Novel Variants in the VCP Gene Causing Multisystem Proteinopathy 1
Abstract
1. Introduction
1.1. Pathology of MSP1
1.2. VCP Variants
2. Case Report
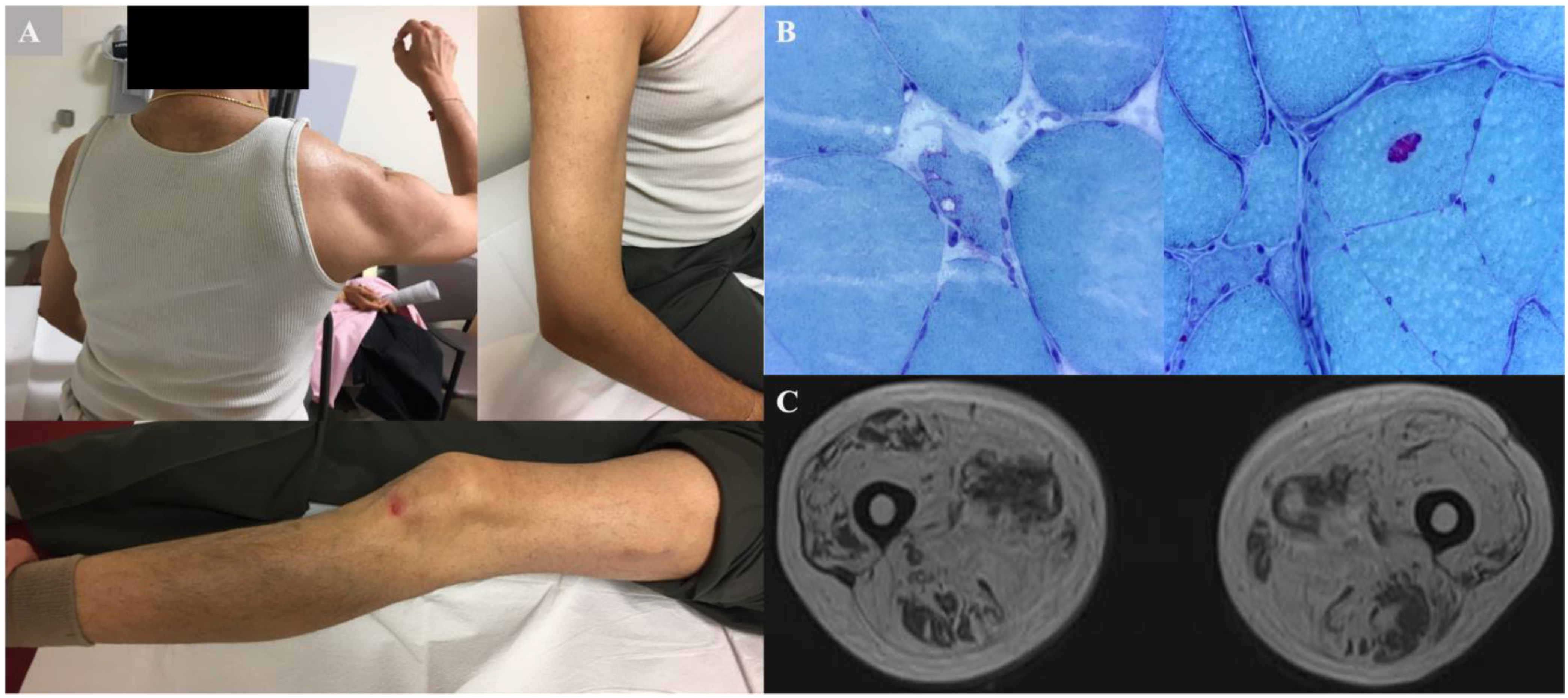
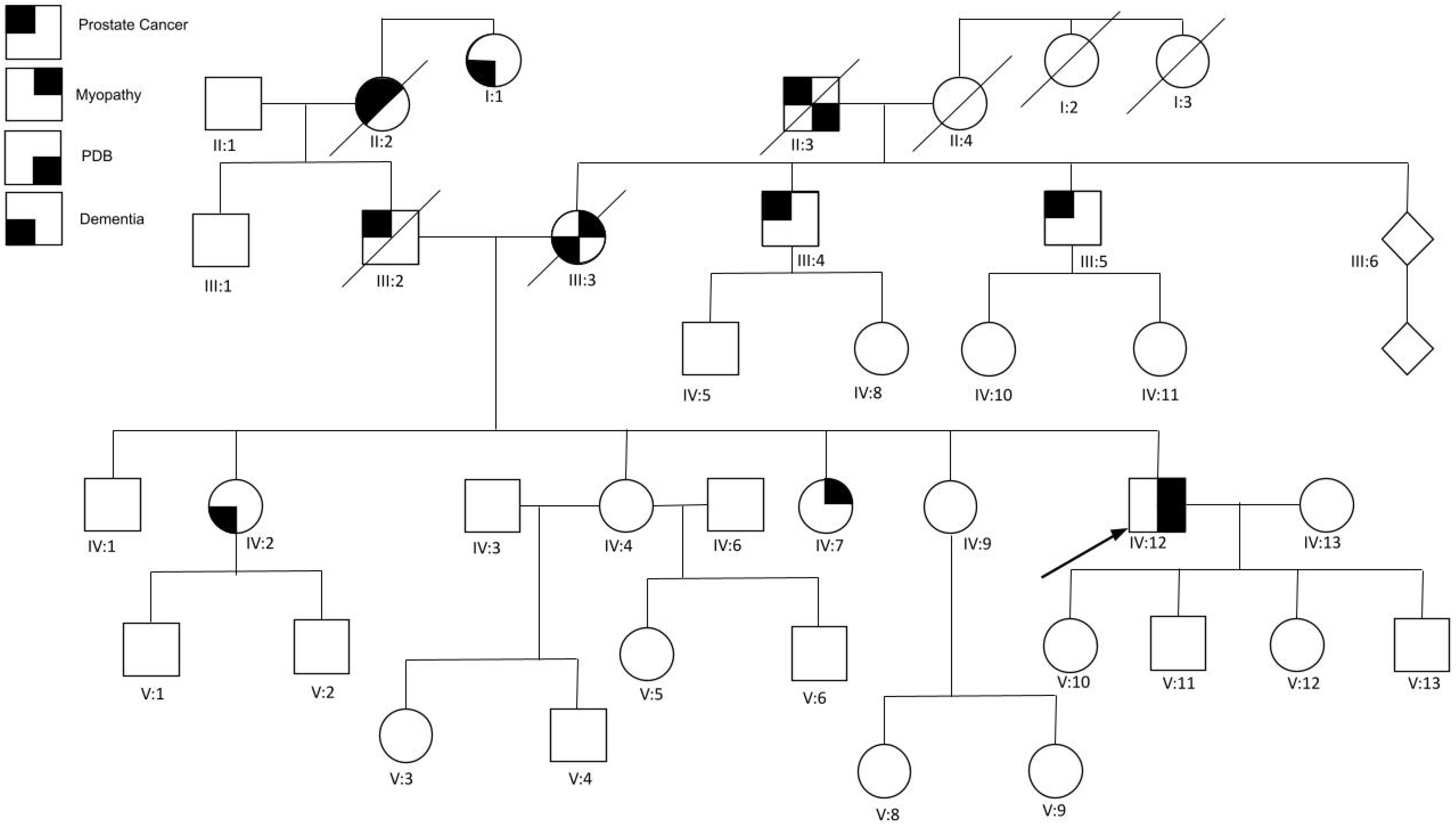
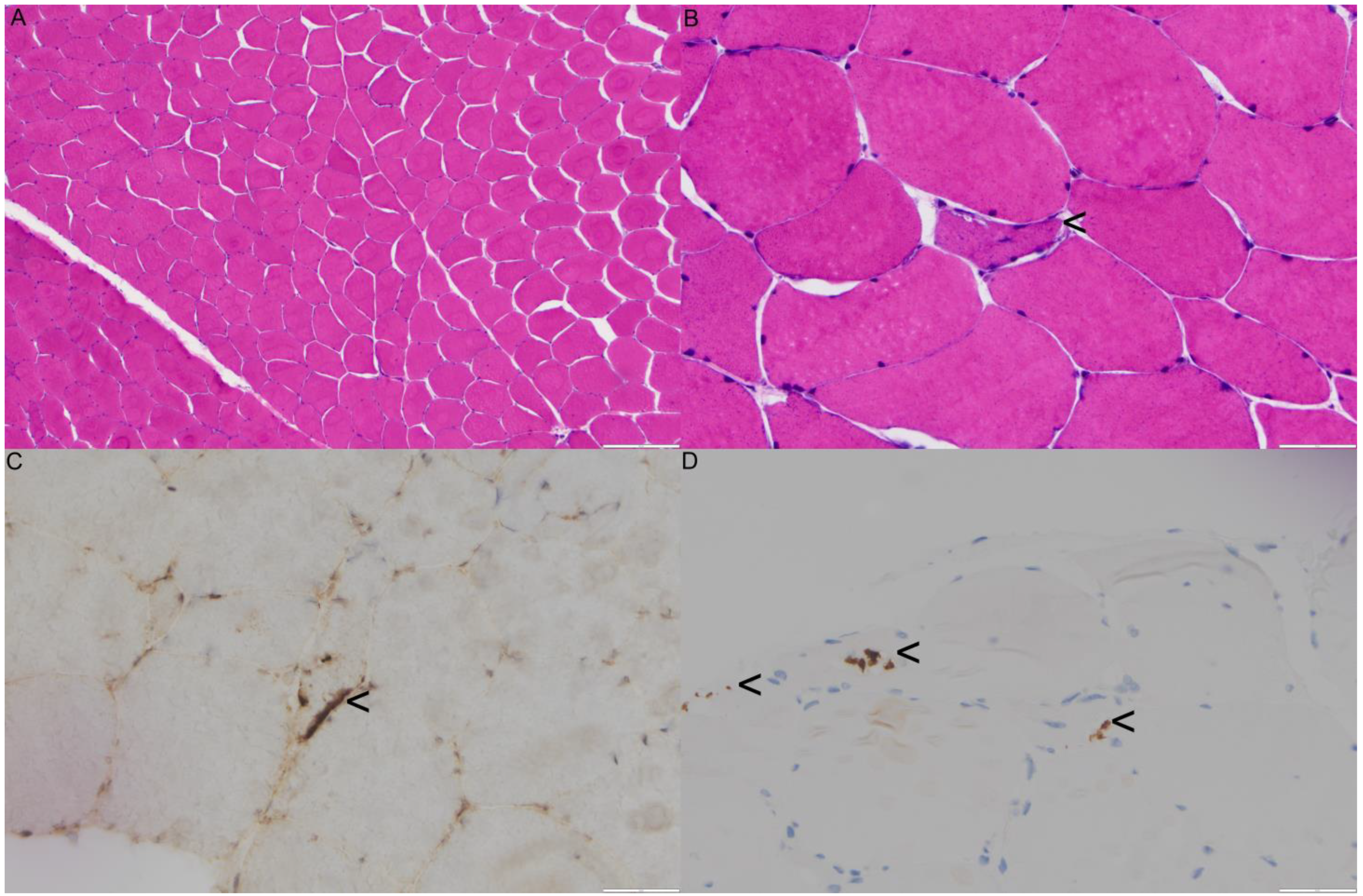
2.1. Family 1 Proband 1 (c.1106T>C; p.Ile369Thr)
2.2. Family 2 (c.478 G>A; p.A160T)
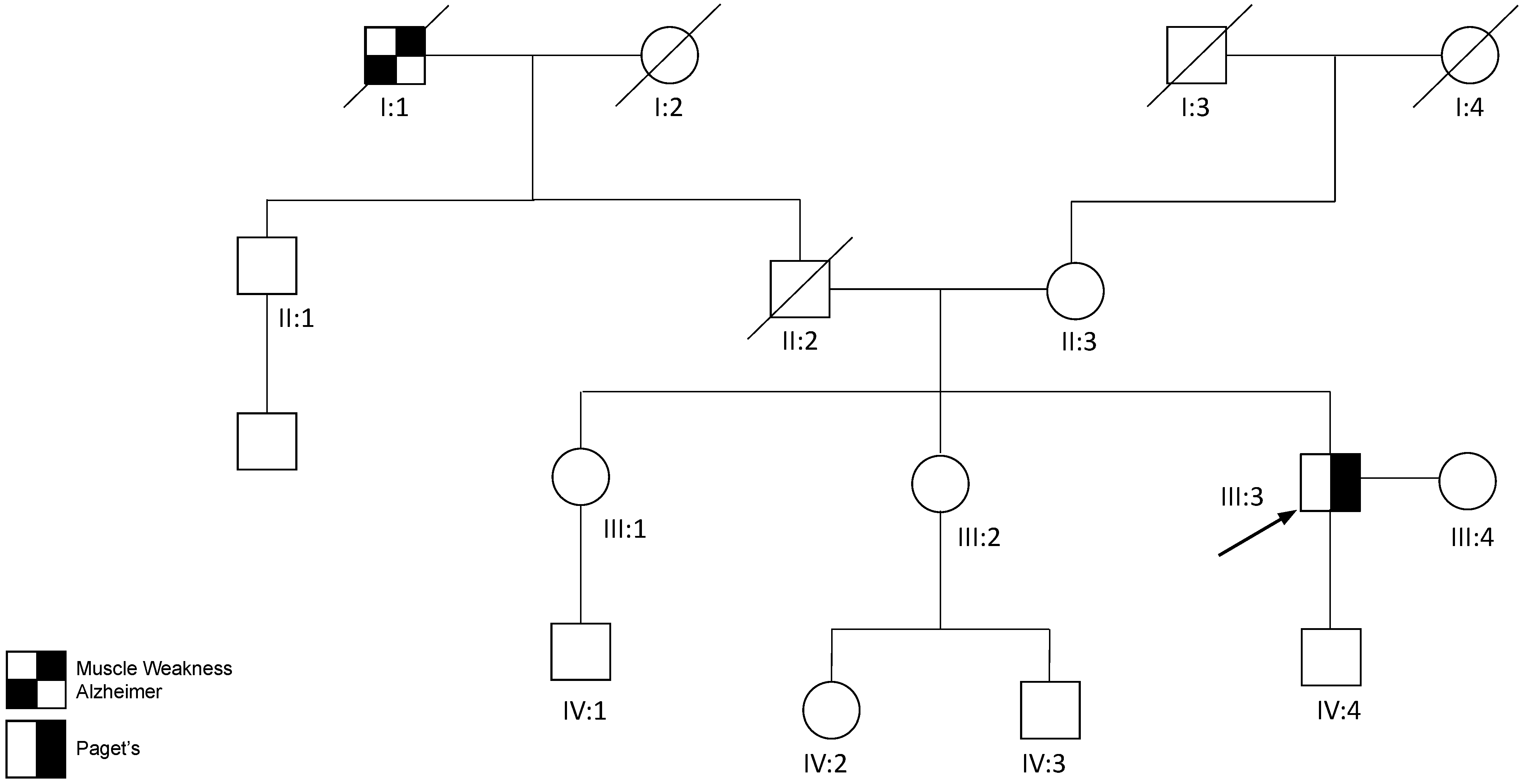
2.3. Family 3 Proband 3 (c.760A>T; p.Ile254Phe)
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimonis, V.E.; Kovach, M.J.; Waggoner, B.; Leal, S.; Salam, A.; Rimer, L.; Davis, K.; Khardori, R.; Gelber, D. Clinical and molecular studies in a unique family with autosomal dominant limb-girdle muscular dystrophy and Paget disease of bone. Genet. Med. 2000, 2, 232–241. [Google Scholar] [CrossRef]
- Kimonis, V.E.; Mehta, S.G.; Fulchiero, E.C.; Thomasova, D.; Pasquali, M.; Boycott, K.; Neilan, E.G.; Kartashov, A.; Forman, M.S.; Tucker, S.; et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am. J. Med. Genet. Part A 2008, 146, 745–757. [Google Scholar] [CrossRef]
- Korb, M.; Peck, A.; Alfano, L.N.; Berger, K.I.; James, M.K.; Ghoshal, N.; Healzer, E.; Henchcliffe, C.; Khan, S.; Mammen, P.P.A.; et al. Development of a standard of care for patients with valosin-containing protein associated multisystem proteinopathy. Orphanet J. Rare Dis. 2022, 17, 23. [Google Scholar] [CrossRef]
- Schiava, M.; Ikenaga, C.; Villar-Quiles, R.N.; Caballero-Ávila, M.; Topf, A.; Nishino, I.; Kimonis, V.; Udd, B.; Schoser, B.; Zanoteli, E.; et al. Genotype-phenotype correlations in valosin-containing protein disease: A retrospective muticentre study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1099–1111. [Google Scholar] [CrossRef]
- Al-Obeidi, E.; Al-Tahan, S.; Surampalli, A.; Goyal, N.; Wang, A.; Hermann, A.; Omizo, M.; Smith, C.; Mozaffar, T.; Kimonis, V. Genotype-phenotype study in patients with valosin-containing protein mutations associated with multisystem proteinopathy. Clin. Genet. 2018, 93, 119–125. [Google Scholar] [CrossRef]
- Farpour, F.; Tehranzadeh, J.; Donkervoort, S.; Smith, C.; Martin, B.; Vanjara, P.; Osann, K.; Kimonis, V.E. Radiological features of Paget disease of bone associated with VCP myopathy. Skelet. Radiol. 2012, 41, 329–337. [Google Scholar] [CrossRef]
- Nalbandian, A.; Donkervoort, S.; Dec, E.; Badadani, M.; Katheria, V.; Rana, P.; Nguyen, C.; Mukherjee, J.; Caiozzo, V.; Martin, B.; et al. The Multiple Faces of Valosin-Containing Protein-Associated Diseases: Inclusion Body Myopathy with Paget’s Disease of Bone, Frontotemporal Dementia, and Amyotrophic Lateral Sclerosis. J. Mol. Neurosci. 2011, 45, 522–531. [Google Scholar] [CrossRef]
- Mehta, S.; Khare, M.; Ramani, R.; Watts, G.; Simon, M.; Osann, K.; Donkervoort, S.; Dec, E.; Nalbandian, A.; Platt, J.; et al. Genotype–phenotype studies of VCP-associated inclusion body myopathy with Paget disease of bone and/or frontotemporal dementia. Clin. Genet. 2013, 83, 422–431. [Google Scholar] [CrossRef]
- Weiss, L.; Jung, K.-M.; Nalbandian, A.; Llewellyn, K.; Yu, H.; Ta, L.; Chang, I.; Migliore, M.; Squire, E.; Ahmed, F.; et al. Ceramide contributes to pathogenesis and may be targeted for therapy in VCP inclusion body myopathy. Hum. Mol. Genet. 2021, 29, 3945–3953. [Google Scholar] [CrossRef]
- Dardis, C.; Antezana, A.; Tanji, K.; Maccabee, P.J. Inclusion Body Myositis: A Case Presenting with Respiratory Failure and Autopsy Findings Leading to the Hypothesis of a Paraneoplastic Cause. Am. J. Case Rep. 2017, 18, 700–706. [Google Scholar] [CrossRef]
- Dimachkie, M.M.; Barohn, R.J. Inclusion body myositis. Neurol. Clin. 2014, 32, 629–646. [Google Scholar] [CrossRef]
- Wang, S.C.; Smith, C.D.; Lombardo, D.M.; Kimonis, V. Characteristics of VCP mutation-associated cardiomyopathy. Neuromuscul. Disord. 2021, 31, 701–705. [Google Scholar] [CrossRef]
- Ikenaga, C.; Findlay, A.R.; Seiffert, M.; Peck, A.; Peck, N.; Johnson, N.E.; Statland, J.M.; Weihl, C.C. Phenotypic diversity in an international Cure VCP Disease registry. Orphanet J. Rare Dis. 2020, 15, 267. [Google Scholar] [CrossRef]
- Naddaf, E.; Barohn, R.J.; Dimachkie, M.M. Inclusion Body Myositis: Update on Pathogenesis and Treatment. Neurotherapeutics 2018, 15, 995–1005. [Google Scholar] [CrossRef]
- Bolaños-Toro, F.; Arias-Jaramillo, D.; Aun-Mejía, M.; Castro-Henao, J.; Fernández-Ávila, D.G.; Saldarriaga-Rivera, L.M. Inclusion body myositis: A differential diagnosis to consider in patients with myopathy refractory to immunosuppressants. Two case reports. Rev. Colomb. Reumatol. Engl. Ed. 2021, 28, 300–305. [Google Scholar] [CrossRef]
- Shaker, J.L. Paget’s Disease of Bone: A Review of Epidemiology, Pathophysiology and Management. Ther. Adv. Musculoskelet. Dis. 2009, 1, 107–125. [Google Scholar] [CrossRef]
- Ralston, S.H.; Corral-Gudino, L.; Cooper, C.; Francis, R.M.; Fraser, W.D.; Gennari, L.; Guañabens, N.; Javaid, M.K.; Layfield, R.; O’Neill, T.W.; et al. Diagnosis and Management of Paget’s Disease of Bone in Adults: A Clinical Guideline. J. Bone Miner. Res. 2019, 34, 579–604. [Google Scholar] [CrossRef]
- Paget’s Disease—Pathology—Orthobullets. Available online: https://www.orthobullets.com/pathology/8040/pagets-disease (accessed on 11 November 2022).
- Griepp, D.W.; Sajan, A.; Sighary, M. Diffuse Paget’s Disease of the Skull with Intense Uptake of Technetium-99m-Labeled Diphosphonate Tracer in Bone Scintigraphy. World Neurosurg. 2021, 151, 89–90. [Google Scholar] [CrossRef]
- Rosen, H.J.; Allison, S.C.; Schauer, G.F.; Gorno-Tempini, M.L.; Weiner, M.W.; Miller, B.L. Neuroanatomical correlates of behavioural disorders in dementia. Brain J. Neurol. 2005, 128, 2612–2625. [Google Scholar] [CrossRef]
- Knopman, D.S.; Boeve, B.F.; Parisi, J.E.; Dickson, D.W.; Smith, G.E.; Ivnik, R.J.; Josephs, K.A.; Petersen, R.C. Antemortem diagnosis of frontotemporal lobar degeneration. Ann. Neurol. 2005, 57, 480–488. [Google Scholar] [CrossRef]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.-Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M.; et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 2020, 131, 1975–1978. [Google Scholar] [CrossRef]
- Meyer, H.; Weihl, C.C. The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 2014, 127, 3877–3883. [Google Scholar] [CrossRef]
- Rabinovich, E.; Kerem, A.; Fröhlich, K.U.; Diamant, N.; Bar-Nun, S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 2002, 22, 626–634. [Google Scholar] [CrossRef]
- Rape, M.; Hoppe, T.; Gorr, I.; Kalocay, M.; Richly, H.; Jentsch, S. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 2001, 107, 667–677. [Google Scholar] [CrossRef]
- Shih, Y.T.; Hsueh, Y.P. VCP and ATL1 regulate endoplasmic reticulum and protein synthesis for dendritic spine formation. Nat. Commun. 2016, 7, 11020. [Google Scholar] [CrossRef]
- Ju, J.-S.; Fuentealba, R.; Miller, S.E.; Jackson, E.; Piwnica-Worms, D.; Baloh, R.H.; Weihl, C.C. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J. Cell Biol. 2009, 187, 875–888. [Google Scholar] [CrossRef]
- Watts, G.D.J.; Wymer, J.; Kovach, M.J.; Mehta, S.G.; Mumm, S.; Darvish, D.; Pestronk, A.; Whyte, M.P.; E Kimonis, V. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 2004, 36, 377–381. [Google Scholar] [CrossRef]
- Dalal, S.; Rosser, M.F.N.; Cyr, D.M.; Hanson, P.I. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell. 2004, 15, 637–648. [Google Scholar] [CrossRef]
- Xia, D.; Tang, W.K.; Ye, Y. Structure and function of the AAA+ ATPase p97/Cdc48p. Gene 2016, 583, 64–77. [Google Scholar] [CrossRef]
- Buchberger, A.; Schindelin, H.; Hänzelmann, P. Control of p97 function by cofactor binding. FEBS Lett. 2015, 589, 2578–2589. [Google Scholar] [CrossRef]
- Yeung, H.O.; Kloppsteck, P.; Niwa, H.; Isaacson, R.L.; Matthews, S.; Zhang, X.; Freemont, P.S. Insights into adaptor binding to the AAA protein p97. Biochem. Soc. Trans. 2008, 36, 62–67. [Google Scholar] [CrossRef]
- Chapman, E.; Maksim, N.; de la Cruz, F.; La Clair, J.J. Inhibitors of the AAA+ chaperone p97. Molecules 2015, 20, 3027–3049. [Google Scholar] [CrossRef]
- Niwa, H.; Ewens, C.A.; Tsang, C.; Yeung, H.O.; Zhang, X.; Freemont, P.S. The role of the N-domain in the ATPase activity of the mammalian AAA ATPase p97/VCP. J. Biol. Chem. 2012, 287, 8561–8570. [Google Scholar] [CrossRef]
- Tang, W.K.; Xia, D. Altered intersubunit communication is the molecular basis for functional defects of pathogenic p97 mutants. J. Biol. Chem. 2013, 288, 36624–36635. [Google Scholar] [CrossRef]
- Dreveny, I.; Kondo, H.; Uchiyama, K.; Shaw, A.; Zhang, X.; Freemont, P.S. Structural basis of the interaction between the AAA ATPase p97/VCP and its adaptor protein p47. EMBO J. 2004, 23, 1030–1039. [Google Scholar] [CrossRef]
- Hänzelmann, P.; Buchberger, A.; Schindelin, H. Hierarchical binding of cofactors to the AAA ATPase p97. Structure 2011, 19, 833–843. [Google Scholar] [CrossRef]
- Tang, W.K.; Li, D.; Li, C.-C.; Esser, L.; Dai, R.; Guo, L.; Xia, D. A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants. EMBO J. 2010, 29, 2217–2229. [Google Scholar] [CrossRef]
- Zhang, X.; Shaw, A.; Bates, P.A.; Newman, R.H.; Gowen, B.; Orlova, E.; Gorman, M.A.; Kondo, H.; Dokurno, P.; Lally, J.; et al. Structure of the AAA ATPase p97. Mol. Cell. 2000, 6, 1473–1484. [Google Scholar] [CrossRef]
- Saracino, D.; Clot, F.; Camuzat, A.; Anquetil, V.; Hannequin, D.; Guyant-Maréchal, L.; Didic, M.; Guillot-Noël, L.; Rinaldi, D.; Latouche, M.; et al. Novel VCP mutations expand the mutational spectrum of frontotemporal dementia. Neurobiol. Aging 2018, 72, 187.e11–187.e14. [Google Scholar] [CrossRef]
- Nandi, P.; Li, S.; Columbres, R.C.A.; Wang, F.; Williams, D.; Poh, Y.-P.; Chou, T.-F.; Chiu, P.-L. Structural and Functional Analysis of Disease-Linked p97 ATPase Mutant Complexes. Int J. Mol. Sci. 2021, 22, 8079. [Google Scholar] [CrossRef]
- Pfeffer, G.; Lee, G.; Pontifex, C.S.; Fanganiello, R.D.; Peck, A.; Weihl, C.C.; Kimonis, V. Multisystem Proteinopathy Due to VCP Mutations: A Review of Clinical Heterogeneity and Genetic Diagnosis. Genes 2022, 13, 963. [Google Scholar] [CrossRef]
- Al-Tahan, S.; Al-Obeidi, E.; Yoshioka, H.; Lakatos, A.; Weiss, L.; Grafe, M.; Palmio, J.; Wicklund, M.; Harati, Y.; Omizo, M.; et al. Novel valosin-containing protein mutations associated with multisystem proteinopathy. Neuromuscul. Disord. 2018, 28, 491–501. [Google Scholar] [CrossRef]
- Surampalli, A. A Clinicopathologic Case Report of a Female with Valosin-Containing Protein (VCP) Gene Mutation Related Disease. Int. J. Neurodegener. Dis. 2018, 1, 6. [Google Scholar] [CrossRef]
- De Ridder, W.; Azmi, A.; Clemen, C.S.; Eichinger, L.; Hofmann, A.; Schröder, R.; Johnson, K.; Töpf, A.; Straub, V.; De Jonghe, P.; et al. Multisystem proteinopathy due to a homozygous p.Arg159His VCP mutation: A tale of the unexpected. Neurology 2020, 94, e785–e796. [Google Scholar] [CrossRef]
- Jerath, N.U. Resolving a Multi-Generational Neuromuscular Mystery in a Family Presenting with a Variable Scapuloperoneal Syndrome in a c.464G>A, p.Arg155His VCP Mutation. Case Rep. Genet. 2019, 2019, 2403024. [Google Scholar] [CrossRef]
- Feng, S.Y.; Lin, H.; Che, C.H.; Huang, H.P.; Liu, C.Y.; Zou, Z.Y. Phenotype of VCP Mutations in Chinese Amyotrophic Lateral Sclerosis Patients. Front. Neurol. 2022, 13, 790082. [Google Scholar] [CrossRef]
- Choy, N.; Wang, S.; Abbona, P.; Leffler, D.; Kimonis, V. Severe cardiomyopathy associated with the VCP p.R155C and c.177_187del MYBPC3 gene variants. Eur. J. Med. Genet. 2022, 65, 104480. [Google Scholar] [CrossRef]
- Matsubara, T.; Izumi, Y.; Oda, M.; Takahashi, M.; Maruyama, H.; Miyamoto, R.; Watanabe, C.; Tachiyama, Y.; Morino, H.; Kawakami, H.; et al. An autopsy report of a familial amyotrophic lateral sclerosis case carrying VCP Arg487His mutation with a unique TDP-43 proteinopathy. Neuropathology 2021, 41, 118–126. [Google Scholar] [CrossRef]
- Pellerin, D.; Ellezam, B.; Korathanakhun, P.; Renaud, M.; Dicaire, M.-J.; Pilote, L.; Levy, J.P.; Karamchandani, J.; Ducharme, S.; Massie, R.; et al. Multisystem Proteinopathy Associated with a VCP G156S Mutation in a French Canadian Family. Can. J. Neurol. Sci. 2020, 47, 412–415. [Google Scholar] [CrossRef]
- Falcão de Campos, C.; de Carvalho, M. Distal myopathy and rapidly progressive dementia associated with a novel mutation in the VCP gene: Expanding inclusion body myopathy with early-onset Paget disease and frontotemporal dementia spectrum. J. Clin. Neurosci. 2019, 64, 8–10. [Google Scholar] [CrossRef]
- Shmara, A.; Perez-Rosendahl, M.; Murphy, K.; Kwon, A.; Smith, C.; Kimonis, V. A clinicopathologic study of malignancy in VCP-associated multisystem proteinopathy. Orphanet J. Rare Dis. 2022, 17, 272. [Google Scholar] [CrossRef]
- Tang, W.K.; Xia, D. Structural and functional deviations in disease-associated p97 mutants. J. Struct. Biol. 2012, 179, 83–92. [Google Scholar] [CrossRef]
- Bayraktar, O.; Oral, O.; Kocaturk, N.M.; Akkoc, Y.; Eberhart, K.; Kosar, A.; Gozuacik, D. IBMPFD Disease-Causing Mutant VCP/p97 Proteins Are Targets of Autophagic-Lysosomal Degradation. PLoS ONE 2016, 11, e0164864. [Google Scholar] [CrossRef]
- Bersano, A.; Del Bo, R.; Lamperti, C.; Ghezzi, S.; Fagiolari, G.; Fortunato, F.; Ballabio, E.; Moggio, M.; Candelise, L.; Galimberti, D.; et al. Inclusion body myopathy and frontotemporal dementia caused by a novel VCP mutation. Neurobiol. Aging 2009, 30, 752–758. [Google Scholar] [CrossRef]
- Spina, S.; Van Laar, A.D.; Murrell, J.R.; Hamilton, R.L.; Kofler, J.K.; Epperson, F.; Farlow, M.R.; Lopez, O.L.; Quinlan, J.; DeKosky, S.T.; et al. Phenotypic variability in three families with valosin-containing protein mutation. Eur. J. Neurol. 2013, 20, 251–258. [Google Scholar] [CrossRef]
- González-Pérez, P.; Cirulli, E.T.; Drory, V.E.; Dabby, R.; Nisipeanu, P.; Carasso, R.L.; Sadeh, M.; Fox, A.; Festoff, B.W.; Sapp, P.C.; et al. Novel mutation in VCP gene causes atypical amyotrophic lateral sclerosis. Neurology 2012, 79, 2201–2208. [Google Scholar] [CrossRef]
- Shmara, A.; Gibbs, L.; Mahoney, R.P.; Hurth, K.; Goodwill, V.S.; Cuber, A.; Im, R.; Pizzo, D.P.; Brown, J.; Laukaitis, C.; et al. Prevalence of Frontotemporal Dementia in Females of 5 Hispanic Families With R159H VCP Multisystem Proteinopathy. Neurol. Genet. 2023, 9, e200037. [Google Scholar] [CrossRef]
- Manno, A.; Noguchi, M.; Fukushi, J.; Motohashi, Y.; Kakizuka, A. Enhanced ATPase activities as a primary defect of mutant valosin-containing proteins that cause inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Genes Cells 2010, 15, 911–922. [Google Scholar] [CrossRef]
- Mehta, S.G.; Watts, G.D.; Adamson, J.L.; Hutton, M.; Umberger, G.; Xiong, S.; Ramdeen, S.; Lovell, M.A.; Kimonis, V.E.; Smith, C.D. APOE is a potential modifier gene in an autosomal dominant form of frontotemporal dementia (IBMPFD). Genet. Med. 2007, 9, 9–13. [Google Scholar] [CrossRef]
- Stojkovic, T.; Hammouda, E.H.; Richard, P.; de Munain, A.L.; Ruiz-Martinez, J.; Gonzalez, P.C.; Laforêt, P.; Pénisson-Besnier, I.; Ferrer, X.; Lacour, A.; et al. Clinical outcome in 19 French and Spanish patients with valosin-containing protein myopathy associated with Paget’s disease of bone and frontotemporal dementia. Neuromuscul. Disord. 2009, 19, 316–323. [Google Scholar] [CrossRef]
- Haubenberger, D.; Bittner, R.E.; Rauch-Shorny, S.; Zimprich, F.; Mannhalter, C.; Wagner, L.; Mineva, I.; Vass, K.; Auff, E. Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology. 2005, 65, 1304–1305. [Google Scholar] [CrossRef]
- Wang, Q.; Song, C.; Li, C.C.H. Hexamerization of p97-VCP is promoted by ATP binding to the D1 domain and required for ATPase and biological activities. Biochem. Biophys. Res. Commun. 2003, 300, 253–260. [Google Scholar] [CrossRef]
- Wong, T.H.; Pottier, C.; Hondius, D.C.; Meeter, L.H.; van Rooij, J.G.; Melhem, S.; van Minkelen, R.; van Duijn, C.M.; Rozemuller, A.J.; Seelaar, H.; et al. Three VCP Mutations in Patients with Frontotemporal Dementia. J. Alzheimers Dis. 2018, 65, 1139–1146. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, H. Valosin-Containing Protein, a Calcium-Associated ATPase Protein, in Endoplasmic Reticulum and Mitochondrial Function and Its Implications for Diseases. Int. J. Mol. Sci. 2020, 21, 3842. [Google Scholar] [CrossRef]
- Watts, G.; Thomasova, D.; Ramdeen, S.; Fulchiero, E.; Mehta, S.; Drachman, D.; Weihl, C.; Jamrozik, Z.; Kwiecinski, H.; Kaminska, A.; et al. Novel VCP mutations in inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Clin. Genet. 2007, 72, 420–426. [Google Scholar] [CrossRef]
- Bruno, F.; Conidi, M.E.; Puccio, G.; Frangipane, F.; Laganà, V.; Bernardi, L.; Smirne, N.; Mirabelli, M.; Colao, R.; Curcio, S.; et al. A Novel Mutation (D395A) in Valosin-Containing Protein Gene Is Associated With Early Onset Frontotemporal Dementia in an Italian Family. Front. Genet. 2021, 12, 2384. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Ralston, S.H. A New Gene for Susceptibility to Paget’s Disease of Bone and for Multisystem Proteinopathy. J. Bone Miner. Res. 2020, 35, 1385–1386. [Google Scholar] [CrossRef]

| Case | Age (Y) | Sex | DNA Mutation | ProteinMutation | Onset, Myopathy (Y) | Onset, PDB(Y) | Onset, FTD (Y) | ALP (Normal 30 to 130 IU/L) | CK (Normal 20 to 222 IU/L) | Family History of Associated Disease/Genetic Confirmation Not Performed |
|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 Proband 1 (III:3) | 71 | M | c.1106T>C | I369T | 60 | - | - | 71 | 117 | Dementia (maternal uncle (II:6) and aunt (II:7)) |
| Family 2 | ||||||||||
| Proband 2(IV:12) | 56 | M | c.478G>A | A160T | 50 | 44 | - | 146 | 318 | Inclusion body myopathy and dementia (mother III:3) |
| (IV:2) | 54 | F | - | - | 52 | - | - | |||
| (IV:7) | 48 | F | 45 | - | - | 44 | 83 | |||
| Family 3 Proband 3 (III:3) | 49 | M | c.760A>T | I254F | 49 | 45 | - | 263 | 2378 | Muscular dystrophy and Alzheimer’s disease (paternal grandfather I:1) |
| Proband 1 | Family 2 | Proband 3 | |
|---|---|---|---|
| Transcript ID | NM_007126.5(VCP):c.1106T>C | NM_007126.5(VCP):c.478G>C | NM_007126.5(VCP):c.760A>T |
| Protein | NP_009057.1:p.Ile369Thr | NP_009057.1:p.Ala160Pro | NP_009057.1:p.Ile254Phe |
| Protein domain of variant localization | D1 | NTD | D1 |
| Type of single nucleotide variant | Missense | Missense | Missense |
| Allelic status | Heterozygous | Heterozygous | Heterozygous |
| Ascertainment | Clinical exome | Research exome | Gene panel |
| gnomAD V.2.1.1. | Absent | Absent | Absent |
| Ancestry | South Asian Indian | European, Ashkenazi Jewish | Chinese |
| ClinVar classification as of 2/3/22 | Uncertain significance. Accession: VCV000963526.7 | Uncertain significance (4 total: 3 entries (2017–2021) IBMPFD (2 individuals), condition not provided (1 individual), and pathogenic (2018) (1 individual). Accession: VCV000532761.20 | Uncertain significance (1 entry IBMPFD). Accession: RCV002301822.1 |
| MutationTaster (v2021) prediction | Deleterious Tree vote: 84|16 (del|benign) | Deleterious Tree vote: 87|13 (del|benign) | Deleterious Tree vote: 80|20 (del|benign). |
| Conservation between multiple species | Ile present in 12/12 (total species) | Ala present in 10/12 (total species) Absent in fruit fly, C. elegans | Ile present in 12/12 (total species) |
| PolyPhen-2 v2.2.3r406 prediction and score | Possibly damaging, 1.00 | Benign, 0.002 | Possibly damaging, 0.873 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Columbres, R.C.A.; Chin, Y.; Pratti, S.; Quinn, C.; Gonzalez-Cuyar, L.F.; Weiss, M.; Quintero-Rivera, F.; Kimonis, V. Novel Variants in the VCP Gene Causing Multisystem Proteinopathy 1. Genes 2023, 14, 676. https://doi.org/10.3390/genes14030676
Columbres RCA, Chin Y, Pratti S, Quinn C, Gonzalez-Cuyar LF, Weiss M, Quintero-Rivera F, Kimonis V. Novel Variants in the VCP Gene Causing Multisystem Proteinopathy 1. Genes. 2023; 14(3):676. https://doi.org/10.3390/genes14030676
Chicago/Turabian StyleColumbres, Rod Carlo Agram, Yue Chin, Sanjana Pratti, Colin Quinn, Luis F. Gonzalez-Cuyar, Michael Weiss, Fabiola Quintero-Rivera, and Virginia Kimonis. 2023. "Novel Variants in the VCP Gene Causing Multisystem Proteinopathy 1" Genes 14, no. 3: 676. https://doi.org/10.3390/genes14030676
APA StyleColumbres, R. C. A., Chin, Y., Pratti, S., Quinn, C., Gonzalez-Cuyar, L. F., Weiss, M., Quintero-Rivera, F., & Kimonis, V. (2023). Novel Variants in the VCP Gene Causing Multisystem Proteinopathy 1. Genes, 14(3), 676. https://doi.org/10.3390/genes14030676







