Abstract
Interleukin 6 (IL-6) and C-Reactive Protein (CRP) play an important role in chronic periodontitis with coronary artery disease (CAD). Genetic factors can affect a person’s risk of CAD, which affects one-third of the population. This study investigated the role of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms. IL-6 and CRP levels on the severity of periodontitis in CAD in Indonesia were also evaluated. This case-control study was conducted with mild and moderate–severe chronic periodontitis groups. A path analysis test was conducted with Smart PLS with a 95% confidence interval to determine the significant variable for chronic periodontitis. Our study revealed that the effects of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms on IL-6 levels and CRP levels were not significant. IL-6 and CRP levels were not significantly different between the two groups. We found that IL-6 levels had a significant effect on CRP levels in periodontitis patients with CAD (path coefficient 0.322, p = 0.003). IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms had no effect on the severity of chronic periodontitis in CAD patients in the Indonesian population. We also observed no apparent effects of the influence of gene polymorphisms in IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C genes. Although the IL-6 and CRP levels were not significantly different between the two groups, IL-6 levels affected CRP levels in periodontitis patients with CAD.
1. Introduction
Coronary artery disease (CAD) is a cardiovascular disorder due to atherosclerosis or atherosclerotic occlusion of the coronary arteries [1]. It is estimated that 17.9 million people died from cardiovascular disease, heart attacks, and strokes in 2019 [2]. Several risk factors contribute to the development of coronary atherosclerosis, including diabetes mellitus, hypertension, smoking, a diet high in saturated fat, a lack of exercise, inflammatory factors [3], endothelial progenitor cells (EPCs) [4], and chronic periodontitis [5].
Periodontitis is defined as a chronic infectious disease of supporting tissues of teeth. It is associated with gene variations and environmental interaction [6,7]. This disease is a health problem, especially in developing countries [8]. The prevalence and severity of periodontitis increase with age, and this disease occurs frequently in males [9,10]. Moreover, periodontitis is associated with CAD [11,12] and an increase in early mortality [8].
The relationship between periodontitis and cardiovascular disease can develop through direct and indirect pathways. The direct pathways include bacterial invasion and infection, and the indirect pathways include increases in C-Reactive protein (CRP) and interleukin-6 (IL-6) [13,14]. Several studies reported that the involvement of CRP and CAD is associated with periodontitis [15,16,17,18]. Additionally, inflammatory mediators are also related to periodontitis [19,20].
Polymorphisms in the promoter region of the IL-6 gene trigger variations in the transcription [21]. The most frequent polymorphisms occur in 572 C/G and 174 G/C genes. The two SNPs influencing IL-6 expression and serum levels are associated with periodontitis susceptibility; however, their geographic and ethnic distribution is distinct [22,23]. The associations between IL-6 polymorphisms and periodontitis have been extensively studied. IL-6 -572 may be a genetic risk factor for periodontitis patients in Asian populations, especially the Chinese population [24]. The IL-6 -174 polymorphism is rare in the Chinese population [25]. Several studies have also shown a relationship between IL-6 polymorphisms and CAD, especially IL-6 -572 C/G [26,27,28]. Furthermore, a study reported the polymorphism of IL-6 -572 G/C is correlated with plasma concentrations of IL-6 [29].
CRP gene polymorphisms affect CRP levels in serum. Moreover, about 35–40% of variations in CRP levels between individuals are inherited [19]. Polymorphisms are associated with increased plasma CRP levels, -717 A/G, and -757 T/C genes [30]. A significant relationship has been reported between CRP -757 T/C and chronic periodontitis [31]. They play an important role in the pathophysiology of carotid atherosclerosis [32]. Meanwhile, the CRP -717 polymorphisms have been shown to be associated with CAD [33] and periodontal health in Indonesia [34]. Studies about the relationship between IL-6 polymorphisms, CRP with chronic periodontitis, and CAD are limited, including studies on polymorphisms of IL-6-572C/G and CRP+1444 C/T [25,35].
To our knowledge, there are no studies on IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels in chronic periodontitis related to CAD patients in Indonesia. Therefore, we investigated the roles of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels on the occurrence of chronic periodontitis related to CAD patients.
2. Materials and Methods
2.1. Study Design
This study was an analytic observational study with a case-control study. The. inclusion criteria were CAD patients with at least 20 teeth who underwent coronary angiography from December 2021 to October 2022 at dr. Doris Sylvanus Palangka Raya, Indonesia, and did not undergo six months of periodontal treatment. CAD patients with moderate–severe periodontitis were used as a case group, and patients with mild chronic periodontitis were used as a control group. The exclusion criteria were not using antibiotics and anti-inflammatories in the last three months, acute myocardial infarction, pregnancy, fever, pneumonia including COVID-19, and kidney failure. The sample size of each group was 40, and the samples were obtained by consecutive sampling.
Chronic periodontitis was diagnosed according to the assessment of the probing depth in 6 index teeth (16, 21, 24, 36, 41, 44) by measuring the depth of the buccal, mesial, lingual, and distal areas [36] with the Hu-Friedy PCPUNC instrument and conducting panoramic photo examination to determine alveolar reabsorption. A mean probing depth limit of 4 mm if ≥4 mm was included in the case group (moderate-severe periodontitis), while <4 mm was included in the control group (mild periodontitis).
Stenosis was considered significant if at least 70% of the main coronary arteries were in one angiographic projection, at least 50% of two projections, and 50% of the main coronary arteries [37] using Quantitative Coronary Angiography (QCA) Allura FC. Interviews, physical examinations, and clinical laboratories were conducted to determine gender, age, dyslipidemia [38], consumption of lipid-lowering drugs, hypertension (systolic >140 and diastolic >90 mmHg measured twice/previous history/consumption of anti-hypertensives), smoking [39], obesity and diabetes mellitus (diagnosed by Internist/previous history/consumption anti-DM drugs). This study was approved by the Research Ethics at RSUD, dr. Doris Sylvanus Palangka Raya, Indonesia (Number: 5641/UM-TU/RSUD/11-2021). All subjects received and signed the informed consent form.
2.2. DNA Isolation
Blood samples were collected through the cubital vein. Samples were put into each vacutainer containing EDTA anticoagulant. Peripheral blood mononuclear cell (PBMC) isolation with the addition of Ficoll was performed using the Genomic DNA Mini Kit (Blood/Cultured Cell) (Cat. No. GB100, Geneaid Biotech Ltd., New Taipei City, Taiwan), according to the manufacturer’s recommended procedures. The results of DNA extraction were stored at −20 °C until further analysis. DNA quantification was measured using the NanoDrop TM One spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, NC, USA).
2.3. Genotyping Polymorphism of IL-6 and CRP
The PCR mix consisted of 12.5 μL TaqMan® GTXpress Master Mix 2X (Cat. No. 4403311, Lot: 01324621, Applied Biosystem, Foster City, CA, USA), 1.25 μL TaqMan® SNP Genotyping Assay 20X, 1-20 ng DNA sample, and nuclease-free water to a final volume of 25 μL. Amplification was performed using a CFX 96 Touch™ Real-Time PCR (Bio-Rad, Hercules, CA, USA) with a setting of 20 s of enzyme activation at 95 °C, followed by 40 cycles of denaturation for 15 s at 95 °C and annealing extension for 1 min at 60 °C. Sample genotype was measured based on each allele’s Relative Fluorescence Unit (RFU). Materials for real-time PCR were TaqMan® Pre-Designed SNP Genotyping Assay Reagent, Thermo Fisher Scientific Baltics UAB V. Graiciuno 8 LT-02241 Vilnius Lithuania, cat No. 4351379, C32343415_10 (size S) (rs3093059), C318207_10 (size S) (rs2794521), and C__11326893_10 (size S) (rs1800796). QuantStudio 5 (Applied Biosystems, Waltham, MA, USA) was used as a thermocycler.
Forward Primer (with M13 tail) IL-6 -572 C/G gene polymorphism (rs1800796): 5′-GTAAAACGACGGCCAGTAGTGGGCTGAAGCAGGTGA-3′ (36-mer). Reverse Primer (with M13 tail): 5′-GCGGATAACAATTTCACACAGGCTTTGTTGGAGGGTGAGGG-3′ (41 -mer).
Forward Primer (with M13 tail) CRP -757 A/G gene polymorphism (rs3093059): 5′-GTAAAACGACGGCCAGTCCTTTGGAAAAGATGTATTCGG-3′ (39-mer). Reverse Primer (with M13 tail): 5′-GCGGATAACAATTTCACACAGGGACTCTACTACAAAGGATACGG-3′ (44-mer).
Forward Primer (with M13 tail) CRP -717 T/C gene polymorphism (rs2794521): 5′-GTAAAACGACGGCCAGTCCTTTGGAAAAGATGTATTCGG-3’ (39-mer. Reverse Primer (with M13 tail): 5′-GCGGATAACAATTTCACACAGGGACTCTACTACAAAGGATACGG-3′ (44-mer). DNA sequencing confirmation was conducted by 1st BASE DNA Sequencing Division, Apical Scientific Laboratory, Selangor, Malaysia.
2.4. IL-6 Levels
IL-6 levels in blood serum were measured by electrochemiluminescence immunoassay (ECLIA) analysis. The result was expressed with pg/mL units. Additionally, the result was determined by a calibration curve with a two-point calibration and a master curve provided through a reagent barcode, with normal values ≤7.00 pg/mL (Ref. 05109442 190, Roche Diagnostics GMBH Sandhofer Str 116 -68305 Mannheim).
2.5. CRP Levels
CRP levels were determined based on quantitative HsCRP in serum with the latex immunoassay principle. Agglutination was detected as a change in absorbance at a wavelength of 572 nm with the turbidimetric or immunoturbidimetric method and normal values ≤10 ng/mL (Ref. 6K26-30, Sentinel CH SpA Via Robert Koch 2 Milan 20152 Italy).
2.6. Data Analysis
Data were analyzed using SPSS statistics software for Windows, version 26, IBM Corp, Armonk, NY, USA. A chi-square test was used to determine the relationship between polymorphisms and chronic periodontitis. Continuous data were presented as mean ± standard deviation (SD) while dichotomous with frequency and percentage. Continuous data were tested for normality distribution with the Shapiro–Wilk test. An ANOVA test was performed if the distribution was normal, while Kruskal–Wallis test was used if data were not normally distributed. The relationship between IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels was determined. Genotype and allele frequencies were compared by Hardy–Weinberg equilibrium. t-test was used if data distribution was normal. The Mann–Whitney test was used if data were not normally distributed. The path analysis test was conducted with Smart PLS 3.04 GmbH, Gewerbering, Oststeibek, Germany, to determine the dominant variable for chronic periodontitis. A 95% confidence interval was used and was considered a significant difference if p ≤ 0.05.
3. Results
3.1. Subject Characteristics
Our subjects were predominantly male (72.5%) patients who were over <60 years (83.8%) and had hypertension (78.8%). Our study showed that they did not suffer from diabetes mellitus (DM) (70%), and the statuses of dyslipidemia, smoking or non-smoking, and obesity were almost the same in both groups. We found the IL-6 -572 C/G homozygote minor GG 5 gene polymorphism (6.3%), the CRP -717 T/C homozygote CC 5 gene (6.3%), and the CRP -757 A/G minor homozygote GG 2 gene (2.5%), as shown in Table 1. The frequencies of the C and G alleles and the A and G alleles appeared similar to the frequencies in Asian populations (https://www.ncbi.nlm.nih.gov/snp/rs1800796, accessed on 23 February 2023) (https://www.ncbi.nlm.nih.gov /snp/rs3093059, accessed on 23 February 2023). The frequency of the C allele was higher than the T allele compared to Asian populations (https://www.ncbi.nlm.nih.gov/snp/rs2794521, accessed on 23 February 2023).

Table 1.
Subject characteristics.
The frequency distributions of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms were 0.540, 0.764; 0.099, 0.144; 0.196, 0.907 (x2, p-value, respectively), according to the Hardy–Weinberg equilibrium. We observed no significant difference between the mild and moderate–severe chronic periodontitis groups. Therefore, the two groups were considered homogeneous (p > 0.05).
3.2. Polymorphism Analysis of the IL-6 -572 C/G Gene, the CRP -757 A/G Gene, and the CRP -717 T/C Gene for the Severity of Periodontitis, IL-6 Levels, and CRP Levels
Table 2 shows the percentage of genotypes and alleles of the IL-6 -572 C/G gene polymorphism in patients with moderate–severe periodontitis and mild periodontitis with CAD. The genotypes and alleles of the two study groups were not statistically different (p = 0.233).

Table 2.
Relationship between polymorphisms and alleles of the IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C genes with chronic periodontitis.
Regarding the IL-6 -572 C/G gene polymorphism, the percentage of the GG genotype was higher in the moderate–severe periodontitis group (10%) than in the mild periodontitis group (2.5%). Our study demonstrated that 5% of the GG genotype was found in the moderate–severe periodontitis group, while none of the GG genotype was found in the mild periodontitis group based on the CRP -757 A/G gene. We also found that the percentage of the CC genotype was lower in the moderate–severe periodontitis group (5%) than in the mild periodontitis group (7.5%) based on the CRP -717 T/C gene. Genotypes CC, CG, and GG and alleles C and G in the IL-6 -572 C/G gene; genotypes AA, AG, and GG and alleles A and G in the CRP -757 A/G gene; and genotypes TT, TC, and CC and alleles T and C in the CRP -717 T/C gene were not significantly different between the two groups (p > 0.05).
Table 3 shows the polymorphism of the IL-6 -572 C/G gene. We found that the average IL-6 level was higher in the GG genotype than in the CC and CG genotypes; however, it was not statistically significant (p = 0.579). The CRP -757 A/G gene polymorphism showed a lower average HsCRP level in the GG genotype compared to the AA and AG genotypes. However, it was not significantly different (p = 0.842). The average HsCRP level was lower in the CC genotype (4.580 mg/L) compared to the TT and TC genotypes (8.002 mg/L; 9.842 mg/L); however, there was no apparent significant difference (p = 0.490).

Table 3.
Relationship between IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels in periodontitis and CAD.
3.3. Correlation between IL-6 Levels, CRP Levels, and the Severity of Periodontitis in CAD
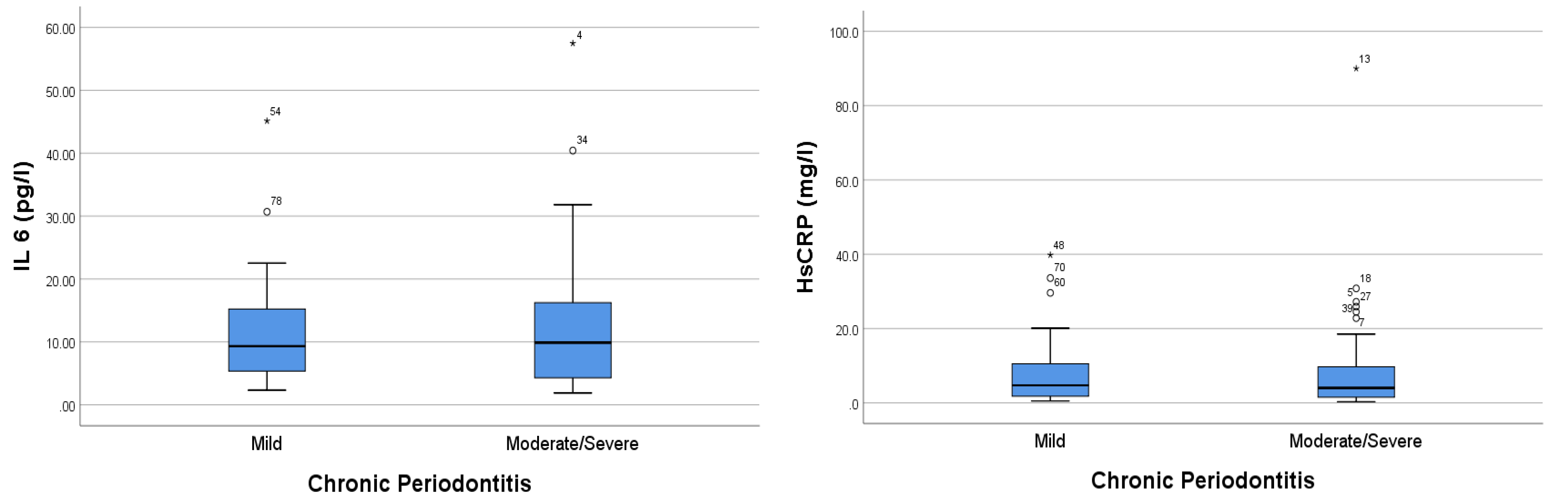
IL-6 and HsCRP levels were higher in the moderate–severe chronic periodontitis group than in the mild periodontitis group. Our results showed that the IL-6 levels in the mild and moderate–severe periodontitis groups were 11.245 ± SD 8.646 and 12.982 ± 11.752, respectively. Moreover, the CRP levels in the mild periodontitis and moderate–severe periodontitis groups were 7.675 ± SD 9.143 and 9.420 ± 15.548, respectively (Figure 1).
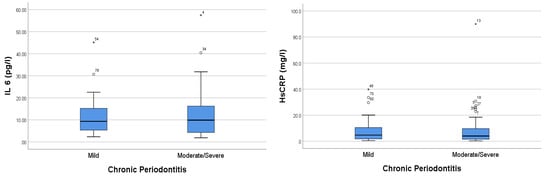
Figure 1.
Comparison analysis of IL-6 and HsCRP levels in periodontitis patients with CAD. The * sign indicates the sample data number, which is an extreme outlier.
3.4. Path Analysis Effects of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C Gene Polymorphisms; IL-6 Levels; and CRP Levels on Chronic Periodontitis in CAD
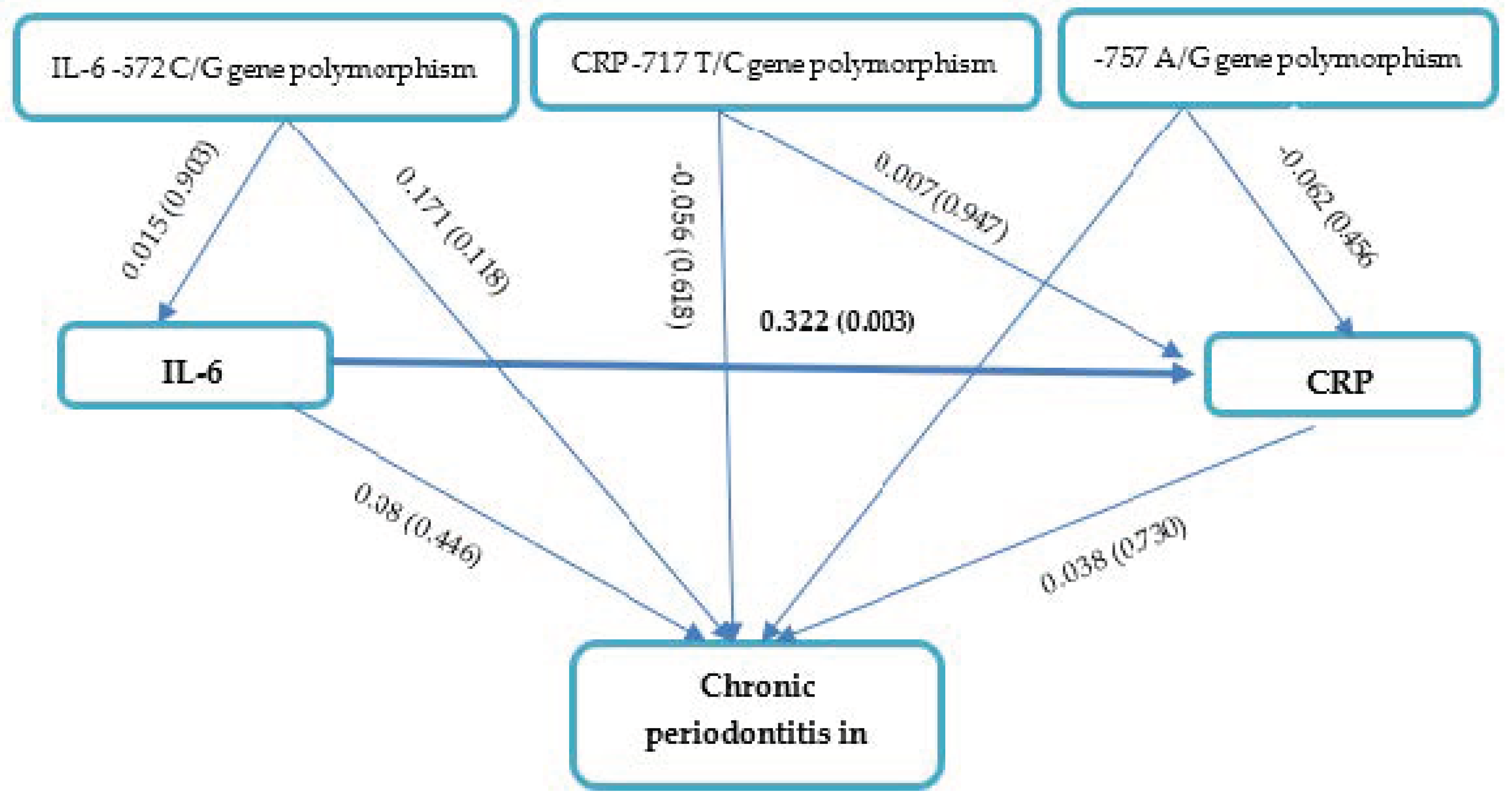
IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels had no apparent effects on the severity of chronic periodontitis in CAD based on the path analysis (Figure 2 and Table 4). Our results also demonstrated that CRP -757 A/G, CRP-717 T/C, and IL-6 -572 C/G gene polymorphisms had no effect on IL-6 levels (p > 0.05). Interestingly, IL-6 levels affected CRP levels in periodontitis patients with CAD (path coefficient 0.322, p = 0.003).
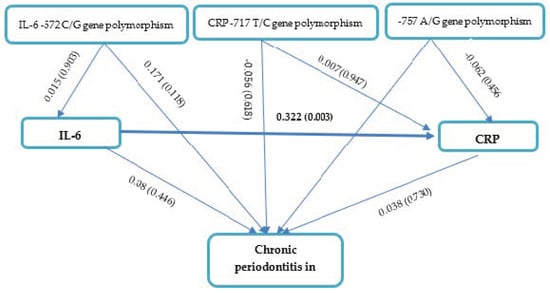
Figure 2.
Path Analysis Effects of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels on chronic periodontitis in CAD.

Table 4.
Path Coefficients (Direct and Indirect effects).
4. Discussion
4.1. Gene Polymorphisms in IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C
The present study found no association between IL-6-572 C⁄G gene polymorphism and severe chronic periodontitis-related CAD in Indonesia. The mild and. moderate–severe periodontitis groups showed no significant differences in the. proportions of genotypes CC, CG, and GG or alleles C and G. We also found that IL-6 levels were the highest in the GG genotype compared to AA and AG; however, this was not statistically significant.
The etiology and pathogenesis of periodontal disease are complex [40]. The individual risk of periodontitis can be influenced by genetic factors [41], and the disease affects one-third of the population [42]. The study focuses on the effects of IL-6 -572 C/G gene polymorphisms on chronic periodontitis in CAD, which remain unclear. Based on the previous study, the IL-6 -572 C ⁄G gene polymorphism did not correlate with chronic periodontitis susceptibility, but it was significantly different between the CAD and non-CAD groups [25].
Polymorphisms have been reported to be related with periodontitis. These genetic risk factors play an important role in Asian populations, especially the Chinese population [24], and in Europeans [43]. In contrast, polymorphism IL-6 -572 is not associated with periodontitis in the Iranian population [25]. Polymorphisms are associated with a susceptibility and risk factors of CAD [44]. Additionally, it is also associated with family histories of CAD [45]. A recent study showed a relationship between the IL-6 -572 C/G polymorphism and CAD. The CG and GG genotypes increase the risk of periodontitis-related CAD [25]. Gene polymorphisms in IL-6 influence IL-6 expression and cause. variations in transcription and expression between individuals [46,47]. The increase in IL-6 levels can be influenced by the IL-6 -572 gene polymorphism [29], idiopathic pulmonary arterial hypertension [48], DVT [49], and the interaction of the IL-6 gene promoter haplotype [45].
No studies have focused on the CRP -757 A/G and CRP -717 T/C gene polymorphisms for chronic periodontitis in CAD. Our study showed no significant difference in the. proportions of the AA, AG, and GG genotypes or the A and G alleles (-757 A/G). We did not observe significant differences in the proportions of the genotypes TT, TC, CC, T, and C (-717 T/C) and the CRP levels in both groups. The highest mean CRP levels were in the AA genotype in the CRP -757 gene and the TC genotype in the CRP -717 gene, but they were not statistically significant. Similar studies also reported a significant association of CRP -757 T/C with chronic periodontitis in South India and high-risk TT genotypes [30]. The C allele is associated with increased CRP levels [33,50]. However, other studies have found an interaction effect between gender and obesity on increasing HsCRP [51]. This condition reinforces the genetic influence on CRP production [52].
The CRP-717 T/C gene is not related to periodontitis [31,53] including in the Indonesian population [54]. It provides a complementary indicator of periodontitis risk [33]. Another study reported that the CRP -717 polymorphism predisposes to CAD allele A [32]. A relationship was also reported between CRP gene polymorphisms and increased CRP lebels in several populations, including -717 and -757 [29]. Our study reveals no effects of polymorphism on the severity of periodontitis in CAD patients. The results might have been affected by the sample size, the interaction of environmental and confounding factors [55], the differences in allele and genotype frequencies, and the heterogeneity of genetic susceptibility in several ethnic groups and races [49,56].
In the present study, the frequency of the G allele at IL-6 -572 was lower than in the Asian population. In contrast, its frequency in the European population reached 95% [57]. Allele G at CRP -757 showed a frequency of 14.4%, which is lower than the frequencies in Asia (16%) and the European population (6%) [58]. The frequency of the C allele in CRP -717 is 26.9% higher than in the Asian population (16%) and in Europe (28%) [59]. A study reported that -717 C/T dominant C allele with homozygote recessive in Iranian population with chronic periodontitis [60]. Periodontitis was found in -717 dominant genotypes AA (46.1%), AG (43%), GG (10.7%), 757 TT (81.5%) TC (16.9%), and CC (1.5%) in the South Indian population [25]. The IL-6 and CRP gene polymorphisms might be influenced by IL-6 and CRP levels, especially in terms of the sample size, the limited number of polymorphism loci, and the multifactorial influences regulating these inflammatory mediators.
4.2. IL-6 and CRP Levels
Our study emphasizes the previous study concerning the significant effect of IL-6 on increasing CRP levels in chronic periodontitis with CAD. IL-6 is an inflammatory mediator encoded by the IL-6 gene. It is involved in immunological, infection, inflammatory mechanisms, and polymorphisms in the IL-6 gene region that affect physiological function and other cytokine imbalances [61,62].
Periodontitis is associated with increased IL-6 [16] and CRP levels [17,63,64]. A relationships between IL-6 and CRP were extensively studied. CRP is an inflammatory marker and is synthesized in the liver. CRP can trigger several cytokines, such as IL-6, IL-1, TNF-α, and IFN-α [65]. Furthermore, previous studies reported a relationship between IL-6 levels and increased CRP levels in the early stages of inflammation. IL-6 is synthesized and induces CRP [66,67].
Increased CRP levels in periodontitis have been reported [68,69,70]. Subgingival biofilm bacteria increase the total leukocyte count, including CRP, which is closely related to the patient’s systemic status [71]. IL-6 induces vascular endothelial growth factor (VEGF), matrix-metalloproteinase-1 (MMP-1), cathepsin L production [72], osteoclast differentiation, and bone resorption [73,74]. These conditions trigger the loss of the periodontal ligament and alveolar bone [41]. Furthermore, RANKL or OPG has a potential marker of alveolar bone damage [75,76,77].
IL-6 and CRP play important roles in forming CAD plaques [17,26,78,79,80]. CRP is a major parameter for treating CAD patients with periodontitis [17,81,82]. In contrast, CRP is not associated with periodontitis and CAD risk [49,58,83,84]. Another study showed the presence of CRP and IL-6 in periodontitis patients with CAD [85].
The results of the current study showed that the CAD with moderate–severe periodontitis group presented a higher level of IL-6 and CRP in comparison with the mild periodontitis group; however, our results were not statistically significant. This difference could be due to differences in examination methods, especially the probing depth limit [86], the involvement of Acute Myocardial Infarction [87], and the use of statins in CHD patients to reduce levels of inflammatory mediators [88]. In addition, IL-6 and CRP can be influenced by psychosocial or environmental pressure [89].
This study is the first study to analyze the effects of IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C gene polymorphisms; IL-6 levels; and CRP levels on the severity of periodontitis in CAD, especially in the Indonesian population. However, there are several limitations. First, the sample size was only obtained from one center. Second, the type of polymorphisms studied were limited to the IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C genes. Third, our study focused on the severity of chronic periodontitis in CAD and did not involve a normal group (without periodontitis). Further studies are needed to involve healthy controls and more centers.
5. Conclusions
The chronic periodontitis population with CAD had the majority of CC, AA, and TT genotypes in IL-6 -572 C/G, CRP -757 A/G, and CRP -717 T/C C/G gene polymorphisms. Polymorphisms were not affected by the severity of chronic periodontitis, IL-6 levels, and CRP levels. In addition, IL-6 levels affected CRP levels in moderate–severe periodontitis patients with CAD. Further studies are warranted to involve IL-6 and CRP polymorphisms at other loci and with larger samples.
Author Contributions
Conceptualization: S.I.S., B.S.P. and A.; methodology: S.I.S., S.K.S., B.S.P. and A.; sample collection: M.E.S. and S.I.S.; formal analysis: S.I.S. and M.E.S.; writing—original draft preparation: S.I.S. and C.D.K.W.; writing—review and editing: C.D.K.W. and A.; supervision: B.S.P. and A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of dr. Doris Sylvanus Hospital (ethical clearance number 5641/UM-TU/RSUD/11-2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the paper.
Acknowledgments
The authors thank Yusuf Galenta, Andreriyanto, Nia Aprilia Holli, Ripka Br Tarigan, Rina Triana, Winih Ayu Jati who kindly helped in this study. We also sincerely thank to all participant in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 23 February 2023).
- Dean, K.; Triposciadis, F.; Geleris, P.; Boudoulas, H. ScienceDirect Coronary Atherosclerosis: Pathophysiologic Basis for Diagnosis and Management. Prog. Cardiovasc. Dis. 2016, 58, 1–17. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Hutomo, S.A.; Luke, K. The Role of Endothelial Progenitor Cells in Coronary Artery Disease: Basic Molecular Mechanisms and Its Clinical Potentials. Indones. Biomed. J. 2021, 13, 106–113. [Google Scholar] [CrossRef]
- Meregildo-Rodriguez, E.D.; Robles-Arce, L.G.; Chunga-Chévez, E.V.; Asmat-Rubio, M.G.; Zavaleta-Alaya, P.; Vásquez-Tirado, G.A. Periodontal disease as a non-traditional risk factor for acute coronary syndrome: A systematic review and meta-analysis. Infez. Med. 2022, 30, 501–515. [Google Scholar] [CrossRef]
- Laine, M.L.; Crielaard, W.; Loos, B.G. Genetic susceptibility to periodontitis. Periodontology 2000 2012, 58, 37–68. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 1–14. [Google Scholar] [CrossRef]
- Bokhari, S.A.; Khan, A.; Leung, W.; Wajid, G. Association of periodontal and cardiovascular diseases: South-Asian studies 2001–2012. J. Indian Soc. Periodontol. 2015, 19, 495–500. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontology 2000 2020, 84, 202–214. [Google Scholar] [CrossRef]
- Wulandari, P.; Widkaja, D.; Nasution, A.H.; Syahputra, A.; Gabrina, G. Association between age, gender and education level with the severity of periodontitis in pre-elderly and elderly patients. Maj. Kedokt. Gigi 2022, 16, 16–20. [Google Scholar] [CrossRef]
- Byun, S.H.; Lee, S.; Kang, S.H.; Choi, H.G.; Hong, S.J. Cross-sectional analysis of the association between periodontitis and cardiovascular disease using the korean genome and epidemiology study data. Int. J. Environ. Res. Public Health 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Ketabi, M.; Meybodi, F.R.; Asgari, M.R. The association between periodontal disease parameters and severity of atherosclerosis. Dent. Res. J. 2016, 13, 250–255. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Bansal, T.; Pandey, A.; Deepa, D.; Asthana, A.K. C-reactive protein (CRP) and its association with periodontal disease: A brief review. J. Clin. Diagn. Res. 2014, 8, 21–24. [Google Scholar] [CrossRef]
- Morozumi, T.; Yashima, A.; Gomi, K.; Ujiie, Y.; Izumi, Y.; Akizuki, T.; Mizutani, K.; Takamatsu, H.; Minabe, M.; Miyauchi, S.; et al. Increased systemic levels of inflammatory mediators following one-stage full-mouth scaling and root planing. J. Periodontal Res. 2018, 53, 536–544. [Google Scholar] [CrossRef]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Tabeta, K.; Hiromasa Yoshie Yamazaki, K. Current evidence and biological plausibility linking periodontitis to atherosclerotic cardiovascular disease. Jpn. Assoc. Dent. Sci. 2014, 50, 55–62. [Google Scholar] [CrossRef]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Richardson, D.R.; Morris, D.L. Gene of the month: Interleukin 6 (IL-6). J. Clin. Pathol. 2014, 67, 932–937. [Google Scholar] [CrossRef]
- Ma, H.; Sun, G.; Wang, W.; Zhou, Y.; Liu, D.; Tong, Y.; Lu, Z. Association between interleukin-6 −572 C>G and −174 G>C polymorphisms and hypertension: A meta-analysis of case-control studies. Medicine 2016, 95, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Dabrowska-Zamojcin, E.; Mazurek-Mochol, M.; Pawlik, A. Cytokines and Their Genetic Polymorphisms Related to Periodontal Disease. J. Clin. Med. 2020, 9, 4045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, X.; Li, R. Genetic Relationship Between IL-6 rs1800796 Polymorphism and Susceptibility to Periodontitis. Immunol. Investig. 2019, 48, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.H.; Liu, D.L.; Xiao, L.M.; Xie, C.J.; Sun, S.Y.; Zhang, J.C. Coronary heart disease and chronic periodontitis: Is polymorphism of interleukin-6 gene the common risk factor in a Chinese population? Oral Dis. 2011, 17, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Mansouri, K.; Hosseinian-far, A.; Ghasemi, H.; Mohammadi, M. The effect of polymorphisms (174G > C and 572C > G) on the Interleukin-6 gene in coronary artery disease: A systematic review and meta-analysis. Genes Environ. 2021, 43, 1. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.L.; Zhao, C.R.; Pan, C.L.; Zhang, Z. Interleukin-6 as a Predictor of the Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Epidemiological Studies. Immunol. Investig. 2018, 47, 689–699. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Skrip, L.; Lei, H.; Wang, Y.; Hu, D.; Ding, R. IL-6 gene polymorphisms and CAD risk: A meta-analysis. Mol. Biol. Rep. 2013, 40, 2589–2598. [Google Scholar] [CrossRef]
- Malarstig, A.; Wallentin, L.; Siegbahn, A. Genetic variation in the interleukin-6 gene in relation to risk and outcomes in acute coronary syndrome. Thromb. Res. 2017, 119, 467–473. [Google Scholar] [CrossRef]
- Suk Danik, J.; Ridker, P.M. Genetic determinants of C-reactive protein. Curr. Atheroscler. Rep. 2007, 9, 195–203. [Google Scholar] [CrossRef]
- Selvaraj, S.M.; Christina, J.J.; Gurumoorthy, S.; Jayaraman, B.G.; Vellaichamy, A. Association of −757T > C polymorphism of C-reactive protein gene with chronic periodontitis of South Indian population. Inflamm. Res. 2019, 68, 347–349. [Google Scholar] [CrossRef]
- Ben Assayag, E.; Shenhar-Tsarfaty, S.; Bova, I.; Berliner, S.; Usher, S.; Peretz, H.; Shapira, I.; Bornstein, N.M. Association of the −757T > C polymorphism in the CRP gene with circulating C-reactive protein levels and carotid atherosclerosis. Thromb. Res. 2009, 124, 458–462. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Huang, J.; Su, S.; Qiang, B.; Gu, D. −717A > G polymorphism of human C-reactive protein gene associated with coronary heart disease in ethnic Han Chinese: The Beijing atherosclerosis study. J. Mol. Med. 2005, 83, 72–78. [Google Scholar] [CrossRef]
- Auerkari, E.; Suhartono, A.; Djamal, N.; Verisqa, F.; Suryandari, D.; Kusdhany, L.; Masulili, S.; Talbot, C. CRP and IL-1B Gene Polymorphisms and CRP in Blood in Periodontal Disease. Open Dent. J. 2013, 7, 88–93. [Google Scholar] [CrossRef]
- Rocha, L.O.; Rocha, E.; Succi, G.D.M.; Brito Junior, R.B.D. Association between Periodontitis, Genetic Polymorphisms and Presence of Coronary Artery Disease in Southern Brazil. Arq. Bras. Cardiol. 2020, 114, 268–272. [Google Scholar] [CrossRef]
- Amabile, N.; Susini, G.; Bonello, L.; Gil, J.; Arques, S.; Bonfil, J.J.; Paganelli, F. Severity of periodontal disease correlates to inflammatory systemic status and independently predicts the presence and angiographic extent of stable coronary artery disease. J. Intern. Med. 2008, 263, 644–652. [Google Scholar] [CrossRef]
- Ford, T.J.; Corcoran, D.; Berry, C. Stable coronary syndromes: Pathophysiology, diagnostic advances and therapeutic need. Heart 2018, 104, 284–292. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Deanfield, J.; Bittencourt, S.; Tokg, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- PERKENI. Pedoman Pengelolaan Dislipidemi di Indonesia 2019; Pb. Perkeni Publishing: Jakarta, Indonesia, 2019. [Google Scholar]
- Doll, R.; Hill, A.B. Mortality in Relation to Smoking: Ten Years’ Observations of British Doctors. Br. Med. J. 1976, 2, 1525–1536. [Google Scholar] [CrossRef]
- Gandhi, M.; Kothiwale, S. Association of Periodontal Diseases with Genetic Polymorphisms. Int. J. Genet. Eng. 2012, 2, 19–27. [Google Scholar] [CrossRef]
- Nibali, L.; Fedele, S.; D’Aiuto, F.; Donos, N. Interleukin-6 in oral diseases: A review. Oral Dis. 2012, 18, 236–243. [Google Scholar] [CrossRef]
- Nibali, L.; Bayliss-Chapman, J.; Almofareh, S.A.; Zhou, Y.; Divaris, K.; Vieira, A.R. What Is the Heritability of Periodontitis? A Systematic Review. J. Dent. Res. 2019, 98, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Gyu, G.; Sung, S.; Choi, J.; Dae, J. Association between tumor necrosis factor—A promoter 2 308 A/G, 2 238 A/G, interleukin-6 2 174 G/C and 2 572 G/C polymorphisms and periodontal disease: A meta-analysis. Mol. Biol. Rep. 2013, 40, 5191–5203. [Google Scholar] [CrossRef]
- Salman, B.N.; Vahabi, S.; Biglari, A.; Salavitabar, S.; Doabsar, M.H. Correlation of interleukin-6-174 GC and interleukin-6-572 GC gene polymorphisms with periodontal disease in an Iranian population. Dent. Res. J. Orig. 2016, 13, 354–361. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Wang, Y.; Hu, J.; Wu, D.; Liu, S.; Zeng, X.; Yu, G.; Wang, Y.; Wang, Z. The IL-6 rs1800795 and rs1800796 polymorphisms are associated with coronary artery disease risk. J. Cell Mol. 2020, 24, 6191–6207. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.; Özçetin, M.; Ateş, Ö.; Altunkaş, F.; Karaman, K.; Akar, İ.; İnce, İ.; Yalçın, M.; Karayakalı, M.; Ceyhan, K.; et al. Analyses of C-reactive protein, endothelial nitric oxide synthase and interleukin-6 gene polymorphisms in adolescents with a family history of premature coronary artery disease: A pilot study. Balk. Med. J. 2015, 32, 397–402. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Masuda, K.; Kishimoto, T. Regulation of IL-6 in immunity and diseases. Adv. Exp. Med. Biol. 2016, 941, 79–88. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Xiao, L.; Xie, C.; Xuan, D.; Luo, G. Gene polymorphisms and periodontitis. Periodontology 2000 2011, 56, 102–124. [Google Scholar] [CrossRef]
- Fang, M.; Huang, Y.; Zhang, Y. Interleukin-6 −572C/G polymorphism is associated with serum interleukin-6 levels and risk of idiopathic pulmonary arterial hypertension. J. Am. Soc. Hypertens. 2017, 11, 171–177. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Biswas, A.; Ranjan, R.; Kishor, K.; Pandey, H.; Kumar, R.; Mahapatra, M.; Oldenburg, J.; Saxena, R. Impact of interleukin 6 promoter polymorphisms (−174 G > C, −572 G > C and −597 G > A) on plasma IL-6 levels and their influence on the development of DVT: A study from India. Hematology 2018, 23, 833–838. [Google Scholar] [CrossRef]
- Sheu WH, H.; Wang, W.C.; Wu, K.D.; He, C.T.; Hwu, C.M.; Quertermous, T.; Hsieh, W.S.; Lee, W.J.; Ting, C.T.; Chen YD, I.; et al. CRP-level-associated polymorphism rs1205 within the CRP gene is associated with 2-hour glucose level: The SAPPHIRe study. Sci. Rep. 2017, 7, 7987. [Google Scholar] [CrossRef]
- Shen, C.; Sun, X.; Wang, H.; Wang, B.; Xue, Y.; Li, Y.; Chen, J.; Jiang, Y. Association Study of CRP Gene and Ischemic Stroke in a Chinese Han Population. J. Mol. Neurosci. 2013, 49, 559–566. [Google Scholar] [CrossRef]
- Wu, F.Y.; Li, C.I.; Liao, L.N.; Liu, C.S.; Lin, W.Y.; Lin, C.H.; Yang, C.W.; Li, T.C.; Lin, C.C. Evaluation of single nucleotide polymorphisms in 6 candidate genes and carotid intima-media thickness in community-dwelling residents. PLoS ONE 2020, 15, e0230715. [Google Scholar] [CrossRef]
- Najar, R.A.; Ghaderian, S.M.H.; Panah, A.S.T. C-reactive protein (CRP) gene polymorphisms: Implication in CRP plasma levels and susceptibility to acute myocardial infarction. Mol. Biol. Rep. 2012, 39, 3705–3712. [Google Scholar] [CrossRef]
- Surena, V.; Bahareh, N.; Mahsa, K.; Sepanta, H. Association of Matrix Metalloproteinase-1 1607 1G 2G and CReactive Protein -717 C/T Gene Polymorphism in Irania Patients with Chronic Periodontitis: A Clinical Trial. Iran Red Crescent. Med. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Suhartono, A.W.; Sulijaya, B.; Djamal, N.Z.; Lelyati, S.; Masulili, C.; Talbot, C. Serum C-reactive protein and C-reactive gene (−717C > T) polymorphism are not associated with periodontitis in Indonesian male patients. Dent. J. 2015, 48, 113–118. [Google Scholar] [CrossRef]
- Shaddox, L.M.; Morford, L.A.; Nibali, L. Periodontal health and disease: The contribution of genetics. Periodontology 2000 2021, 85, 161–181. [Google Scholar] [CrossRef]
- Nibali, L.; D’Aiuto, F.; Donos, N.; Griffiths, G.S.; Parkar, M.; Tonetti, M.S.; Humphries, S.E.; Brett, P.M. Association between periodontitis and common variants in the promoter of the interleukin-6 gene. Cytokine 2009, 45, 50–54. [Google Scholar] [CrossRef]
- National Library of Medicine. dbSNP rs1800796. Released 21 September 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1800796 (accessed on 24 February 2023).
- National Library of Medicine. dbSNP rs2794521. Released 21 September 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2794521 (accessed on 24 February 2023).
- National Library of Medicine. dbSNP rs3093059. Released 21 September 2022. Available online: https://www.ncbi.nlm.nih.gov/snp/rs3093059 (accessed on 24 February 2023).
- Ghanemi, A.; St-Amand, J. Interleukin-6 as a “metabolic hormone”. Cytokine 2018, 112, 132–136. [Google Scholar] [CrossRef]
- Kushwah, A.S.; Banerjee, M. Role of Interleukin 6 (IL-6) and Genetic Variants in Complex Diseases. In Advances in Health and Disease, 26th ed.; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 1–31. [Google Scholar]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef]
- Pejcic, A.; Kesic, L.; Milasin, J. Association between Periodontopathogens and CRP Levels in Patients with Periodontitis in Serbia. J. Dent. Res. Dent. Clin. Dent. Prospect. 2011, 5, 10–16. [Google Scholar] [CrossRef]
- Cruz-Ávila, J.; Hernández-Pérez, E.; González-González, R.; Bologna-Molina, R.; Molina-Frechero, N. Periodontal Disease in Obese Patients; Interleukin-6 and C-Reactive Protein Study: A Systematic Review. Dent. J. 2022, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Billmeier, U.; Waldner, M.J.; Atreya, R.; Neurath, M.F. From physiology to disease and targeted therapy: Interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch. Toxicol. 2015, 89, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Kondapally, M.; KSV, R.; NVS, S.; Penmetsa, G.S.; Mohan, P.K.; Gangolu, M. C-Reactive Protein and Periodontal Disease. Eur. J. Mol. Clin. Med. 2020, 7, 1664–1670. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein; StatPearls: Treasure Island, CA, USA, 2022. [Google Scholar]
- Chandy, S.; Joseph, K.; Sankaranarayanan, A.; Issac, A.; Babu, G.; Wilson, B.; Joseph, J. Evaluation of c-reactive protein and fibrinogen in patients with chronic and aggressive periodontitis: A clinico-biochemical study. J. Clin. Diagn. Res. 2017, 11, ZC41–ZC45. [Google Scholar] [CrossRef]
- Esteves-Lima, R.P.; Reis, C.S.; Santirocchi-Júnior, F.; Abreu, L.G.; Costa, F.O. Association between periodontitis and serum c-reactive protein levels. J. Clin. Exp. Dent. 2020, 12, e838–e843. [Google Scholar] [CrossRef]
- Martu, S.; Nicolaiciuc, O.; Solomon, S.; Sufaru, I.; Scutariu, M.; Rezus, C.; Popescu, E. The evaluation of the c reactive protein levels in the context of the periodontal pathogens presence in cardiovascular risk patients. Rev. De Chim. 2017, 68, 1081–1084. [Google Scholar] [CrossRef]
- Naruishi, K. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J. Cell Physiol. 2018, 233, 6393–6400. [Google Scholar] [CrossRef]
- Shao, M.Y.; Huang, P.; Cheng, R.; Hu, T. Interleukin-6 polymorphisms modify the risk of periodontitis: A systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 2009, 10, 920–927. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef]
- Diyatri, I. Analisis Polimorfisme Gen OPG dan MMP-1, Serta Profil OPG, RANKL, MMP-I, danTIMP-1 pada Penderita Periodontitis Agresif di Surabaya. Ph.D. Thesis, ADLN Perpustakaan Universitas Airlangga, Surabaya, Indonesia, 2013. [Google Scholar]
- Delange, N.; Lindsay, S.; Lemus, H.; Finlayson, T.L.; Kelley, S.T.; Gottlieb, R.A. Periodontal disease and its connection to systemic biomarkers of cardiovascular disease in young american Indian/Alaskan natives. J. Periodontol. 2018, 89, 219–227. [Google Scholar] [CrossRef]
- Rodrigues, W.; Miguel, C.; Lazo-Chica, J.; Trindade Da Silva, C.; Vieira, C.; Clemente-Napimoga, J.; Freire Oliveira, C.; Napimoga, M. Interleukin-6, tumor necrosis factor-α, C-reactive protein, and hematological parameters in experimental periodontal disease after β-adrenergic blockade. J. Indian Soc. Periodontol. 2019, 23, 511–516. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690. [Google Scholar] [CrossRef]
- Held, C.; White, H.D.; Stewart RA, H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6, e005077. [Google Scholar] [CrossRef]
- Susilo, H.; Thaha, M.; Pikir, B.S.; Alsagaff, M.Y.; Suryantoro, S.D.; Wungu CD, K.; Pratama, N.R.; Pakpahan, C.; Oceandy, D. The Role of Plasma Interleukin-6 Levels on Atherosclerotic Cardiovascular Disease and Cardiovascular Mortality Risk Scores in Javanese Patients with Chronic Kidney Disease. J. Pers. Med. 2022, 12, 1122. [Google Scholar] [CrossRef]
- Jia, R.F.; Li, L.; Li, H.; Cao, X.J.; Ruan, Y.; Meng, S.; Wang, J.Y.; Jin, Z.N. Meta-analysis of C-Reactive Protein and Risk of Angina Pectoris. Am. J. Cardiol. 2020, 125, 1039–1045. [Google Scholar] [CrossRef]
- Liu, C.; Shi, F.; Li, W.; Chen, J. Efficacy of non-surgical periodontal treatment on patients with coronary artery disease: A meta-analysis of randomized controlled trials. Med. Oral Patol. Oral Y Cir. Bucal 2022, 27, e578–e587. [Google Scholar] [CrossRef]
- Yakob, M.; Meurman, J.H.; Söder, B. C-reactive protein in relation to early atherosclerosis and periodontitis. Clin. Oral Investig. 2012, 16, 259–265. [Google Scholar] [CrossRef]
- Etemadifar, R.; Konarizadeh, S.; Zarei, A.; Farshidi, H.; Sobhani, A. Relationship between periodontal status and C-reactive protein and interleuckin-6 levels among atherosclerotic patients in Bandar Abbas, Iran in 2014. Electron. Physician 2015, 7, 1010–1106. [Google Scholar] [CrossRef]
- Widén, C.; Holmer, H.; Coleman, M.; Tudor, M.; Ohlsson, O.; Sättlin, S.; Renvert, S.; Persson, G.R. Systemic inflammatory impact of periodontitis on acute coronary syndrome. J. Clin. Periodontol. 2016, 43, 713–719. [Google Scholar] [CrossRef]
- Kampits, C.; Montenegro, M.M.; Ribeiro, I.W.; Furtado, M.V.; Polanczyk, C.A.; Rösing, C.K.; Haas, A. Periodontal disease and inflammatory blood cytokines in patients with stable coronary artery disease. J. Appl. Oral Sci. 2016, 24, 352–358. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).