5 (19.96) | 53.5 (14.67) | 38.3 (22.8) | 43.4 (19.9) | 0.147 | |
| Gender | M | 10 | 10 | 7 | 27 | 0.415 |
| F | 2 | 0 | 1 | 3 | ||
| Activity before death | Rest | 9 | 7 | 3 | 19 | 0.222 |
| Moderate activity | 2 | 3 | 2 | 7 | ||
| Physical activity | - | - | 2 | 2 | ||
| Psychic stress | 1 | - | 1 | 2 | ||
| Medical history | Neurologic/psychiatry | 3 | - | 1 | 4 | 0.298 |
| RF | - | 3 | 1 | 4 | ||
| Substance use disorder | 2 | 2 | 1 | 5 | ||
| None | 6 | 2 | 4 | 12 | ||
| Unknown | 1 | 3 | 1 | 5 | ||
| Toxicology | Positive | 4 | 2 | 2 | 8 | 0.744 |
| Negative | 8 | 8 | 6 | 22 | ||
| Autoptic Subgroup | Variant | Type Variant | Gene | Gene Function | p 1 | p 2 |
|---|---|---|---|---|---|---|
| Other known CoD | rs10752613 | Intergenic variant | - | - | 0.022 | 0.014 |
| rs12986742 | Intron variant | LINC01122 | Long non-coding RNA | - | 0.046 | |
| rs6746883 | Ncte variant * | SULT1C2 | Drug metabolism | 0.04 | 0.009 | |
| rs16831114 | Intergenic variant | - | - | 0.04 | - | |
| rs2602877 | Intron variant | LOC100507053 | Long non-coding RNA, alcohol addiction | 0.031 | 0.001 | |
| rs7734083 | Intron variant | RGS7BP | Brain functions, neuropsychiatric disorders, drug addiction | 0.019 | 0.041 | |
| rs2092585 | Ncte variant * | LOC105374869 | Long non-coding RNA | - | 0.027 | |
| rs7741026 | TF binding site | CARMIL1 SCGN | Cellular components organization Cellular stress response, diabetes | - | 0.03 | |
| rs3131931 | Intergenic variant | - | - | 0.022 | 0.001 | |
| rs831510 | Missense variant | FGD2 | Intracellular signaling | 0.31 | 0.008 | |
| rs747199 | Intron variant | SLC29A1 POLR1C | Adenosine transport across membranes RNA polymerase I and III subunit C | - | 0.033 | |
| rs7761731 | Missense variant | CYP39A1 | Cholesterol clearance, drug metabolism | 0.016 | 0.013 | |
| rs952884 | Intron variant | CYP39A1 | - | 0.031 | - | |
| rs9446917 | Intron variant | LOC124901342 | Long non-coding RNA | 0.022 | 0.014 | |
| rs12056033 | Intergenic variant | - | - | 0.016 | 0.018 | |
| rs10091356 | Intron variant | LOC101929028 | Long non-coding RNA | - | 0.039 | |
| rs10087388 | 3′ UTR variant | RNF170 | IP3 receptors degradation | - | 0.042 | |
| rs3747532 | Missense variant | CER1 | Embryonal development | - | 0.042 | |
| rs1818809 | Intergenic variant | - | - | 0.024 | - | |
| rs1801041 | 3′ UTR variant | DNA2 | DNA replication | - | 0.026 | |
| rs10500633 | Intron variant | MMP26 | Extracellular proteins cleavage, inflammation | 0.04 | 0.013 | |
| rs2024301 | Missense variant | CLEC4A | Immune response | - | 0.046 | |
| rs10842971 | Missense variant | PZP | Proteinase inhibition | 0.016 | 0.008 | |
| rs2306894 | Missense variant | CLEC1A | Immune response | - | 0.048 | |
| rs1971911 | 3′ UTR variant | DNM1L | Mitochondrial and peroxisomal division, apoptosis | - | 0.02 | |
| rs2288035 | 3′ UTR variant | WWOX | Neurodegeneration, cholesterol and glucose metabolisms | - | 0.044 | |
| rs2228100 | Missense variant | ALDH3A1 | Xenobiotics metabolism, cornea protection | - | 0.043 | |
| rs12951993 | Intergenic variant | - | - | 0.02 | 0.024 | |
| rs10409101 | TF binding site | - | - | - | 0.043 | |
| rs12151363 | Missense variant | TDRD12 | piRNAs metabolic processes | 0.046 | - | |
| rs400058 | Intron variant | CADM4 | Cell–cell adhesion | 0.04 | - | |
| rs4148125 | Intron variant | ABCG1 | Cholesterol metabolism | - | 0.007 | |
| Coronary artery disease | rs6746883 | Ncte variant * | SULT1C2 | Drug metabolism | - | 0.036 |
| rs4685744 | 3′ UTR variant | SUMF1 | Protein metabolism | 0.029 | 0.018 | |
| rs2477642 | Intron variant | MRC1 | Glycoprotein endocytosis by macrophages | - | 0.042 | |
| rs10852287 | Intergenic variant | - | - | 0.031 | 0.018 | |
| rs17651507 | Intron variant | MAPT | Tau protein, neurodegenerative disorders | 0.013 | 0.009 | |
| Normal heart | rs1551634 | Intergenic variant | - | - | - | 0.046 |
| rs4933754 | Intron variant | EXOC6 | Exocytosis, glucose metabolism | - | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccacece, L.; Abondio, P.; Giorgetti, A.; Bini, C.; Pelletti, G.; Luiselli, D.; Pelotti, S. A Genome-Wide Analysis of a Sudden Cardiac Death Cohort: Identifying Novel Target Variants in the Era of Molecular Autopsy. Genes 2023, 14, 1265. https://doi.org/10.3390/genes14061265
Beccacece L, Abondio P, Giorgetti A, Bini C, Pelletti G, Luiselli D, Pelotti S. A Genome-Wide Analysis of a Sudden Cardiac Death Cohort: Identifying Novel Target Variants in the Era of Molecular Autopsy. Genes. 2023; 14(6):1265. https://doi.org/10.3390/genes14061265
Chicago/Turabian StyleBeccacece, Livia, Paolo Abondio, Arianna Giorgetti, Carla Bini, Guido Pelletti, Donata Luiselli, and Susi Pelotti. 2023. "A Genome-Wide Analysis of a Sudden Cardiac Death Cohort: Identifying Novel Target Variants in the Era of Molecular Autopsy" Genes 14, no. 6: 1265. https://doi.org/10.3390/genes14061265
APA StyleBeccacece, L., Abondio, P., Giorgetti, A., Bini, C., Pelletti, G., Luiselli, D., & Pelotti, S. (2023). A Genome-Wide Analysis of a Sudden Cardiac Death Cohort: Identifying Novel Target Variants in the Era of Molecular Autopsy. Genes, 14(6), 1265. https://doi.org/10.3390/genes14061265






