Identification of MicroRNA Expression Profiles Related to the Aggressiveness of Salivary Gland Adenoid Cystic Carcinomas
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. RNA Isolation from FFPE Samples
2.3. NanoString nCounter miRNA and mRNA Assay
2.4. NanoString Data Analysis
2.5. miRNA Functional Enrichment Analysis
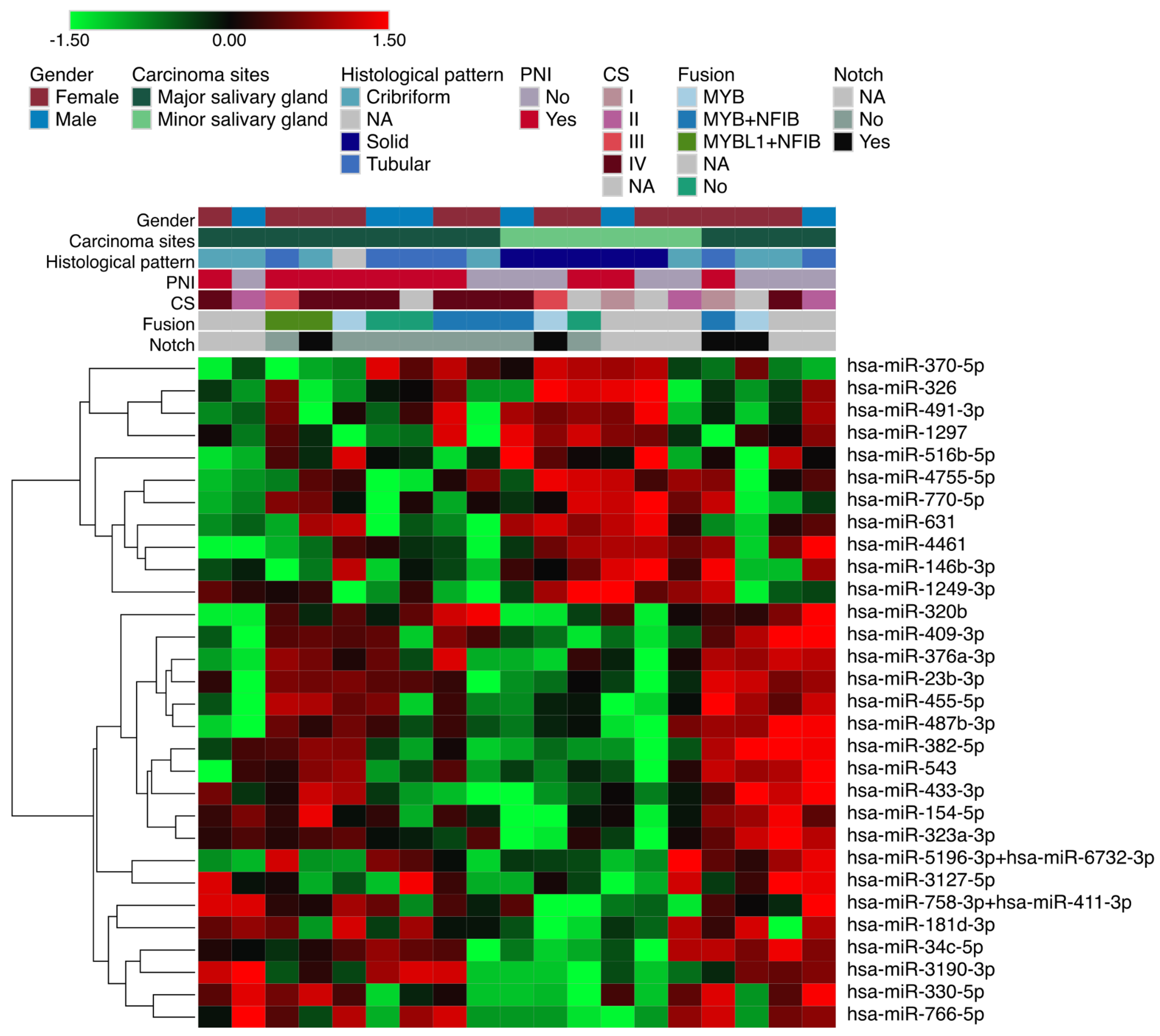
3. Results
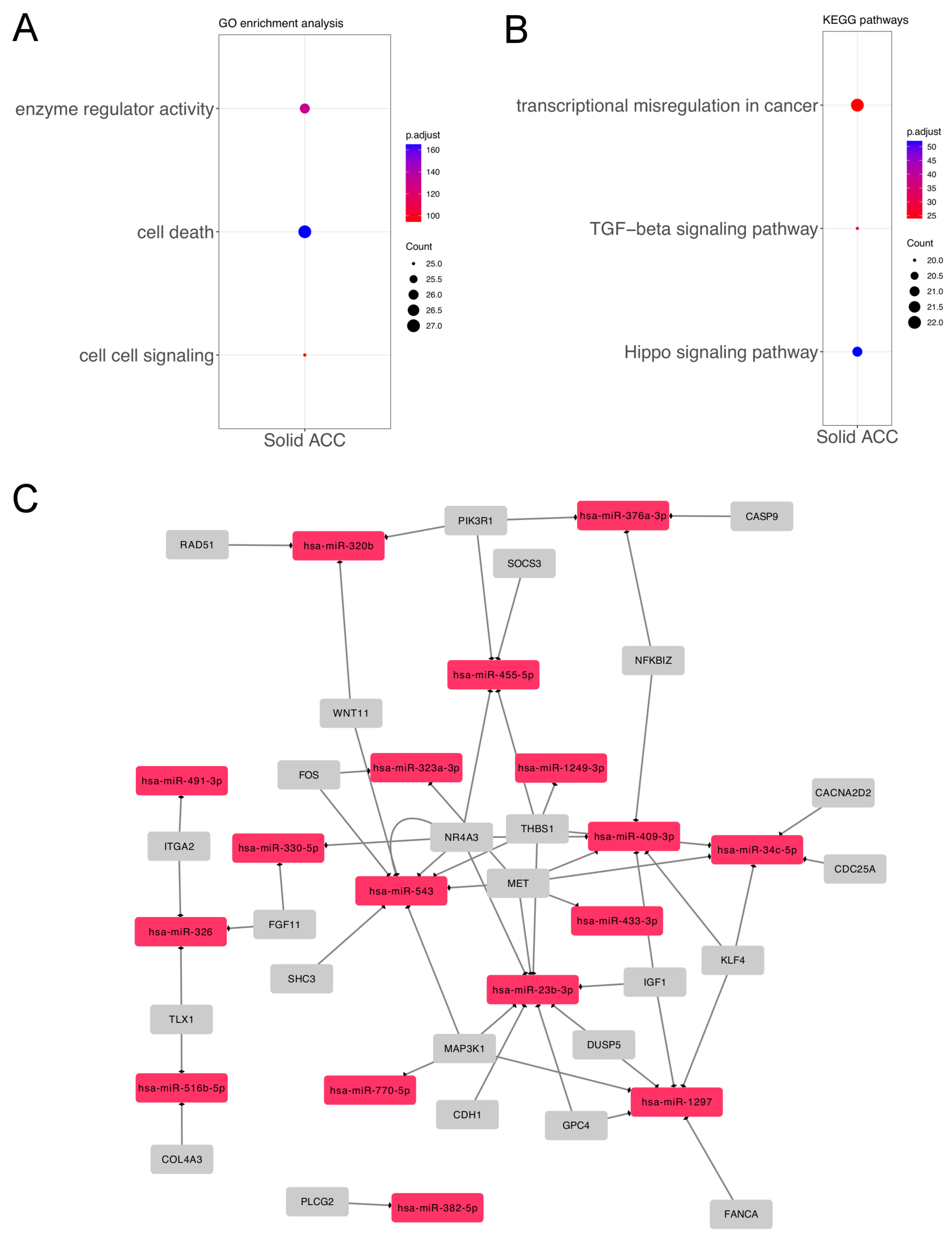
3.1. Differentially Expressed miRNAs in Solid ACC Compared to Other Growth Patterns
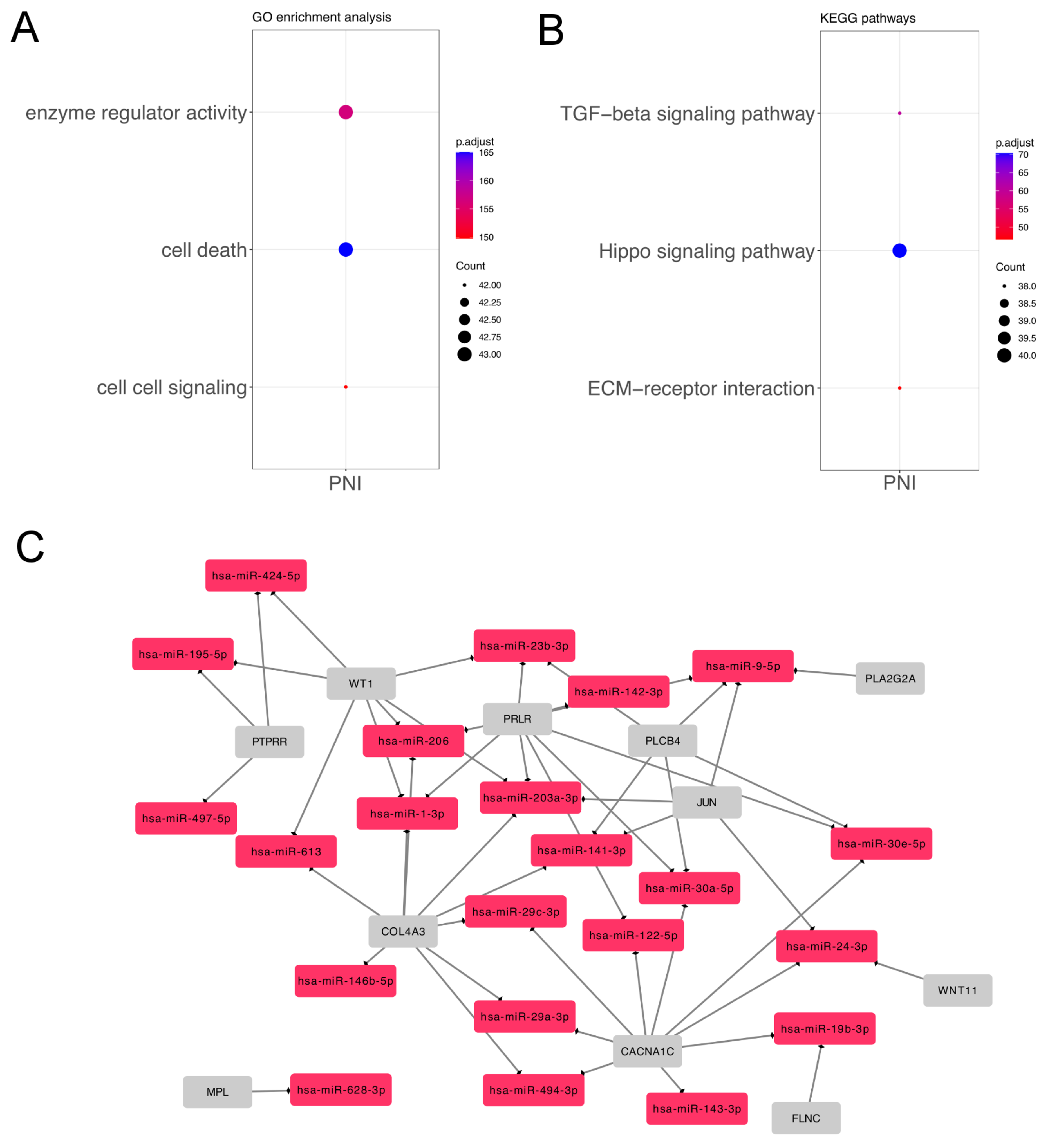
3.2. Differentially Expressed miRNAs in Salivary Gland ACC According to Perineural Invasion
3.3. MicroRNA Expression between Tumors Arising in Major and Minor Salivary Glands
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am. J. Surg. 1974, 128, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006, 85, 74–75. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Chan, J.; Grandis, J.; Takata, T.; Slootweg, P. WHO Classification of Head and Neck Tumours; IARC Publictions: Lyon, France, 2017. [Google Scholar]
- Lloyd, S.; Yu, J.B.; Wilson, L.D.; Decker, R.H. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am. J. Clin. Oncol. 2011, 34, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.N.; Morais, E.F.D.; Macedo, R.A.D.P.; Tinôco, J.M.D.L.; Morais, M.D.L.S.D.A. Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: A systematic review. Braz. J. Otorhinolaryngol. 2015, 81, 329–335. [Google Scholar] [CrossRef]
- van Weert, S.; van der Waal, I.; Witte, B.I.; Leemans, C.R.; Bloemena, E. Histopathological grading of adenoid cystic carcinoma of the head and neck: Analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015, 51, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Talbot, C.C.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.J.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.T.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef]
- Persson, M.; Andrén, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef]
- Mitani, Y.; Liu, B.; Rao, P.H.; Borra, V.J.; Zafereo, M.; Weber, R.S.; Kies, M.; Lozano, G.; Futreal, P.A.; Caulin, C.; et al. Novel MYBL1 Gene Rearrangements with Recurrent MYBL1-NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations. Clin. Cancer Res. 2016, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Frerich, C.A.; Brayer, K.J.; Painter, B.M.; Kang, H.; Mitani, Y.; El-Naggar, A.K.; Ness, S.A. Transcriptomes define distinct subgroups of salivary gland adenoid cystic carcinoma with different driver mutations and outcomes. Oncotarget 2018, 9, 7341–7358. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, P.T.; Izumchenko, E.; Meir, J.; Ha, P.K.; Sidransky, D.; Brait, M. Adenoid cystic carcinoma: Emerging role of translocations and gene fusions. Oncotarget 2016, 7, 66239–66254. [Google Scholar] [CrossRef]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. 2011, 717, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S. Molecular features of adenoid cystic carcinoma with an emphasis on microRNA expression. APMIS 2018, 126, 7–57. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Hansen, T.V.O.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; et al. MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: A cohort study. Virchows Arch. 2018, 473, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; Ulhøi, B.P.; et al. Adenoid cystic carcinomas of the salivary gland, lacrimal gland, and breast are morphologically and genetically similar but have distinct microRNA expression profiles. Mod. Pathol. 2018, 31, 1211–1225. [Google Scholar] [CrossRef]
- Kiss, O.; Tőkés, A.M.; Vranic, S.; Gatalica, Z.; Vass, L.; Udvarhelyi, N.; Szász, A.M.; Kulka, J. Expression of miRNAs in adenoid cystic carcinomas of the breast and salivary glands. Virchows Arch. 2015, 467, 551–562. [Google Scholar] [CrossRef]
- Kiss, O.; Tőkés, A.M.; Spisák, S.; Szilágyi, A.; Lippai, N.; Szász, A.M.; Kulka, J. MicroRNA-profiling in breast- and salivary gland-derived adenoid cystic carcinomas. Orvosi Hetil. 2013, 154, 963–968. [Google Scholar] [CrossRef]
- Zhang, X.; Cairns, M.; Rose, B.; O’Brien, C.; Shannon, K.; Clark, J.; Gamble, J.; Tran, N. Alterations in miRNA processing and expression in pleomorphic adenomas of the salivary gland. Int. J. Cancer 2009, 124, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Boštjančič, E.; Hauptman, N.; Grošelj, A.; Glavač, D.; Volavšek, M. Expression, Mutation, and Amplification Status of EGFR and Its Correlation with Five miRNAs in Salivary Gland Tumours. Biomed. Res. Int. 2017, 2017, 9150402. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.R.B.; Coutinho-Camillo, C.M.; Soares, F.A.; Freitas, V.S.; Vilas-Bôas, D.S.; Xavier, F.C.A.; Rocha, C.A.G.; de Araújo, I.B.; Dos Santos, J.N. MicroRNAs expression pattern related to mast cell activation and angiogenesis in paraffin-embedded salivary gland tumors. Pathol. Res. Pract. 2017, 213, 1470–1476. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhang, J.; Zhang, H.; Tang, H. miR-24-3p Suppresses Malignant Behavior of Lacrimal Adenoid Cystic Carcinoma by Targeting PRKCH to Regulate p53/p21 Pathway. PLoS ONE 2016, 11, e0158433. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yu, X.; Li, Y.; Ma, H.; Fan, S.; Chen, W.; Pan, G.; Wang, W.; Zhang, H.; Li, J.; et al. Upregulation of lncRNA ADAMTS9-AS2 Promotes Salivary Adenoid Cystic Carcinoma Metastasis via PI3K/Akt and MEK/Erk Signaling. Mol. Ther. 2018, 26, 2766–2778. [Google Scholar] [CrossRef]
- Yan, F.; Wang, C.; Li, T.; Cai, W.; Sun, J. Role of miR-21 in the growth and metastasis of human salivary adenoid cystic carcinoma. Mol. Med. Rep. 2018, 17, 4237–4244. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Dong, Z.; Cao, G.; Zhang, S. Identification of microRNA profiles in salivary adenoid cystic carcinoma cells during metastatic progression. Oncol. Lett. 2014, 7, 2029–2034. [Google Scholar] [CrossRef]
- Denaro, M.; Navari, E.; Ugolini, C.; Seccia, V.; Donati, V.; Casani, A.P.; Basolo, F. A microRNA signature for the differential diagnosis of salivary gland tumors. PLoS ONE 2019, 14, e0210968. [Google Scholar] [CrossRef]
- Brayer, K.J.; Kang, H.; El-Naggar, A.K.; Andreasen, S.; Homøe, P.; Kiss, K.; Mikkelsen, L.; Heegaard, S.; Pelaez, D.; Moeyersoms, A.; et al. Dominant Gene Expression Profiles Define Adenoid Cystic Carcinoma (ACC) from Different Tissues: Validation of a Gene Signature Classifier for Poor Survival in Salivary Gland ACC. Cancers 2023, 15, 1390. [Google Scholar] [CrossRef]
- Neuber, A.C.; Tostes, C.H.; Ribeiro, A.G.; Marczynski, G.T.; Komoto, T.T.; Rogeri, C.D.; da Silva, V.D.; Mauad, E.C.; Reis, R.M.; Marques, M.M.C. The biobank of barretos cancer hospital: 14 years of experience in cancer research. Cell Tissue Bank. 2022, 23, 271–284. [Google Scholar] [CrossRef]
- Evangelista, A.F.; Zanon, M.F.; Carloni, A.C.; de Paula, F.E.; Morini, M.A.; Ferreira-Neto, M.; Soares, I.C.; Miziara, J.E.; de Marchi, P.; Scapulatempo-Neto, C.; et al. Detection of ALK fusion transcripts in FFPE lung cancer samples by NanoString technology. BMC Pulm. Med. 2017, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Pessôa-Pereira, D.; Evangelista, A.F.; Causin, R.L.; da Costa Vieira, R.A.; Abrahão-Machado, L.F.; Santana, I.V.V.; da Silva, V.D.; de Souza, K.C.B.; de Oliveira-Silva, R.J.; Fernandes, G.C.; et al. miRNA expression profiling of hereditary breast tumors from BRCA1- and BRCA2-germline mutation carriers in Brazil. BMC Cancer 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Waggott, D.; Chu, K.; Yin, S.; Wouters, B.G.; Liu, F.F.; Boutros, P.C. NanoStringNorm: An extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics 2012, 28, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, J.; Gao, Y.; Cui, C.; Zhang, S.; Li, J.; Zhou, Y.; Cui, Q. HMDD v3.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2019, 47, D1013–D1017. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Yan, F.; Cai, W.; Zheng, J.; Jiang, X.; Sun, J. Effect of simvastatin and microRNA-21 inhibitor on metastasis and progression of human salivary adenoid cystic carcinoma. Biomed. Pharmacother. 2018, 105, 1054–1061. [Google Scholar] [CrossRef]
- Flores, B.d.C.T.d.C.P.; Lourenço, S.V.; Damascena, A.S.; Kowaslki, L.P.; Soares, F.A.; Coutinho-Camillo, C.M. Altered expression of apoptosis-regulating miRNAs in salivary gland tumors suggests their involvement in salivary gland tumorigenesis. Virchows Arch. 2017, 470, 291–299. [Google Scholar] [CrossRef]
- Nascimento, A.G.; Amaral, A.L.; Prado, L.A.; Kligerman, J.; Silveira, T.R. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 1986, 57, 312–319. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Morris, C.G.; Amdur, R.J.; Werning, J.W.; Hinerman, R.W.; Villaret, D.B. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004, 26, 154–162. [Google Scholar] [CrossRef]
- Amit, M.; Eran, A.; Billan, S.; Fridman, E.; Na’ara, S.; Charas, T.; Gil, Z. Perineural Spread in Noncutaneous Head and Neck Cancer: New Insights into an Old Problem. J. Neurol. Surg. B Skull Base 2016, 77, 86–95. [Google Scholar] [CrossRef]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Enokida, H.; Chiyomaru, T.; Kinoshita, T.; Nakagawa, M.; Naya, Y.; Ichikawa, T.; Seki, N. The microRNA-23b/27b/24-1 cluster is a disease progression marker and tumor suppressor in prostate cancer. Oncotarget 2014, 5, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhu, L.; Xu, J.; Lu, B.; Yang, Y.; Liu, F.; Wang, Z. MicroRNA-409-3p functions as a tumor suppressor in human lung adenocarcinoma by targeting c-Met. Cell. Physiol. Biochem. 2014, 34, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Huang, K.; Zhang, Q.; Zhang, Y.; Niu, J. miR-154 targeting ZEB2 in hepatocellular carcinoma functions as a potential tumor suppressor. Oncol. Rep. 2015, 34, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Xu, H.; Yang, Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem. Biophys. Res. Commun. 2016, 469, 189–195. [Google Scholar] [CrossRef]
- Ishihara, T.; Seki, N.; Inoguchi, S.; Yoshino, H.; Tatarano, S.; Yamada, Y.; Itesako, T.; Goto, Y.; Nishikawa, R.; Nakagawa, M.; et al. Expression of the tumor suppressive miRNA-23b/27b cluster is a good prognostic marker in clear cell renal cell carcinoma. J. Urol. 2014, 192, 1822–1830. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, X.F.; Li, P.Z.; Zhang, X.; Xu, Y.; Jin, X. MicroRNA-23b regulates nasopharyngeal carcinoma cell proliferation and metastasis by targeting E-cadherin. Mol. Med. Rep. 2016, 14, 537–543. [Google Scholar] [CrossRef]
- Loftus, J.C.; Ross, J.T.D.; Paquette, K.M.; Paulino, V.M.; Nasser, S.; Yang, Z.; Kloss, J.; Kim, S.; Berens, M.E.; Tran, N.L. miRNA expression profiling in migrating glioblastoma cells: Regulation of cell migration and invasion by miR-23b via targeting of Pyk2. PLoS ONE 2012, 7, e39818. [Google Scholar] [CrossRef]
- Rice, M.A.; Ishteiwy, R.A.; Magani, F.; Udayakumar, T.; Reiner, T.; Yates, T.J.; Miller, P.; Perez-Stable, C.; Rai, P.; Verdun, R.; et al. The microRNA-23b/-27b cluster suppresses prostate cancer metastasis via Huntingtin-interacting protein 1-related. Oncogene 2016, 35, 4752–4761. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, J.y.; Meng, X.N.; Xiu, Y.L.; Zong, Z.H. MiR-23b targets cyclin G1 and suppresses ovarian cancer tumorigenesis and progression. J. Exp. Clin. Cancer Res. 2016, 35, 31. [Google Scholar] [CrossRef]
- Cao, J.; Liu, J.; Long, J.; Fu, J.; Huang, L.; Li, J.; Liu, C.; Zhang, X.; Yan, Y. microRNA-23b suppresses epithelial-mesenchymal transition (EMT) and metastasis in hepatocellular carcinoma via targeting Pyk2. Biomed. Pharmacother. 2017, 89, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Tan, X.; Zhang, Q.; Liu, C.; Zhang, Y. MiR-23b-3p induces the proliferation and metastasis of esophageal squamous cell carcinomas cells through the inhibition of EBF3. Acta Biochim. Biophys. Sin. 2018, 50, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Weng, C.; Dong, H.; Li, S.; Chen, G.; Xu, Z. MicroRNA-409-3p suppresses colorectal cancer invasion and metastasis partly by targeting GAB1 expression. Int. J. Cancer 2015, 137, 2310–2322. [Google Scholar] [CrossRef]
- Josson, S.; Gururajan, M.; Hu, P.; Shao, C.; Chu, G.Y.; Zhau, H.E.; Liu, C.; Lao, K.; Lu, C.L.; Lu, Y.T.; et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin. Cancer Res. 2014, 20, 4636–4646. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, M.; Ji, H.; Chen, G.; Jiang, H.; Wang, Z. MicroRNA-181d is a tumor suppressor in human esophageal squamous cell carcinoma inversely regulating Derlin-1. Oncol. Rep. 2016, 36, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, P.S.; Wang, P.W.; Liu, C.J.; Chu, T.H.; Cheng, H.W.; Lin, S.C. miR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, N.; Hsu, B.E.; Istomine, R.; Alvarez, F.; Blagih, J.; Ma, E.H.; Morales, S.V.; Dai, D.L.; Li, G.; Souleimanova, M.; et al. Translational control in the tumor microenvironment promotes lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, E2202–E2209. [Google Scholar] [CrossRef]
- Ko, Y.H.; Lee, M.A.; Hong, Y.S.; Lee, K.S.; Jung, C.K.; Kim, Y.S.; Sun, D.I.; Kim, B.S.; Kim, M.S.; Kang, J.H. Prognostic factors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn. J. Clin. Oncol. 2007, 37, 805–811. [Google Scholar] [CrossRef]
- Jiang, L.H.; Ge, M.H.; Hou, X.X.; Cao, J.; Hu, S.S.; Lu, X.X.; Han, J.; Wu, Y.C.; Liu, X.; Zhu, X.; et al. miR-21 regulates tumor progression through the miR-21-PDCD4-Stat3 pathway in human salivary adenoid cystic carcinoma. Lab. Investig. 2015, 95, 1398–1408. [Google Scholar] [CrossRef]
- Feng, Y.H.; Tsao, C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef]
- Cinpolat, O.; Unal, Z.N.; Ismi, O.; Gorur, A.; Unal, M. Comparison of microRNA profiles between benign and malignant salivary gland tumors in tissue, blood and saliva samples: A prospective, case-control study. Braz. J. Otorhinolaryngol. 2017, 83, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Ito, T.; Mizutani, T.; Minoguchi, S.; Yamamichi, N.; Sakurai, K.; Iba, H. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 2008, 378, 492–504. [Google Scholar] [CrossRef]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A review of its role in cancers. Onco Targets Ther. 2018, 11, 7109–7123. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dai, G.; Yu, L.; Hu, Q.; Chen, J.; Guo, W. miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci. Rep. 2018, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, B.; Wang, Y.; Chen, G.; Lian, Q.; Wang, H. miR-140-3p inhibits breast cancer proliferation and migration by directly regulating the expression of tripartite motif 28. Oncol. Lett. 2019, 17, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Xu, T.; Bruce, J.P.; Yip, K.W.; Zhang, N.; Huang, Z.; Zhang, G.; Liu, F.F.; Liang, J.; Yang, H.; et al. hsa-miR-24 suppresses metastasis in nasopharyngeal carcinoma by regulating the c-Myc/epithelial-mesenchymal transition axis. Oncol. Rep. 2018, 40, 2536–2546. [Google Scholar] [CrossRef]


| Clinical Data | Category * | Frequency |
|---|---|---|
| Sex | Male | 6 (31.57%) |
| Female | 13 (68.4%) | |
| Age | Average (min–max) | 54.0 (30–89) |
| Carcinoma sites | Paranasal sinuses | 5 (26.3%) |
| Floor of the mouth | 3 (15.7%) | |
| Oropharynx | 2 (10.5%) | |
| Parotid gland | 6 (31.5%) | |
| Submandibular gland | 2 (10.5%) | |
| Base of the tongue | 1 (5.2%) | |
| Histological pattern | Cribriform | 7 (36.8%) |
| Tubular | 6 (31.5%) | |
| Solid | 5 (26.3%) | |
| N/A | 1 (5.2%) | |
| Perineural invasion | Yes | 11 (57.8%) |
| No | 8 (42.1%) | |
| Clinical stage | I | 1 (5.2%) |
| II | 5 (26.3%) | |
| III | 2 (10.5%) | |
| IV | 9 (47.3%) | |
| N/A | 2 (10.5%) | |
| Surgery | Yes | 19 (100%) |
| Radiation therapy | Yes | 19 (100%) |
| Chemotherapy | Yes | 1 (5.2%) |
| No | 18 (94.7%) | |
| Follow-up | Alive with disease | 4 (21.0%) |
| Alive without disease | 12 (63.2%) | |
| Died from the disease | 3 (15.8%) |
| Analysis | MicroRNA | HMDD Category | References |
|---|---|---|---|
| Solid growth pattern | hsa-miR-181d | Carcinoma, Adenoid Cystic | [19] |
| Solid growth pattern | hsa-miR-23b | Carcinoma, Adenoid Cystic; | [20] |
| Salivary Gland Neoplasms | [22] | ||
| Solid growth pattern | hsa-miR-455 | Carcinoma, Adenoid Cystic | [19] |
| Solid growth pattern | hsa-mir-154-5p | Salivary Gland Neoplasms | [22] |
| Solid growth pattern | hsa-mir-409 | Salivary Gland Neoplasms | [22] |
| Perineural invasion | hsa-mir-29c | Salivary Gland Neoplasms | [22] |
| Perineural invasion | hsa-mir-140 | Salivary Gland Neoplasms | [23] |
| Perineural invasion | hsa-mir-195 | Salivary Gland Neoplasms | [24] |
| Perineural invasion | hsa-mir-24 | Lacrimal Adenoid Cystic Carcinoma | [25] |
| Salivary Gland Neoplasms | [21] | ||
| Perineural invasion | hsa-mir-143 | Carcinoma, Salivary Adenoid Cystic | [26] |
| Perineural invasion | hsa-mir-21 | Carcinoma, Salivary Adenoid Cystic; | [39] |
| Carcinoma, Adenoid Cystic | [18] | ||
| Salivaray Gland Neoplasms | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanon, M.F.; Scapulatempo-Neto, C.; Gama, R.R.; Marques, M.M.C.; Reis, R.M.; Evangelista, A.F. Identification of MicroRNA Expression Profiles Related to the Aggressiveness of Salivary Gland Adenoid Cystic Carcinomas. Genes 2023, 14, 1220. https://doi.org/10.3390/genes14061220
Zanon MF, Scapulatempo-Neto C, Gama RR, Marques MMC, Reis RM, Evangelista AF. Identification of MicroRNA Expression Profiles Related to the Aggressiveness of Salivary Gland Adenoid Cystic Carcinomas. Genes. 2023; 14(6):1220. https://doi.org/10.3390/genes14061220
Chicago/Turabian StyleZanon, Maicon Fernando, Cristovam Scapulatempo-Neto, Ricardo Ribeiro Gama, Márcia Maria Chiquitelli Marques, Rui Manuel Reis, and Adriane Feijó Evangelista. 2023. "Identification of MicroRNA Expression Profiles Related to the Aggressiveness of Salivary Gland Adenoid Cystic Carcinomas" Genes 14, no. 6: 1220. https://doi.org/10.3390/genes14061220
APA StyleZanon, M. F., Scapulatempo-Neto, C., Gama, R. R., Marques, M. M. C., Reis, R. M., & Evangelista, A. F. (2023). Identification of MicroRNA Expression Profiles Related to the Aggressiveness of Salivary Gland Adenoid Cystic Carcinomas. Genes, 14(6), 1220. https://doi.org/10.3390/genes14061220







