Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hematologic Analysis
2.3. Histology
2.4. Flow Cytometry
2.5. ROS Detection
2.6. Reticulocyte Count
2.7. Purification of Erythroblasts
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Nrf2/miR-144/451 Double-KO Mice Display Similar Hemolysis Levels to miR-144/451 Single-KO Mice though Double-KO Mice Generate More ROS at Baseline
3.2. miR-144/451 and Nrf2 KO Mice Differentially Respond to PHZ-Induced Acute Hemolytic Anemia
3.3. Inactivation of Nrf2 Provokes a Stronger Reticulocytosis in miR-144/451 KO Mice in Response to PHZ-Induced Acute Hemolytic Anemia
3.4. More ROS Accumulation in Newly Generated Erythrocytes from Nrf2/miR-144/451 Double-KO Mice upon PHZ Induction
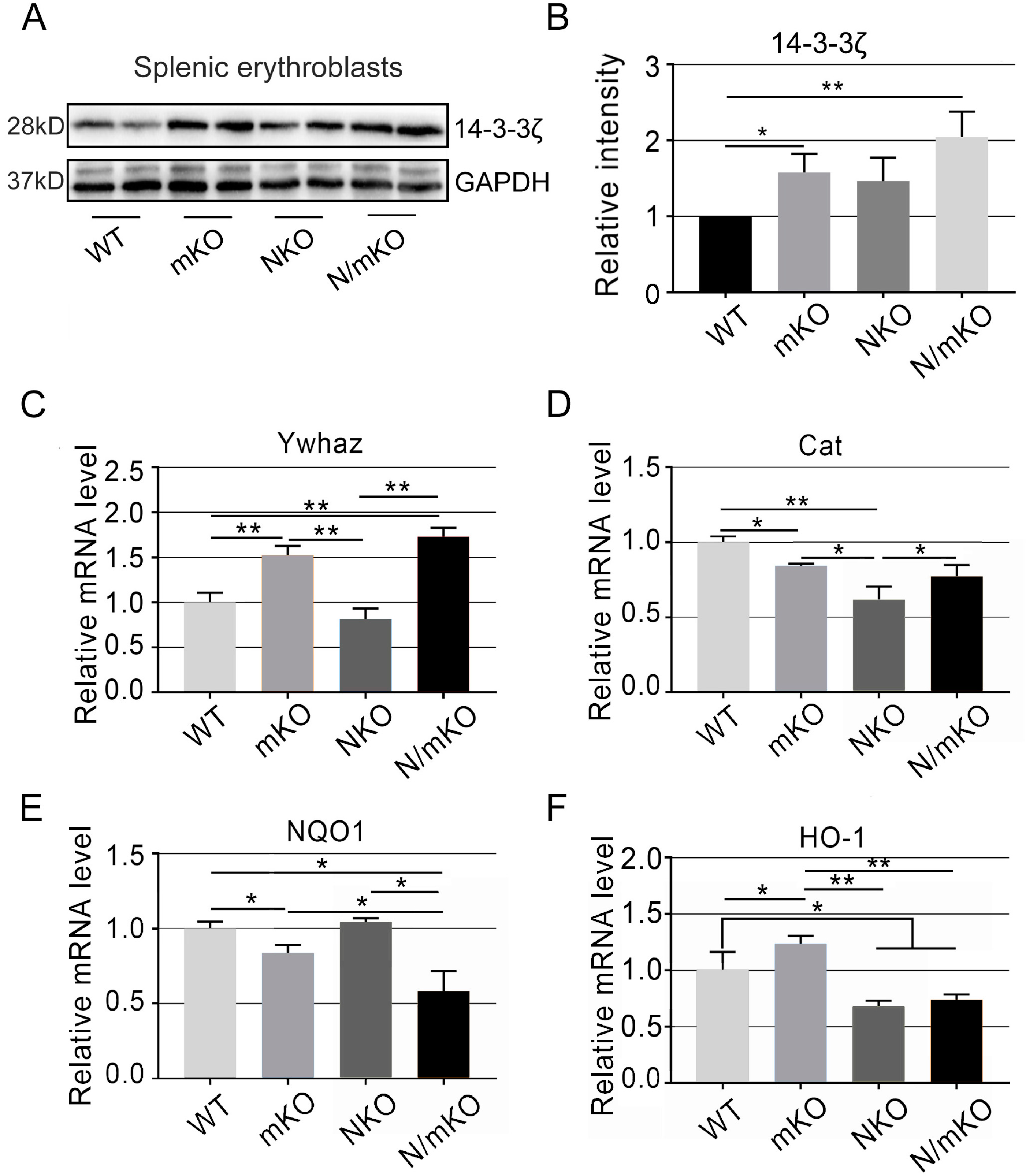
3.5. Altered Expression of miR-144/451-Targeted and Nrf2-Transcribed Detoxifying Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Rachmilewitz, E. The role of oxidative stress in hemolytic anemia. Curr. Mol. Med. 2008, 8, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; dos Santos, C.O.; Zhao, G.; Jiang, J.; Amigo, J.D.; Khandros, E.; Dore, L.C.; Yao, Y.; D’Souza, J.; Zhang, Z.; et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010, 24, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Chan, K.; Kan, Y.W.; Johnson, J.A. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc. Natl. Acad. Sci. USA 2004, 101, 9751–9756. [Google Scholar] [CrossRef]
- Kawatani, Y.; Suzuki, T.; Shimizu, R.; Kelly, V.P.; Yamamoto, M. Nrf2 and selenoproteins are essential for maintaining oxidative homeostasis in erythrocytes and protecting against hemolytic anemia. Blood 2011, 117, 986–996. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Dore, L.C.; Amigo, J.D.; Dos Santos, C.O.; Zhang, Z.; Gai, X.; Tobias, J.W.; Yu, D.; Klein, A.M.; Dorman, C.; Wu, W.; et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3333–3338. [Google Scholar] [CrossRef]
- Patrick, D.M.; Zhang, C.C.; Tao, Y.; Yao, H.; Qi, X.; Schwartz, R.J.; Jun-Shen Huang, L.; Olson, E.N. Defective erythroid differentiation in miR-451 mutant mice mediated by 14-3-3zeta. Genes Dev. 2010, 24, 1614–1619. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Simmini, S.; Abreu-Goodger, C.; Bartonicek, N.; Di Giacomo, M.; Bilbao-Cortes, D.; Horos, R.; Von Lindern, M.; Enright, A.J.; O’Carroll, D. The miR-144/451 locus is required for erythroid homeostasis. J. Exp. Med. 2010, 207, 1351–1358. [Google Scholar] [CrossRef]
- Fang, X.; Shen, F.; Lechauve, C.; Xu, P.; Zhao, G.; Itkow, J.; Wu, F.; Hou, Y.; Wu, X.; Yu, L.; et al. miR-144/451 represses the LKB1/AMPK/mTOR pathway to promote red cell precursor survival during recovery from acute anemia. Haematologica 2018, 103, 406–416. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Gbotosho, O.T.; Kapetanaki, M.G.; Ross, M.; Ghosh, S.; Weidert, F.; Bullock, G.C.; Watkins, S.; Ofori-Acquah, S.F.; Kato, G.J. Nrf2 deficiency in mice attenuates erythropoietic stress-related macrophage hypercellularity. Exp. Hematol. 2020, 84, 19–28.e14. [Google Scholar] [CrossRef]
- Xu, L.; Wu, F.; Yang, L.; Wang, F.; Zhang, T.; Deng, X.; Zhang, X.; Yuan, X.; Yan, Y.; Li, Y.; et al. miR-144/451 inhibits c-Myc to promote erythroid differentiation. FASEB J. 2020, 34, 13194–13210. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef]
- Renard, D.; Rosselet, A. Drug-induced hemolytic anemia: Pharmacological aspects. Transfus. Clin. Biol. 2017, 24, 110–114. [Google Scholar] [CrossRef]
- Karimipour, M.; Dibayi, Z.; Ahmadi, A.; Zirak Javanmard, M.; Hosseinalipour, E. The protective effect of vitamin C on phenylhydrazine-induced hemolytic anemia on sperm quality and in-vitro embryo development in mice. Int. J. Reprod. Biomed. 2018, 16, 3685. [Google Scholar] [CrossRef]
- Nemoto, S.; Finkel, T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002, 295, 2450–2452. [Google Scholar] [CrossRef]
- Hartwig, J.; Loebel, M.; Steiner, S.; Bauer, S.; Karadeniz, Z.; Roeger, C.; Skurk, C.; Scheibenbogen, C.; Sotzny, F. Metformin attenuates ROS via FOXO3 activation in immune cells. Front. Immunol. 2021, 12, 581799. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, J.; Almoiliqy, M.; Wang, Y.; Liu, Z.; Yang, X.; Lu, X.; Meng, Q.; Peng, J.; Lin, Y.; et al. Sesamin protects against and ameliorates rat intestinal ischemia/reperfusion injury with involvement of activating Nrf2/HO-1/NQO1 signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5147069. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.; Han, L.; Fu, L.; Mei, X.; Wang, J.; Itkow, J.; Elabid, A.E.I.; Pang, L.; Yu, D. Activating and sustaining c-Myc by depletion of miR-144/451 gene locus contributes to B-lymphomagenesis. Oncogene 2018, 37, 1293–1307. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.A.; Sanalkumar, R.; O’Geen, H.; Linnemann, A.K.; Chang, C.J.; Bouhassira, E.E.; Farnham, P.J.; Keles, S.; Bresnick, E.H. Autophagy driven by a master regulator of hematopoiesis. Mol. Cell. Biol. 2012, 32, 226–239. [Google Scholar] [CrossRef]
- McIver, S.C.; Kang, Y.A.; DeVilbiss, A.W.; O’Driscoll, C.A.; Ouellette, J.N.; Pope, N.J.; Camprecios, G.; Chang, C.J.; Yang, D.; Bouhassira, E.E.; et al. The exosome complex establishes a barricade to erythroid maturation. Blood 2014, 124, 2285–2297. [Google Scholar] [CrossRef]
- Ramot, Y.; Koshkaryev, A.; Goldfarb, A.; Yedgar, S.; Barshtein, G. Phenylhydrazine as a partial model for beta-thalassaemia red blood cell hemodynamic properties. Br. J. Haematol. 2008, 140, 692–700. [Google Scholar] [CrossRef]
- Matte, A.; Low, P.S.; Turrini, F.; Bertoldi, M.; Campanella, M.E.; Spano, D.; Pantaleo, A.; Siciliano, A.; De Franceschi, L. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic. Biol. Med. 2010, 49, 457–466. [Google Scholar] [CrossRef]
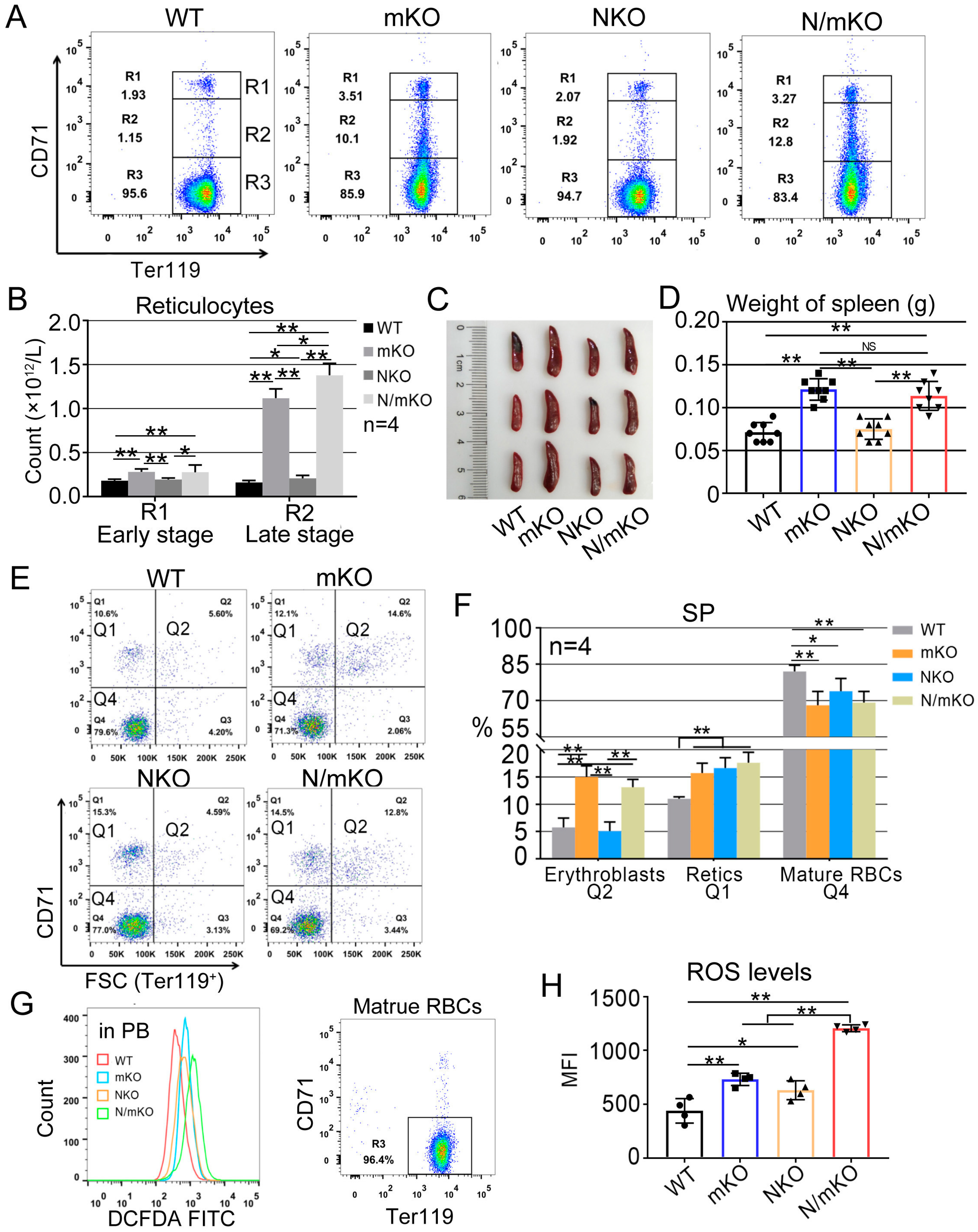
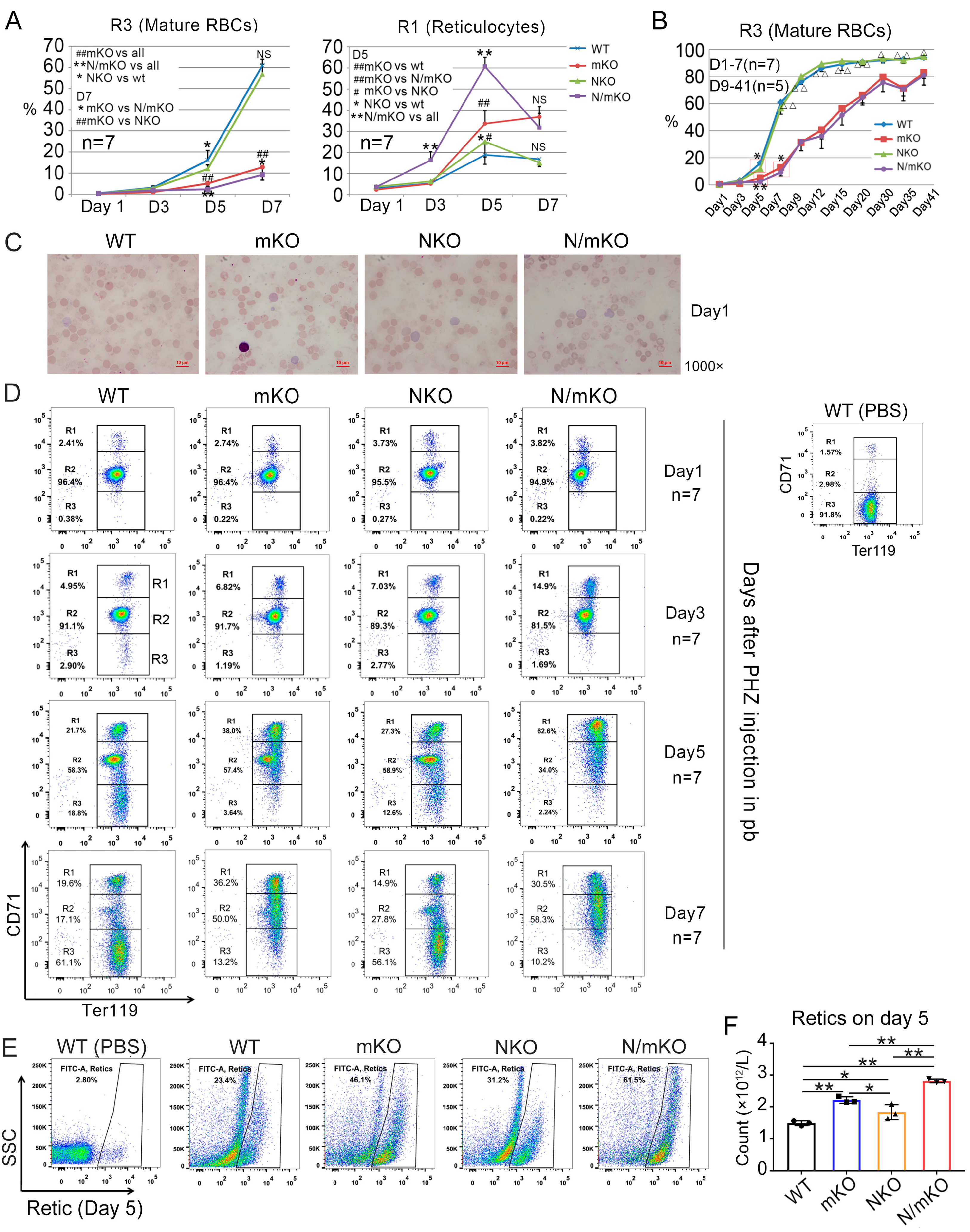
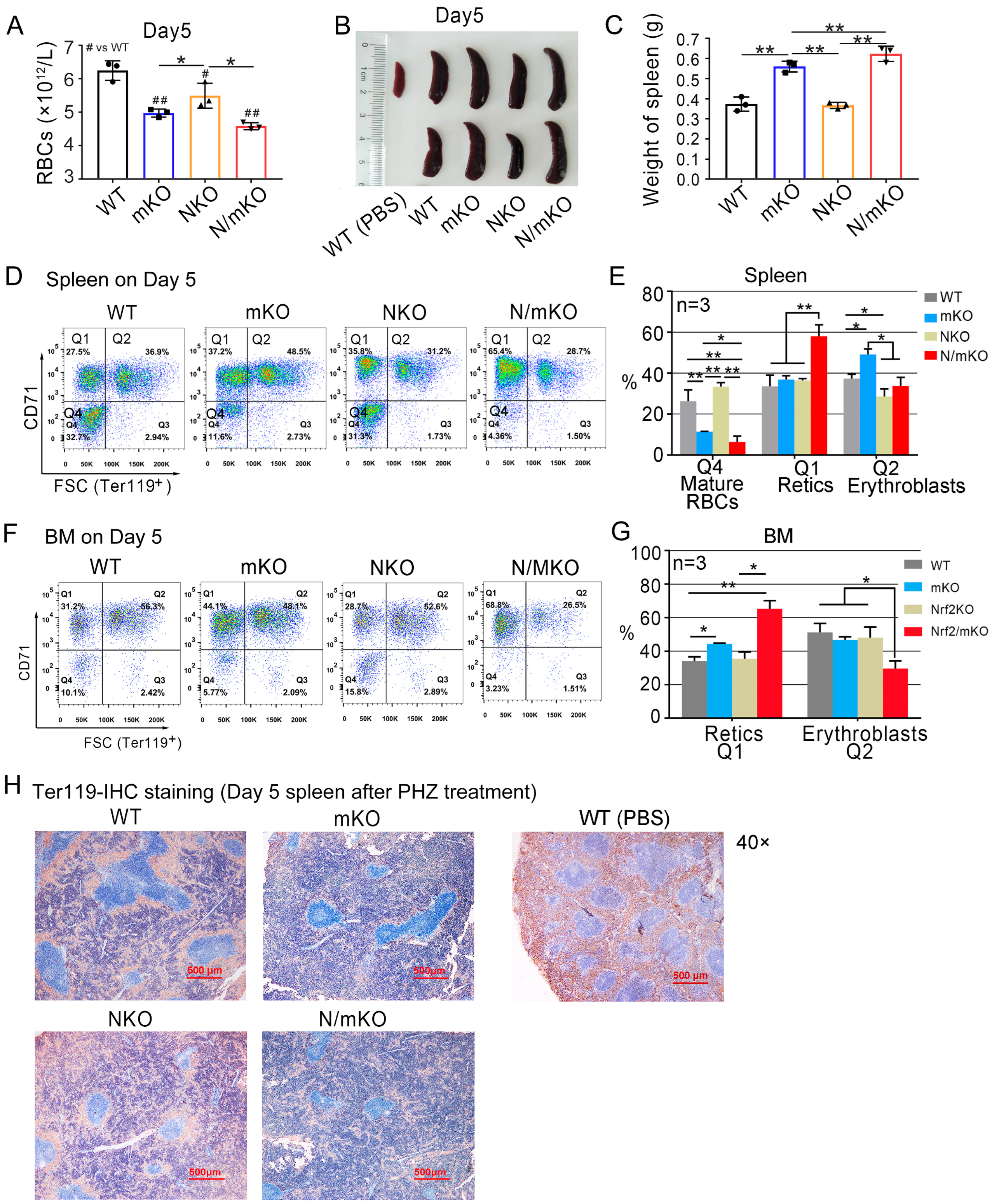
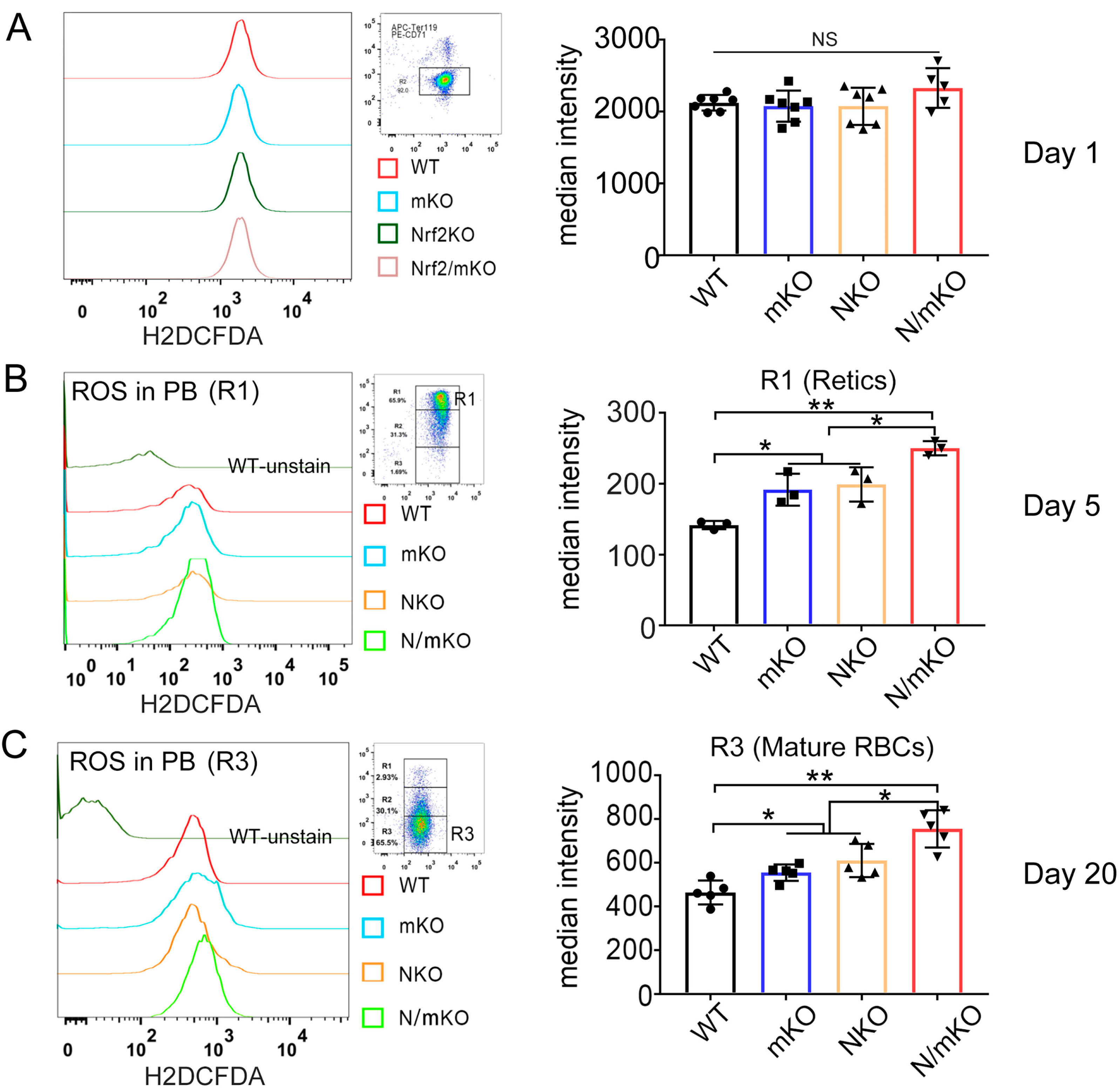
| Index | WT | mKO | NKO | N/mKO |
|---|---|---|---|---|
| RBC (×1012/L) | 9.58 ± 0.06 | 8.51 ± 0.89 * | 9.53 ± 0.19 | 8.64 ± 0.74 * |
| Hb (g/L) | 137.5 ± 3.87 | 124.25 ± 1.71 * | 132.7 ± 12.09 | 124.33 ± 2.52 * |
| HCT (%) | 46.43 ± 1.2 | 42.28 ± 1.73 ** | 44.07 ± 4.8 | 45.65 ± 0.69 # |
| RDW (%) | 17.87 ± 0.06 | 23.15 ± 1.42 ** | 19.15 ± 0.85 * | 23.18 ± 0.17 ** |
| MCV (fl) | 50.6 ± 0.88 | 45.46 ± 2.31 ** | 50.57 ± 1.42 ## | 47.45 ± 0.49 ** |
| MCH (pg) | 14.75 ± 0.34 | 13.45 ± 0.87 * | 15.1 ± 0.48 ## | 13.75 ± 0.21 * |
| MCHC (g/L) | 293.75 ± 4.57 | 295.75 ± 6.6 | 294.75 ± 3.3 | 290.67 ± 2.08 |
| n | 4 | 5 | 5 | 4 |
| Index | WT | mKO | NKO | N/mKO |
|---|---|---|---|---|
| RBC (×1012/L) | 6.25 ± 0.29 | 4.98 ± 0.12 ** | 5.5 ± 0.37 *# | 4.63 ± 0.04 ** |
| Hb (g/L) | 140.67 ± 4.93 | 109.33 ± 2.31 ** | 133.5 ± 3.54 # | 102.5 ± 3.54 **# |
| HCT (%) | 41.43 ± 0.67 | 40.33 ± 1.53 | 38.1 ± 0.28 * | 39.5 ± 0.71 * |
| RDW (%) | 37.67 ± 2.08 | 37.33 ± 2.08 | 37.85 ± 1.2 | 31 ± 1.41 *# |
| MCV (fl) | 67 ± 4.59 | 81.3 ± 5.2 * | 62.87 ± 3.53 ## | 84.95 ± 1.91 ** |
| MCH (pg) | 24.13 ± 0.25 | 22 ± 0.61 ** | 23.33 ± 1.04 | 21.75 ± 0.35 ** |
| MCHC (g/L) | 361.67 ± 22.01 | 271 ± 13.08 ** | 372 ± 38.43 ## | 255 ± 1.41 ** |
| n | 3 | 3 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; He, S.; Ling, L.; Wang, F.; Xu, L.; Fang, L.; Wu, F.; Zhou, S.; Yang, F.; Wei, H.; et al. Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia. Genes 2023, 14, 1011. https://doi.org/10.3390/genes14051011
Yang L, He S, Ling L, Wang F, Xu L, Fang L, Wu F, Zhou S, Yang F, Wei H, et al. Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia. Genes. 2023; 14(5):1011. https://doi.org/10.3390/genes14051011
Chicago/Turabian StyleYang, Lei, Sheng He, Ling Ling, Fangfang Wang, Lei Xu, Lei Fang, Fan Wu, Shuting Zhou, Fan Yang, Hongwei Wei, and et al. 2023. "Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia" Genes 14, no. 5: 1011. https://doi.org/10.3390/genes14051011
APA StyleYang, L., He, S., Ling, L., Wang, F., Xu, L., Fang, L., Wu, F., Zhou, S., Yang, F., Wei, H., & Yu, D. (2023). Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia. Genes, 14(5), 1011. https://doi.org/10.3390/genes14051011








