Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sectioning and Preparation of Skin Tissues
2.3. Isolation and Culture of DPCs
2.4. Exogenous Addition of FGF9 Protein and Inhibition of FGF9 Gene Expression
2.5. Effects of FGF9 on HF Growth In Vitro
2.6. Determination of Cell Proliferation Rate and Cell Cycle
2.7. RNA Extraction, Library Construction, and High-Throughput Sequencing
2.8. Bioinformatics Analysis
2.9. qPCR
2.10. Western Blot
2.11. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
2.12. Statistical Analysis
3. Results
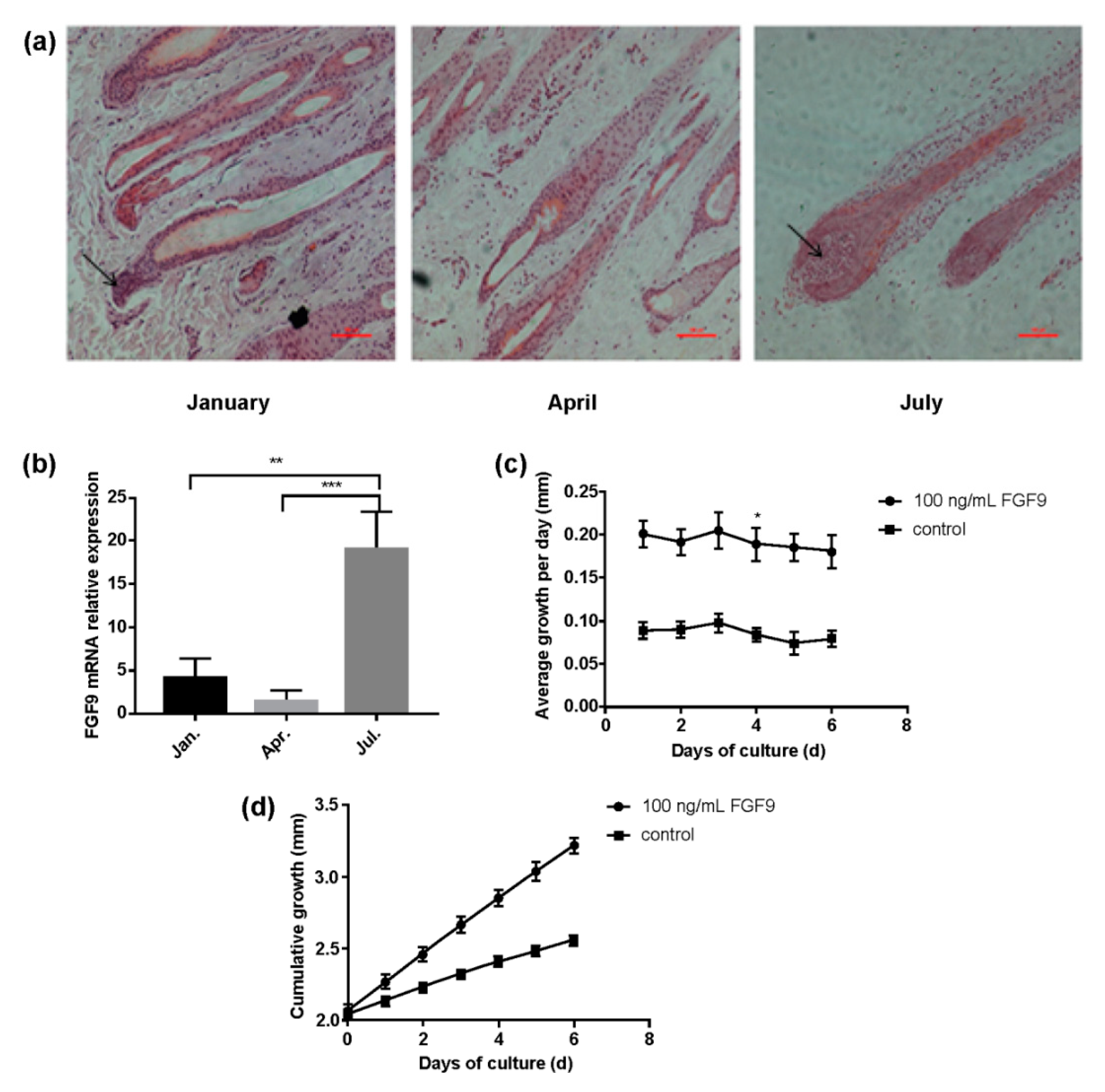
3.1. HF Cycle Phases and FGF9 Expression in Relation to Sheep Wool Growth
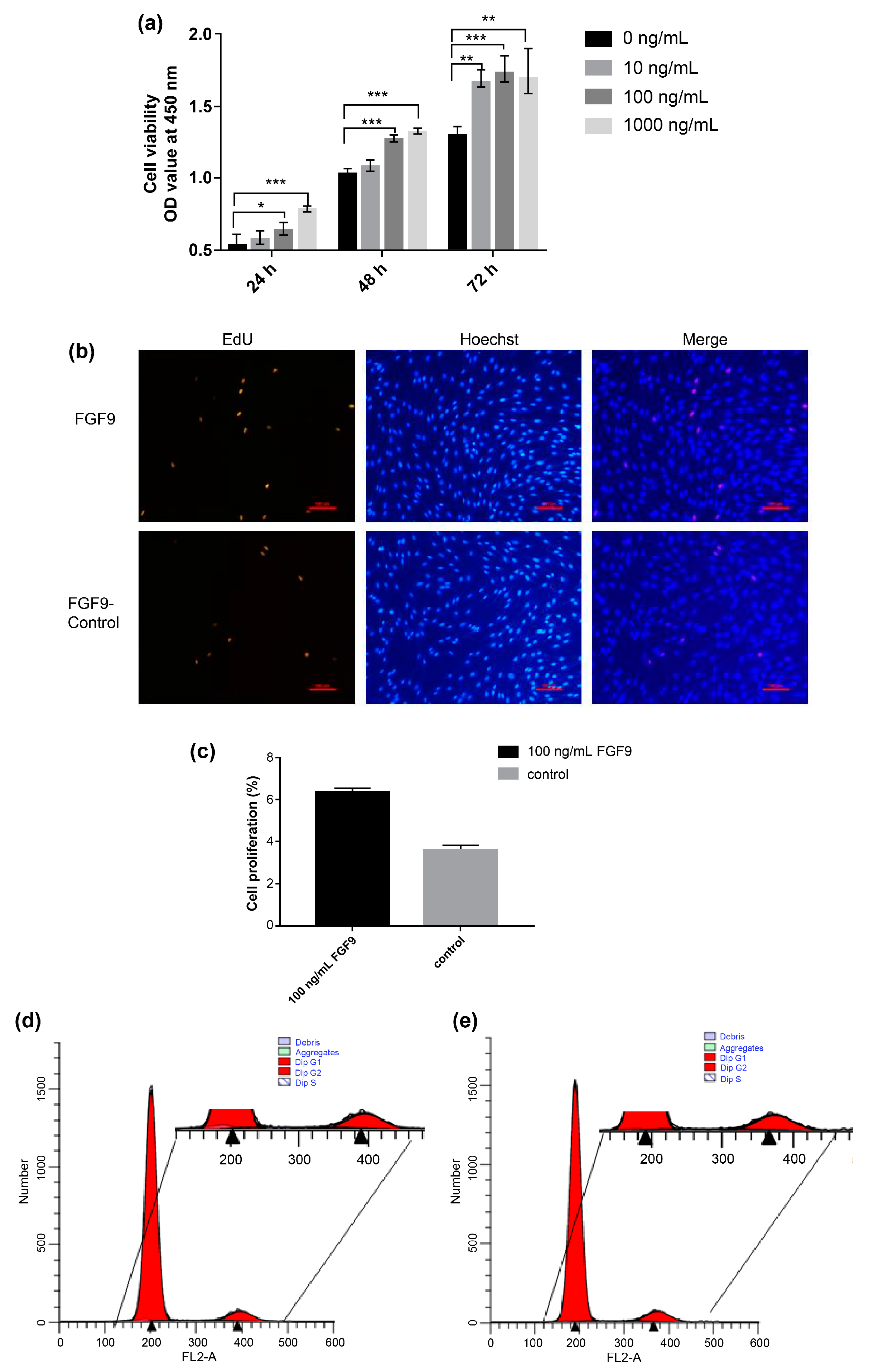
3.2. FGF9 Promotes DPC Proliferation in Small-Tailed Han Sheep
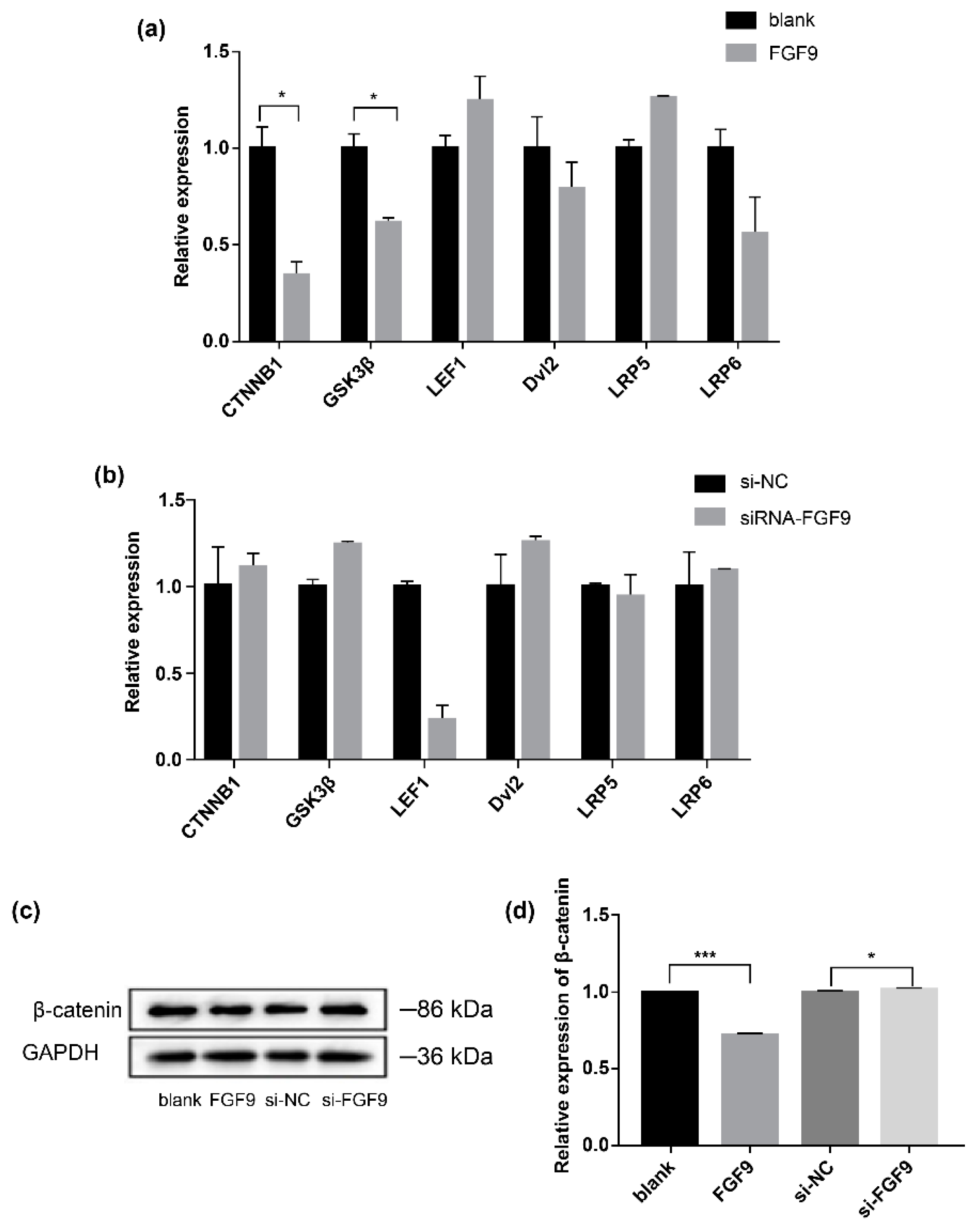
3.3. FGF9 Regulates the Expression of Key Wnt/β-Catenin Signaling Pathway Genes
3.4. Functional Enrichment Analysis of DEGs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, L.C.; Sun, F.L.; Jin, H.G.; Cao, Y.; Wei, T.; Piao, Q.L.; Zhang, M.X. A comparative study of wool and follicle traits of small-tailed Han sheep and Xinji fine wool sheep. Chin. J. Anim. Sci. 2017, 53, 52–56. [Google Scholar] [CrossRef]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Chudova, D.; Hatfield, G.W.; Smyth, P.; Andersen, B. Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance. Proc. Natl. Acad. Sci. USA 2004, 101, 15955–15960. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Foitzik, K. In search of the “hair cycle clock”: A guided tour. Differentiation 2004, 72, 489–511. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef]
- Omagari, N.; Ogawa, K. Three dimensional arrangement of fibrocytes in the dermal papilla of the human sole skin. Okajimas Folia Anat. Jpn. 1990, 67, 195–202. [Google Scholar] [CrossRef]
- Hull, S.M.; Nutbrown, M.; Pepall, L.; Thornton, M.J.; Cunliffe, W.J.; Randall, V.A. Immunohistologic and Ultrastructural Comparison of the Dermal Papilla and Hair Follicle Bulb from “Active” and “Normal” Areas of Alopecia Areata. J. Investig. Dermatol. 1991, 96, 673–681. [Google Scholar] [CrossRef]
- Elliott, K.; Stephenson, T.J.; Messenger, A.G. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef]
- Morgan, B.A. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef]
- Driskell, R.R.; Giangreco, A.; Jensen, K.B.; Mulder, K.W.; Watt, F.M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 2009, 136, 2815–2823. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Wahl, M.B.; Deng, C.; Lewandoski, M.; Pourquie, O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development 2007, 134, 4033–4041. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, W.; Wang, X.; Liu, Q.; Chu, M. Research Progress on Fibroblast Growth Factor Gene Family in Mammalian Ovary Development. Acta Vet. Zootech. Sin. 2017, 48, 1373–1380. [Google Scholar] [CrossRef]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef]
- Housley, D.J.; Venta, P.J. The long and the short of it: Evidence that FGF5 is a major determinant of canine ‘hair’-itability. Anim. Genet. 2006, 37, 309–315. [Google Scholar] [CrossRef]
- Doniach, T. Basic FGF as an inducer of anteroposterior neural pattern. Cell 1995, 83, 1067–1070. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008, 237, 18–27. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Komi-Kuramochi, A.; Kawano, M.; Oda, Y.; Asada, M.; Suzuki, M.; Oki, J.; Imamura, T. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J. Endocrinol. 2005, 186, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Kang, H.Y.; Lee, S.; Kang, S.W.; Goo, B.; Cho, S.B. Up-regulation of fibroblast growth factor (FGF) 9 expression and FGF-WNT/β-catenin signaling in laser-induced wound healing. Wound Repair Regen. 2014, 22, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, H.; Lian, Z. Bioinformatics analysis of evolutionary characteristics and biochemical structure of FGF5 Gene in sheep. Gene 2019, 702, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, X.; Yan, R.; Lu, N.; Zhang, J.; Wu, J.; He, X. Expression of FGF21 and Receptors FGFR1, FGFR2 in the First Hair Follicle Growth Cycle of Mice. Acta Vet. Zootech. Sin. 2019, 50, 534–543. [Google Scholar] [CrossRef]
- Kimura-Ueki, M.; Oda, Y.; Oki, J.; Komi-Kuramochi, A.; Honda, E.; Asada, M.; Suzuki, M.; Imamura, T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Investig. Dermatol. 2012, 132, 1338–1345. [Google Scholar] [CrossRef]
- Niu, S.; Cheng, J.; Gao, S.; Cao, X.; Wu, J.; Lu, N.; Yan, R.; He, X. Expression Profile of Fibroblast Growth Factor 7 Sub-family in the First Hair Follicle Cycle of Mice Skin. Acta Vet. Zootech. Sin. 2018, 49, 515–524. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamanishi, K.; Mori, O.; Kamikawa, M.; Andersen, B.; Kato, S.; Toyoda, T.; Yamada, G. Defective terminal differentiation and hypoplasia of the epidermis in mice lacking theFgf10gene. FEBS Lett. 2000, 481, 53–56. [Google Scholar] [CrossRef]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996, 10, 165–175. [Google Scholar] [CrossRef]
- Kobayashi, K.; Rochat, A.; Barrandon, Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc. Natl. Acad. Sci. USA 1993, 90, 7391–7395. [Google Scholar] [CrossRef]
- Topouzi, H.; Logan, N.J.; Williams, G.; Higgins, C.A. Methods for the isolation and 3D culture of dermal papilla cells from human hair follicles. Exp. Dermatol. 2017, 26, 491–496. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S.; et al. Crosstalk between androgen and Wnt/β-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef]
- Biggs, L.C.; Makela, O.J.; Myllymaki, S.M.; Das Roy, R.; Narhi, K.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. eLife 2018, 7, e36468. [Google Scholar] [CrossRef]
- Cai, J.; Wen, R.; Li, W.; Wang, X.; Tian, H.; Yi, S.; Zhang, L.; Li, X.; Jiang, C.; Li, H. Oil body bound oleosin-rhFGF9 fusion protein expressed in safflower (Carthamus tinctorius L.) stimulates hair growth and wound healing in mice. BMC Biotechnol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Harshuk-Shabso, S.; Dressler, H.; Niehrs, C.; Aamar, E.; Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114. [Google Scholar] [CrossRef]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Wu, E.; Taketo, M.M.; Morgan, B.A. β-catenin activity in the dermal papilla of the hair follicle regulates pigment-type switching. Proc. Natl. Acad. Sci. USA 2010, 107, 21564–21569. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF Signals in Heart Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef]


| Name | Sequences |
|---|---|
| siRNA–FGF9-1 | sense: 5′-GCAGGACUGGAUUUCACUUTT-3′ |
| anti-sense: 5′-AAGUGAAAUCCAGUCCUGCTT-3′ | |
| siRNA–FGF9-2 | sense: 5′-GACUCUACCUCGGCAUGAATT-3′ |
| anti-sense: 5′-UUCAUGCCGAGGUAGAGUCTT-3′ | |
| siRNA–FGF9-3 | sense: 5′-GCACCAGAAAUUCACCCAUTT-3′ |
| anti-sense: 5′-AUGGGUGAAUUUCUGGUGCTT-3′ |
| Gene Name | Sequences (5′→3′) (F: Forward, R: Reverse) | Length (bp) | Tm (°C) |
|---|---|---|---|
| FGF9-C | F: TACGGTCGGATGAGATGAA | 1070 | 60 |
| R: TTGGCAACAATGGATGTG | |||
| FGF9-R | F: AGCCAAAGTTGACAAAGACCGTT | 92 | 60 |
| R: AGCAAATCAATAGGGACCCACCG | |||
| CTNNB1 | F: GGCTACTGTTGGGTTGATTCG | 215 | 60 |
| R: GGATGTGAAGGGCTCCAGTA | |||
| GSK3β | F: ATAATCAAGGTCCTGGGAACAC | 200 | 60 |
| R: TCCAGCAGACGGCTACAAA | |||
| LEF1 | F: GCATCCCTCATCCAGCCATC | 112 | 60 |
| R: GGCTCCTGCTCCTTTCTCTG | |||
| DVL2 | F: TACCTGGTGAAGATCCCCGT | 262 | 60 |
| R: GTGGAGGAGCAAGGTCTGTC | |||
| LRP5 | F: CATCGTGGACTCTGACATTTACTG | 228 | 60 |
| R: TTACAGGCGTGGATGGAGC | |||
| LRP6 | F: TGATCTTTCAGGGGCCAACC | 73 | 60 |
| R: AAACACAGTCAGTCCCACAGG | |||
| ACTB | F: CCGCAAATGCTTCTAGGCGG | 98 | 60 |
| R: TCGCACGAGGCCAATCTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Zhang, S.; Wang, D.; Liu, J.; Luo, X.; Liu, Y.; Li, X.; Sun, F.; Xia, G.; Zhang, L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes 2023, 14, 1106. https://doi.org/10.3390/genes14051106
Jia Q, Zhang S, Wang D, Liu J, Luo X, Liu Y, Li X, Sun F, Xia G, Zhang L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes. 2023; 14(5):1106. https://doi.org/10.3390/genes14051106
Chicago/Turabian StyleJia, Qi, Shuangshuang Zhang, Dan Wang, Jianqiang Liu, Xinhui Luo, Yu Liu, Xin Li, Fuliang Sun, Guangjun Xia, and Lichun Zhang. 2023. "Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep" Genes 14, no. 5: 1106. https://doi.org/10.3390/genes14051106
APA StyleJia, Q., Zhang, S., Wang, D., Liu, J., Luo, X., Liu, Y., Li, X., Sun, F., Xia, G., & Zhang, L. (2023). Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes, 14(5), 1106. https://doi.org/10.3390/genes14051106





