The Klinefelter Syndrome and Testicular Sperm Retrieval Outcomes
Abstract
1. Definition and Clinical Aspects
2. Etiology
3. Phenotype Variability
4. Spermatogenesis
5. Hormonal and Histological Characteristics
6. Testicular Sperm Retrieval
6.1. Predictive Factors of Testicular Sperm Retrieval
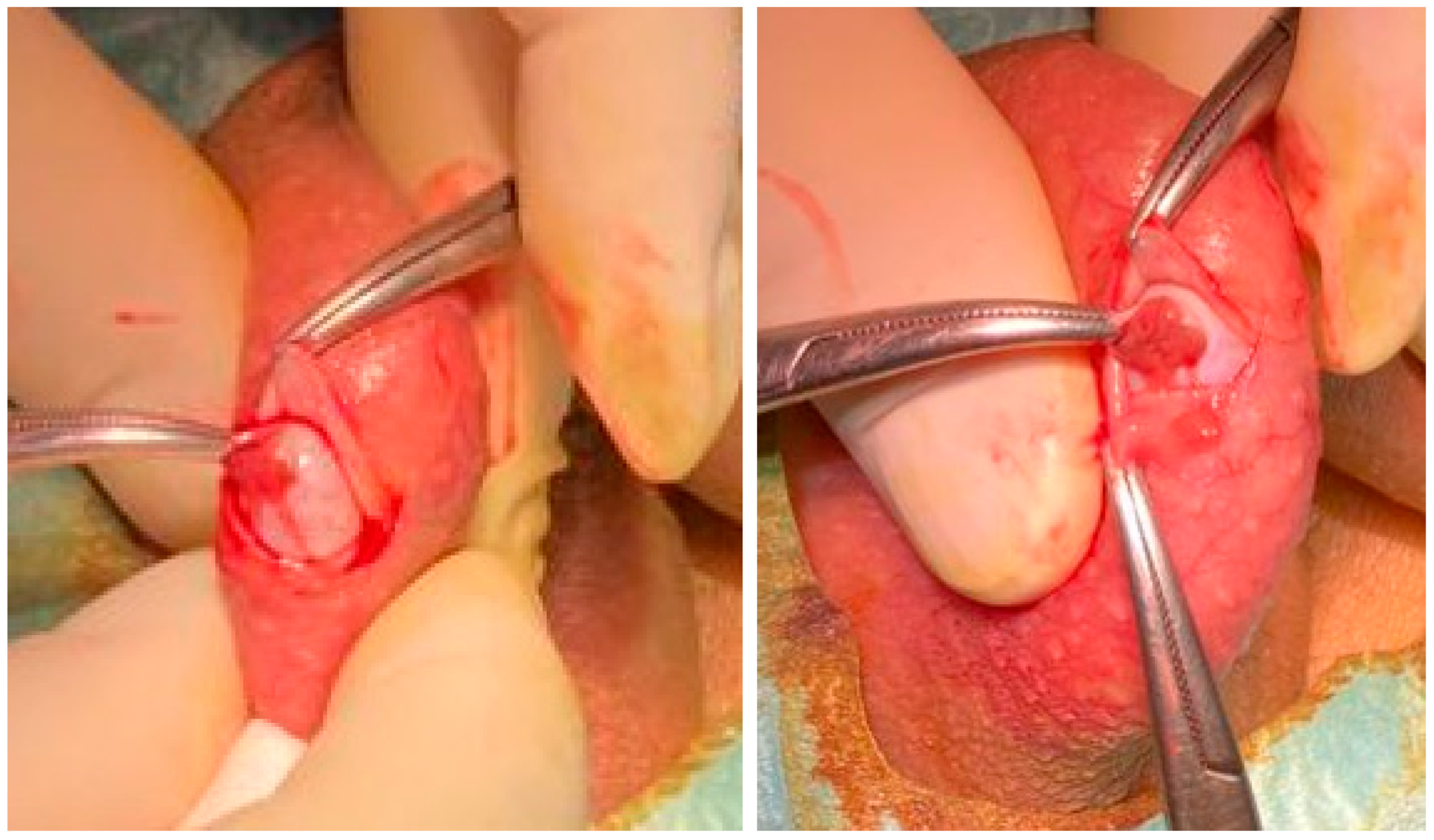
6.2. Techniques of Testicular Sperm Retrieval
6.3. Clinical and Newborn Outcomes
7. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | anti-Müllerian hormone |
| AR | androgen receptor |
| BTB | blood testicular barrier |
| CAG | cytosine-adenine-guanine |
| CP | clinical pregnancy |
| cTESE | conventional TESE |
| DTB | diagnostic testicular biopsy |
| E2 | estradiol |
| ECR | embryo cleavage rate |
| ETC | embryo transfer cycle |
| FNA | fine needle aspiration |
| FR | fertilization rate |
| FSH | Follicular stimulating hormone |
| GnRH | gonadotropin releasing hormone |
| HCG | human chorionic gonadotropin |
| HPG | Hypothalamic-pituitary-gonad |
| ICSI | intracytoplasmic sperm injection |
| INSL3 | insulin-like peptide-3 |
| IR | implantation rate |
| KS | Klinefelter syndrome |
| LBDR | live birth delivery rate |
| LC | Leydig cells |
| LH | Luteinizing hormone |
| LHR | Luteinizing hormone receptor |
| mTESE | microsurgical testicular sperm extraction |
| MESA | microscopic epididymal sperm aspiration |
| NB | newborn |
| PAI | plasminogen activator inhibitor |
| PGT | preimplantation genetic testing |
| PESA | percutaneous epididymal sperm aspiration |
| PND | prenatal diagnosis |
| PRL | prolactin |
| SC | Sertoli cell |
| SHBG | sex-hormone-binding globulin |
| SHOX | short-stature Homeobox containing gene on chromosome X |
| SRR | spermatozoa retrieval rate |
| SSR | successful spermatozoa recover |
| T | Testosterone |
| TESA | testicular sperm aspiration |
| TESE | testicular sperm extraction |
References
- Klinefelter, H.F.; Reifenstein, E.C.; Albright, F. Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle stimulating hormone. J. Clin. Endocrinol. Metab. 1942, 2, 615–627. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Strong, J.A. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 1959, 183, 302–303. [Google Scholar] [CrossRef]
- Lanfranco, F.; Kamischke, A.; Zitzmann, M.; Nieschlag, E. Klinefelter syndrome. Lancet 2004, 364, 273–283. [Google Scholar] [CrossRef]
- Bonomi, M.; Rochira, V.; Pasquali, D.; Balercia, G.; Jannini, E.A.; Ferlin, A.; On behalf of the Klinefelter ItaliaN Group (KING). Klinefelter syndrome (KS): Genetics, Clinical phenotype and hypogonadism. J. Endocrinol. Investig. 2017, 40, 123–134. [Google Scholar] [CrossRef]
- Shiraishi, K.; Matsuyama, H. Klinefelter syndrome: From pediatrics to geriatrics. Reprod. Med. Biol. 2019, 18, 140–150. [Google Scholar] [CrossRef]
- Davis, S.M.; Ross, J.L. Klinefelter Syndrome. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 561–567. [Google Scholar] [CrossRef]
- Deebel, N.A.; Bradshaw, A.W.; Sadri-Ardekani, H. Infertility considerations in klinefelter syndrome: From origin to management. Best. Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101480. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Wikström, A.M.; Rajpert-De Meyts, E.; Dunkel, L.; Skakkebaek, N.E.; Juul, A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum. Reprod. Update 2006, 12, 39–48. [Google Scholar] [CrossRef]
- Fullerton, G.; Hamilton, M.; Maheshwari, A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum. Reprod. 2010, 25, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tüttelmann, F.; Gromoll, J. Novel genetic aspects of Klinefelter’s syndrome. Mol. Hum. Reprod. 2010, 16, 386–395. [Google Scholar] [CrossRef]
- Gruchy, N.; Vialard, F.; Decamp, M.; Choiset, A.; Rossi, A.; Le Meur, N.; Moirot, H.; Yardin, C.; Bonnet-Dupeyron, M.N.; Lespinasse, J.; et al. Pregnancy outcomes in 188 French cases of prenatally diagnosed Klinefelter syndrome. Hum. Reprod. 2011, 26, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razic, M.M.; Abdel-Hamid, I.A.; Elsobky, E.; El-Dahtory, F. Further evidence of the clinical, hormonal, and genetic heterogeneity of Klinefelter syndrome: A study of 216 infertile Egyptian patients. J. Androl. 2012, 33, 441–448. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Gravholt, C.H. Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. J. Clin. Endocrinol. Metab. 2003, 88, 622–626. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Chang, S.; Wallentin, M.; Fedder, J.; Moore, P.; Skakkebæk, A. Klinefelter Syndrome: Integrating Genetics, Neuropsychology, and Endocrinology. Endocr. Rev. 2018, 39, 389–423. [Google Scholar] [CrossRef] [PubMed]
- Smyth, C.M.; Bremner, W.J. Klinefelter syndrome. Arch. Intern. Med. 1998, 158, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; de la Cruz, F.; Swerdloff, R.S.; Samango-Sprouse, C.; Skakkebaek, N.E.; Graham, J.M., Jr.; Hassold, T.; Aylstock, M.; Meyer-Bahlburg, H.F.; Willard, H.F.; et al. Klinefelter syndrome: Expanding the phenotype and identifying new research directions. Genet. Med. 2003, 5, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Juul, A. Testicular function and fertility in men with Klinefelter syndrome: A review. Eur. J. Endocrinol. 2013, 168, R67–R76. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Ferlin, A.; Gravholt, C.H.; Gromoll, J.; Köhler, B.; Lejeune, H.; Rogol, A.D.; Wistuba, J. The Klinefelter syndrome: Current management and research challenges. Andrology 2016, 4, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Skakkebaek, N.E.; Almstrup, K.; Juul, A. Clinical and biological parameters in 166 boys, adolescents and adults with nonmosaic Klinefelter syndrome: A Copenhagen experience. Acta Paediatr. 2011, 100, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Dufau, M.L.; Winters, C.A.; Hattori, M.; Aquilano, D.; Barañao, J.L.; Nozu, K.; Baukal, A.; Catt, K.J. Hormonal regulation of androgen production by the Leydig cell. J. Steroid Biochem. 1984, 20, 161–173. [Google Scholar] [CrossRef]
- Lucas, T.F.; Nascimento, A.R.; Pisolato, R.; Pimenta, M.T.; Lazari, M.F.; Porto, C.S. Receptors and signaling pathways involved in proliferation and differentiation of Sertoli cells. Spermatogenesis 2014, 4, e28138. [Google Scholar] [CrossRef]
- Huhtaniemi, I. A short evolutionary history of FSH-stimulated spermatogenesis. Hormones 2015, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the Hypothalamic Pituitary Gonadal Axis in the Male. Urol. Clin. N. Am. 2016, 43, 151–162. [Google Scholar] [CrossRef]
- Groth, K.A.; Skakkebaek, A.; Høst, C.; Gravholt, C.H.; Bojesen, A. Klinefelter syndrome-a clinical update. J. Clin. Endocrinol. Metab. 2013, 98, 20–30. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebaek, N.H.; Gravholt, C.H. Morbidity in Klinefelter syndrome: A Danish register study based on hospital discharge diagnoses. J. Clin. Endocrinol. Metab. 2006, 91, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, A.; Høst, C.; Gravholt, C.H. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: The impact of body composition. Mol. Hum. Reprod. 2010, 16, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wikström, A.M.; Dunkel, L. Klinefelter syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 239–250. [Google Scholar] [CrossRef]
- Chang, S.; Skakkebæk, A.; Trolle, C.; Bojesen, A.; Hertz, J.M.; Cohen, A.; Hougaard, D.M.; Wallentin, M.; Pedersen, A.D.; Østergaard, J.R.; et al. Anthropometry in Klinefelter syndrome—Multifactorial influences due to CAG length, testosterone treatment and possibly intrauterine hypogonadism. J. Clin. Endocrinol. Metab. 2015, 100, E508–E517. [Google Scholar] [CrossRef]
- Madureira, C.; Cunha, M.; Sousa, M.; Neto, A.P.; Pinho, M.J.; Viana, P.; Gonçalves, A.; Silva, J.; Teixeira da Silva, T.; Oliveira, C.; et al. Treatment by testicular sperm extraction and intracytoplasmic sperm injection of 65 azoospermic patients with non-mosaic Klinefelter syndrome with birth of 17 healthy children. Andrology 2014, 2, 623–631. [Google Scholar] [CrossRef]
- Zitzmann, M.; Rohayem, J. Gonadal dysfunction and beyond: Clinical challenges in children, adolescents, and adults with 47,XXY Klinefelter syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 302–312. [Google Scholar] [CrossRef]
- Barros, P.; Cunha, M.; Barros, A.; Sousa, M.; Dória, S. Clinical outcomes of 77 TESE treatment cycles in non-mosaic Klinefelter syndrome patients. JBRA Assist. Reprod. 2022, 26, 412–421. [Google Scholar] [CrossRef]
- Ross, J.L.; Samango-Sprouse, C.; Lahlou, N.; Kowal, K.; Elder, F.F.; Zinn, A. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm. Res. 2005, 64, 39–45. [Google Scholar] [CrossRef]
- Cabrol, S.; Ross, J.L.; Fennoy, I.; Bouvattier, C.; Roger, M.; Lahlou, N. Assessment of Leydig and Sertoli cell functions in infants with nonmosaic Klinefelter syndrome: Insulin-like peptide 3 levels are normal and positively correlated with LH levels. J. Clin. Endocrinol. Metab. 2011, 96, E746–E753. [Google Scholar] [CrossRef]
- Lahlou, N.; Fennoy, I.; Ross, J.L.; Bouvattier, C.; Roger, M. Clinical and hormonal status of infants with nonmosaic XXY karyotype. Acta Paediatr. 2011, 100, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Davis, S.M.; Ross, J.L.; Juul, A. Minipuberty in Klinefelter syndrome: Current status and future directions. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 320–326. [Google Scholar] [CrossRef]
- Zeger, M.P.; Zinn, A.R.; Lahlou, N.; Ramos, P.; Kowal, K.; Samango-Sprouse, C.; Ross, J.L. Effect of ascertainment and genetic features on the phenotype of Klinefelter syndrome. J. Pediatr. 2008, 152, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, N.; Howell, S.; Davis, S.; Kowal, K.; Tanda, T.; Brown, M.; Boada, C.; Alston, A.; Crawford, L.; Thompson, T.; et al. Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 428–443. [Google Scholar] [CrossRef]
- Zinn, A.R.; Ramos, P.; Elder, F.F.; Kowal, K.; Samango-Sprouse, C.; Ross, J.L. Androgen receptor CAGn repeat length influences phenotype of 47,XXY (Klinefelter) syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5041–5046. [Google Scholar] [CrossRef]
- Visootsak, J.; Graham, J.M., Jr. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J. Rare Dis. 2006, 1, 42. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cheng, A.W.; Ahmed, S.F.; Shaw, N.J.; Hughes, I.A. Genital anomalies in Klinefelter’s syndrome. Horm. Res. 2007, 68, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Reynolds, R.M.; Dabelea, D.M.; Zeitler, P.S.; Tartaglia, N.R. Testosterone Treatment in Infants With 47,XXY: Effects on Body Composition. J. Endocr. Soc. 2019, 3, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Kaar, J.L.; Ringham, B.M.; Hockett, C.W.; Glueck, D.H.; Dabelea, D. Sex differences in infant body composition emerge in the first 5 months of life. J. Pediatr. Endocrinol. Metab. 2019, 32, 1235–1239. [Google Scholar] [CrossRef]
- Aksglaede, L.; Skakkebaek, N.E.; Juul, A. Abnormal sex chromosome constitution and longitudinal growth: Serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. J. Clin. Endocrinol. Metab. 2008, 93, 169–176. [Google Scholar] [CrossRef]
- Wattendorf, D.J.; Muenke, M. Klinefelter syndrome. Am. Fam. Physician 2005, 72, 2259–2262. [Google Scholar] [PubMed]
- Temple, C.M.; Sanfilippo, P.M. Executive skills in Klinefelter’s syndrome. Neuropsychologia 2003, 41, 1547–1559. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Boone, K.B.; Miller, B.L.; Swerdloff, R.S. Neurobehavioral phenotype of Klinefelter syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Høst, C.; Skakkebæk, A.; Groth, K.A.; Bojesen, A. The role of hypogonadism in Klinefelter syndrome. Asian J. Androl. 2014, 16, 185–191. [Google Scholar] [CrossRef]
- Zitzmann, M.; Aksglaede, L.; Corona, G.; Isidori, A.M.; Juul, A.; T’Sjoen, G.; Kliesch, S.; D’Hauwers, K.; Toppari, J.; Słowikowska-Hilczer, J.; et al. European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 2021, 9, 145–167. [Google Scholar] [CrossRef]
- Bojesen, A.; Kristensen, K.; Birkebaek, N.H.; Fedder, J.; Mosekilde, L.; Bennett, P.; Laurberg, P.; Frystyk, J.; Flyvbjerg, A.; Christiansen, J.S.; et al. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006, 29, 1591–1598. [Google Scholar] [CrossRef]
- Bojesen, A.; Gravholt, C.H. Klinefelter syndrome in clinical practice. Nat. Clin. Pract. Urol. 2007, 4, 192–204. [Google Scholar] [CrossRef]
- Bearelly, P.; Oates, R. Recent advances in managing and understanding Klinefelter syndrome. F1000Research 2019, 8, 112. [Google Scholar] [CrossRef]
- Chang, S.; Skakkebaek, A.; Davis, S.M.; Gravholt, C.H. Morbidity in Klinefelter syndrome and the effect of testosterone treatment. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.J.; Oskui, P.M.; Hale, S.L.; Kloner, R.A. Testosterone and the cardiovascular system: A comprehensive review of the basic science literature. J. Am. Heart Assoc. 2013, 2, e000271. [Google Scholar] [CrossRef]
- Lin, H.; Xu, L.; Yu, S.; Hong, W.; Huang, M.; Xu, P. Therapeutics targeting the fibrinolytic system. Exp. Mol. Med. 2020, 52, 367–379. [Google Scholar] [CrossRef]
- Nawaz, S.S.; Siddiqui, K. Plasminogen activator inhibitor-1 mediate downregulation of adiponectin in type 2 diabetes patients with metabolic syndrome. Cytokine X 2022, 4, 100064. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, A.H.; McLoughlin, M.; Vogiatzi, M.G. Endocrine aspects of Klinefelter syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Giltay, J.C.; Maiburg, M.C. Klinefelter syndrome: Clinical and molecular aspects. Expert Rev. Mol. Diagn. 2010, 10, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Cederlöf, M.; Ohlsson Gotby, A.; Larsson, H.; Serlachius, E.; Boman, M.; Långström, N.; Landén, M.; Lichtenstein, P. Klinefelter syndrome and risk of psychosis, autism and ADHD. J. Psychiatr. Res. 2014, 48, 128–130. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Campone, B.; Castellana, M.; Salzano, C.; Fisher, A.D.; de Angelis, C.; Pivonello, R.; Colao, A.; Pasquali, D.; Maggi, M.; et al. Neuropsychiatric Aspects in Men with Klinefelter Syndrome. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 109–115. [Google Scholar] [CrossRef]
- Maiburg, M.; Repping, S.; Giltay, J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. Fertil. Steril. 2012, 98, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.S.; Hassold, T.J. Aberrant recombination and the origin of Klinefelter syndrome. Hum. Reprod. Update 2003, 9, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Depenbusch, M.; Gromoll, J.; Nieschlag, E. X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J. Clin. Endocrinol. Metab. 2004, 89, 6208–6217. [Google Scholar] [CrossRef]
- Bojesen, A.; Hertz, J.M.; Gravholt, C.H. Genotype and phenotype in Klinefelter syndrome—impact of androgen receptor polymorphism and skewed X inactivation. Int. J. Androl. 2011, 34 Pt 2, e642–e648. [Google Scholar] [CrossRef]
- Samplaski, M.K.; Lo, K.C.; Grober, E.D.; Millar, A.; Dimitromanolakis, A.; Jarvi, K.A. Phenotypic differences in mosaic Klinefelter patients as compared with non-mosaic Klinefelter patients. Fertil. Steril. 2014, 101, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Bongers, R.; Werler, S.; Bogdanova, N.; Wistuba, J.; Kliesch, S.; Gromoll, J.; Tüttelmann, F. Gene expression patterns in relation to the clinical phenotype in Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E518–E523. [Google Scholar] [CrossRef]
- Ottesen, A.M.; Aksglaede, L.; Garn, I.; Tartaglia, N.; Tassone, F.; Gravholt, C.H.; Bojesen, A.; Sørensen, K.; Jørgensen, N.; Rajpert-De Meyts, E.; et al. Increased number of sex chromosomes affects height in a nonlinear fashion: A study of 305 patients with sex chromosome aneuploidy. Am. J. Med. Genet. A 2010, 152A, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.; Durand, C.; Bender, S.; Rödelsperger, C.; Glaser, A.; Hecht, J.; Schneider, K.U.; Rappold, G. FGFR3 is a target of the homeobox transcription factor SHOX in limb development. Hum. Mol. Genet. 2011, 20, 1524–1535. [Google Scholar] [CrossRef]
- Rocca, M.S.; Pecile, V.; Cleva, L.; Speltra, E.; Selice, R.; Di Mambro, A.; Foresta, C.; Ferlin, A. The Klinefelter syndrome is associated with high recurrence of copy number variations on the X chromosome with a potential role in the clinical phenotype. Andrology 2016, 4, 328–334. [Google Scholar] [CrossRef]
- Skakkebæk, A.; Nielsen, M.M.; Trolle, C.; Vang, S.; Hornshøj, H.; Hedegaard, J.; Wallentin, M.; Bojesen, A.; Hertz, J.M.; Fedder, J.; et al. DNA hypermethylation and differential gene expression associated with Klinefelter syndrome. Sci. Rep. 2018, 8, 13740. [Google Scholar] [CrossRef]
- Zitzmann, M.; Nieschlag, E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int. J. Androl. 2003, 26, 76–83. [Google Scholar] [CrossRef]
- Fainberg, J.; Hayden, R.P.; Schlegel, P.N. Fertility management of Klinefelter syndrome. Expert Rev. Endocrinol. Metab. 2019, 14, 369–380. [Google Scholar] [CrossRef]
- Wikström, A.M.; Painter, J.N.; Raivio, T.; Aittomäki, K.; Dunkel, L. Genetic features of the X chromosome affect pubertal development and testicular degeneration in adolescent boys with Klinefelter syndrome. Clin. Endocrinol. 2006, 65, 92–97. [Google Scholar] [CrossRef]
- Busch, A.S.; Tüttelmann, F.; Zitzmann, M.; Kliesch, S.; Gromoll, J. The FSHB- 211G>T variant attenuates serum FSH levels in the supraphysiological gonadotropin setting of Klinefelter syndrome. Eur. J. Hum. Genet. 2015, 23, 700–703. [Google Scholar] [CrossRef]
- Marques, C.J.; Carvalho, F.; Sousa, M.; Barros, A. Genomic imprinting in disruptive spermatogenesis. Lancet 2004, 363, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.J.; Costa, P.; Vaz, B.; Carvalho, F.; Fernandes, S.; Barros, A.; Sousa, M. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 2008, 14, 67–74. [Google Scholar] [CrossRef]
- Marques, C.J.; João Pinho, M.; Carvalho, F.; Bièche, I.; Barros, A.; Sousa, M. DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 2011, 6, 1354–1361. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, S.; Heckmann, L.; Di Persio, S.; Li, X.; Meyer Zu Hörste, G.; Wistuba, J.; Cremers, J.F.; Gromoll, J.; Kliesch, S.; Schlatt, S.; et al. High-resolution analysis of germ cells from men with sex chromosomal aneuploidies reveals normal transcriptome but impaired imprinting. Clin. Epigenetics 2019, 11, 127. [Google Scholar] [CrossRef]
- Van Assche, E.; Bonduelle, M.; Tournaye, H.; Joris, H.; Verheyen, G.; Devroey, P.; Van Steirteghem, A.; Liebaers, I. Cytogenetics of infertile men. Hum. Reprod. 1996, 11 (Suppl. 4), 1–24, discussion 25–26. [Google Scholar] [CrossRef]
- Vincent, M.C.; Daudin, M.; De, M.P.; Massat, G.; Mieusset, R.; Pontonnier, F.; Calvas, P.; Bujan, L.; Bourrouillout, G. Cytogenetic investigations of infertile men with low sperm counts: A 25-year experience. J. Androl. 2002, 23, 18–22, discussion 44–45. [Google Scholar] [CrossRef]
- Ferlin, A.; Arredi, B.; Foresta, C. Genetic causes of male infertility. Reprod. Toxicol. 2006, 22, 133–141. [Google Scholar] [CrossRef]
- Vignozzi, L.; Corona, G.; Forti, G.; Jannini, E.A.; Maggi, M. Clinical and therapeutic aspects of Klinefelter’s syndrome: Sexual function. Mol. Hum. Reprod. 2010, 16, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quevedo, L.; Blanco, J.; Sarrate, Z.; Català, V.; Bassas, L.; Vidal, F. Hidden mosaicism in patients with Klinefelter’s syndrome: Implications for genetic reproductive counselling. Hum. Reprod. 2011, 26, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.; Aspillaga, M.; Shelley, T.F.; Gardner, L.I. Possible fertility in Klinefelter’s syndrome. Lancet 1963, 1, 506. [Google Scholar] [CrossRef]
- Foss, G.L.; Lewis, F.J. A study of four cases with Klinefelter’s syndrome, showing motile spermatozoa in their ejaculates. J. Reprod. Fertil. 1971, 25, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kamischke, A.; Baumgardt, A.; Horst, J.; Nieschlag, E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J. Androl. 2003, 24, 41–48. [Google Scholar] [CrossRef]
- Selice, R.; Di Mambro, A.; Garolla, A.; Ficarra, V.; Iafrate, M.; Ferlin, A.; Foresta, C. Spermatogenesis in Klinefelter syndrome. J. Endocrinol. Investig. 2010, 33, 789–793. [Google Scholar] [CrossRef] [PubMed]
- SayedElAkhras; ElSaeed, K.O.; Halawa, M.; Elsemary, M.Y.; AlyElakhras; Adam, G.; Tanos, V. Nonmosaic Klinefelter Syndrome Successful Conception after TESE/ICSI: A case report. Open Access J. Reprod. Syst. Sex. Disord. 2018, 1, 42–45. [Google Scholar] [CrossRef]
- Nassau, D.E.; Best, J.C.; Cohen, J.; Gonzalez, D.C.; Alam, A.; Ramasamy, R. Androgenization in Klinefelter syndrome: Clinical spectrum from infancy through young adulthood. J. Pediatr. Urol. 2021, 17, 346–352. [Google Scholar] [CrossRef]
- Boeri, L.; Palmisano, F.; Preto, M.; Sibona, M.; Capogrosso, P.; Franceschelli, A.; Ruiz-Castañé, E.; Sarquella-Geli, J.; Bassas-Arnau, L.; Scroppo, F.I.; et al. Sperm retrieval rates in non-mosaic Klinefelter patients undergoing testicular sperm extraction: What expectations do we have in the real-life setting? Andrology 2020, 8, 680–687. [Google Scholar] [CrossRef]
- Skakkebaek, N.E. Two types of tubules containing only Sertoli cells in adults with Klinefelter’s syndrome. Nature 1969, 223, 643–645. [Google Scholar] [CrossRef]
- Ichioka, K.; Utsunomiya, N.; Kohei, N.; Ueda, N.; Inoue, K.; Terai, A. Adult onset of declining spermatogenesis in a man with nonmosaic Klinefelter’s syndrome. Fertil. Steril. 2006, 85, 1511.e1–1511.e2. [Google Scholar] [CrossRef]
- Van Saen, D.; Tournaye, H.; Goossens, E. Presence of spermatogonia in 47,XXY men with no spermatozoa recovered after testicular sperm extraction. Fertil. Steril. 2012, 97, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Westlander, G.; Ekerhovd, E.; Granberg, S.; Hanson, L.; Hanson, C.; Bergh, C. Testicular ultrasonography and extended chromosome analysis in men with nonmosaic Klinefelter syndrome: A prospective study of possible predictive factors for successful sperm recovery. Fertil. Steril. 2001, 75, 1102–1105. [Google Scholar] [CrossRef]
- Bergère, M.; Wainer, R.; Nataf, V.; Bailly, M.; Gombault, M.; Ville, Y.; Selva, J. Biopsied testis cells of four 47,XXY patients: Fluorescence in-situ hybridization and ICSI results. Hum. Reprod. 2002, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Warburg, E. A fertile patient with Klinefelter’s syndrome. Acta Endocrinol. 1963, 43, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, J.; Chevret, E.; Rousseaux, S.; Pelletier, R.; Benitz, V.; Jalbert, H.; Sèle, B. Achievement of meiosis in XXY germ cells: Study of 543 sperm karyotypes from an XY/XXY mosaic patient. Hum. Genet. 1994, 93, 32–34. [Google Scholar] [CrossRef]
- Chevret, E.; Rousseaux, S.; Monteil, M.; Usson, Y.; Cozzi, J.; Pelletier, R.; Sèle, B. Increased incidence of hyperhaploid 24,XY spermatozoa detected by three-colour FISH in a 46,XY/47,XXY male. Hum. Genet. 1996, 97, 171–175. [Google Scholar] [CrossRef]
- Foresta, C.; Galeazzi, C.; Bettella, A.; Stella, M.; Scandellari, C. High incidence of sperm sex chromosomes aneuploidies in two patients with Klinefelter’s syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 203–205. [Google Scholar] [CrossRef]
- Blanco, J.; Egozcue, J.; Vidal, F. Meiotic behaviour of the sex chromosomes in three patients with sex chromosome anomalies (47,XXY, mosaic 46,XY/47,XXY and 47,XYY) assessed by fluorescence in-situ hybridization. Hum. Reprod. 2001, 16, 887–892. [Google Scholar] [CrossRef]
- Foresta, C.; Galeazzi, C.; Bettella, A.; Marin, P.; Rossato, M.; Garolla, A.; Ferlin, A. Analysis of meiosis in intratesticular germ cells from subjects affected by classic Klinefelter’s syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 3807–3810. [Google Scholar] [CrossRef]
- Sciurano, R.B.; Luna Hisano, C.V.; Rahn, M.I.; Brugo Olmedo, S.; Rey Valzacchi, G.; Coco, R.; Solari, A.J. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum. Reprod. 2009, 24, 2353–2360. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sofikitis, N.; Mio, Y.; Loutradis, D.; Kaponis, A.; Miyagawa, I. Morphometric and cytogenetic characteristics of testicular germ cells and Sertoli cell secretory function in men with non-mosaic Klinefelter’s syndrome. Hum. Reprod. 2002, 17, 886–896. [Google Scholar] [CrossRef]
- Gonsalves, J.; Turek, P.J.; Schlegel, P.N.; Hopps, C.V.; Weier, J.F.; Pera, R.A. Recombination in men with Klinefelter syndrome. Reproduction 2005, 130, 223–229. [Google Scholar] [CrossRef]
- Levron, J.; Aviram-Goldring, A.; Madgar, I.; Raviv, G.; Barkai, G.; Dor, J. Sperm chromosome analysis and outcome of IVF in patients with non-mosaic Klinefelter’s syndrome. Fertil. Steril. 2000, 74, 925–929. [Google Scholar] [CrossRef]
- Harari, O.; Bourne, H.; Baker, G.; Gronow, M.; Johnston, I. High fertilization rate with intracytoplasmic sperm injection in mosaic Klinefelter’s syndrome. Fertil. Steril. 1995, 63, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xi, Q.; Jing, J.; Li, L.; Zhang, H.; Liu, R.; Pan, Y. Intracytoplasmic sperm injection outcome of ejaculated spermatozoa from a man with mosaic Klinefelter’s Syndrome: Case report and literature review. J. Int. Med. Res. 2018, 46, 4323–4331. [Google Scholar] [CrossRef]
- Bielanska, M.; Tan, S.L.; Ao, A. Fluorescence in-situ hybridization of sex chromosomes in spermatozoa and spare preimplantation embryos of a Klinefelter 46,XY/47,XXY male. Hum. Reprod. 2000, 15, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Fuse, H.; Kojima, Y.; Hayashi, M.; Honda, S. Birth after intracytoplasmic sperm injection of ejaculated spermatozoa from a man with mosaic Klinefelter’s syndrome. Asian J. Androl. 2005, 7, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Dickerman, Z.; Zamir, R.; Galatzer, A. Paternity in Klinefelter’s syndrome—A case report. Arch. Androl. 1982, 8, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Terzoli, G.; Lalatta, F.; Lobbiani, A.; Simoni, G.; Colucci, G. Fertility in a 47,XXY patient: Assessment of biological paternity by deoxyribonucleic acid fingerprinting. Fertil. Steril. 1992, 58, 821–822. [Google Scholar] [CrossRef]
- Al-Jehani, M.; Al-Husayni, F.; Alaidarous, S. Fertility in a patient diagnosed with klinefelter syndrome: A rare case report. Endocrinol. Metab. Int. J. 2019, 7, 122–123. [Google Scholar] [CrossRef]
- Xu, W.Q.; Yuan, Y.; Chen, Y.; Luo, T.; Chen, H.Y. Birth of a boy after intracytoplasmic sperm injection using ejaculated spermatozoa from a nonmosaic klinefelter syndrome man with normal sperm motility: A case report. Front. Genet. 2022, 13, 989701. [Google Scholar] [CrossRef]
- Staessen, C.; Tournaye, H.; Van Assche, E.; Michiels, A.; Van Landuyt, L.; Devroey, P.; Liebaers, I.; Van Steirteghem, A. PGD in 47,XXY Klinefelter’s syndrome patients. Hum. Reprod. Update 2003, 9, 319–330. [Google Scholar] [CrossRef]
- Friedler, S.; Raziel, A.; Strassburger, D.; Schachter, M.; Bern, O.; Ron-El, R. Outcome of ICSI using fresh and cryopreserved-thawed testicular spermatozoa in patients with non-mosaic Klinefelter’s syndrome. Hum. Reprod. 2001, 16, 2616–2620. [Google Scholar] [CrossRef]
- Hinney, B.; Guttenbach, M.; Schmid, M.; Engel, W.; Michelmann, H.W. Pregnancy after intracytoplasmic sperm injection with sperm from a man with a 47,XXY Klinefelter’s karyotype. Fertil. Steril. 1997, 68, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Matsumiya, K.; Koga, M.; Nishimura, K.; Miura, H.; Tsuji, T.; Matsumoto, M.; Okamoto, Y.; Okuyama, A. Ejaculated spermatozoa in patients with non-mosaic Klinefelter’s syndrome. Int. J. Urol. 2000, 7, 88–92, discussion 93–94. [Google Scholar] [CrossRef] [PubMed]
- Ulug, U.; Bener, F.; Akman, M.A.; Bahceci, M. Partners of men with Klinefelter syndrome can benefit from assisted reproductive technologies. Fertil. Steril. 2003, 80, 903–906. [Google Scholar] [CrossRef]
- Bourne, H.; Stern, K.; Clarke, G.; Pertile, M.; Speirs, A.; Baker, H.W. Delivery of normal twins following the intracytoplasmic injection of spermatozoa from a patient with 47,XXY Klinefelter’s syndrome. Hum. Reprod. 1997, 12, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Ron-El, R.; Raziel, A.; Strassburger, D.; Schachter, M.; Bern, O.; Friedler, S. Birth of healthy male twins after intracytoplasmic sperm injection of frozen-thawed testicular spermatozoa from a patient with nonmosaic Klinefelter syndrome. Fertil. Steril. 2000, 74, 832–833. [Google Scholar] [CrossRef]
- Crüger, D.; Toft, B.; Agerholm, I.; Fedder, J.; Hald, F.; Bruun-Petersen, G. Birth of a healthy girl after ICSI with ejaculated spermatozoa from a man with non-mosaic Klinefelter’s syndrome. Hum. Reprod. 2001, 16, 1909–1911. [Google Scholar] [CrossRef]
- Tachdjian, G.; Frydman, N.; Morichon-Delvallez, N.; Dû, A.L.; Fanchin, R.; Vekemans, M.; Frydman, R. Reproductive genetic counselling in non-mosaic 47,XXY patients: Implications for preimplantation or prenatal diagnosis: Case report and review. Hum. Reprod. 2003, 18, 271–275. [Google Scholar] [CrossRef]
- Komori, S.; Horiuchi, I.; Hamada, Y.; Hasegawa, A.; Kasumi, H.; Kondoh, N.; Sawai, H.; Toji, H.; Shigeta, M.; Shima, H.; et al. Birth of healthy neonates after intracytoplasmic injection of ejaculated or testicular spermatozoa from men with nonmosaic Klinefelter’s syndrome: A report of 2 cases. J. Reprod. Med. 2004, 49, 126–130. [Google Scholar]
- Ye, H.; Xue1, S.; Kuang, Y.; Sun, L. A patient with non-mosaic 47, XXY karyotype fathering a normal healthy infant using intracytoplasmic sperm injection (ICSI)—A case report. Clin. Exp. Obstet. Gynecol. 2020, 47, 309–311. [Google Scholar] [CrossRef]
- Aksglaede, L.; Jensen, R.B.; Carlsen, E.; Kok, P.; Keenan, D.M.; Veldhuis, J.; Skakkebaek, N.E.; Juul, A. Increased basal and pulsatile secretion of FSH and LH in young men with 47,XXY or 46,XX karyotypes. Eur. J. Endocrinol. 2008, 158, 803–810. [Google Scholar] [CrossRef]
- Sansone, A.; Kliesch, S.; Isidori, A.M.; Schlatt, S. AMH and INSL3 in testicular and extragonadal pathophysiology: What do we know? Andrology 2019, 7, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, P.; Andersson, A.M.; Skakkebaek, N.E. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 888–891. [Google Scholar] [CrossRef]
- Bay, K.; Hartung, S.; Ivell, R.; Schumacher, M.; Jürgensen, D.; Jorgensen, N.; Holm, M.; Skakkebaek, N.E.; Andersson, A.M. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: Relationship to the luteinizing hormone-testosterone axis. J. Clin. Endocrinol. Metab. 2005, 90, 3410–3418. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Christiansen, P.; Sørensen, K.; Boas, M.; Linneberg, A.; Main, K.M.; Andersson, A.M.; Skakkebaek, N.E.; Juul, A. Serum concentrations of Anti-Müllerian Hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism. Acta. Paediatr. 2011, 100, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R. Interpretation of reproductive hormones before, during and after the pubertal transition-Identifying health and disordered puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef]
- Lottrup, G.; Nielsen, J.E.; Maroun, L.L.; Møller, L.M.; Yassin, M.; Leffers, H.; Skakkebæk, N.E.; Rajpert-De Meyts, E. Expression patterns of DLK1 and INSL3 identify stages of Leydig cell differentiation during normal development and in testicular pathologies, including testicular cancer and Klinefelter syndrome. Hum. Reprod. 2014, 29, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.; Gies, I.; Van Saen, D. Germ cell loss in Klinefelter syndrome: When and why? Am. J. Med. Genet. C. Semin. Med. Genet. 2020, 184, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Edelsztein, N.Y.; Valeri, C.; Lovaisa, M.M.; Schteingart, H.F.; Rey, R.A. AMH Regulation by Steroids in the Mammalian Testis: Underlying Mechanisms and Clinical Implications. Front. Endocrinol. 2022, 13, 906381. [Google Scholar] [CrossRef]
- Assis, L.H.; Crespo, D.; Morais, R.D.; França, L.R.; Bogerd, J.; Schulz, R.W. INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio). Cell Tissue Res. 2016, 363, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bastida, M.G.; Rey, R.A.; Bergadá, I.; Bedecarrás, P.; Andreone, L.; del Rey, G.; Boywitt, A.; Ropelato, M.G.; Cassinelli, H.; Arcari, A.; et al. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin. Endocrinol. 2007, 67, 863–870. [Google Scholar] [CrossRef]
- Oates, R.D. The natural history of endocrine function and spermatogenesis in Klinefelter syndrome: What the data show. Fertil. Steril. 2012, 98, 266–273. [Google Scholar] [CrossRef]
- Hammond, G.L. Diverse roles for sex hormone-binding globulin in reproduction. Biol. Reprod. 2011, 85, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Baker, H.W.; Burger, H.G.; De Kretser, D.M.; Hudson, B. Hormonal studies in Klinefelter’s syndrome. Clin. Endocrinol. 1975, 4, 399–411. [Google Scholar] [CrossRef]
- Pacenza, N.; Pasqualini, T.; Gottlieb, S.; Knoblovits, P.; Costanzo, P.R.; Stewart Usher, J.; Rey, R.A.; Martínez, M.P.; Aszpis, S. Clinical Presentation of Klinefelter’s Syndrome: Differences According to Age. Int. J. Endocrinol. 2012, 2012, 324835. [Google Scholar] [CrossRef]
- Foresta, C.; Caretta, N.; Palego, P.; Ferlin, A.; Zuccarello, D.; Lenzi, A.; Selice, R. Reduced artery diameters in Klinefelter syndrome. Int. J. Androl. 2012, 35, 720–725. [Google Scholar] [CrossRef]
- Tuttelmann, F.; Damm, O.S.; Luetjens, C.M.; Baldi, M.; Zitzmann, M.; Kliesch, S.; Nieschlag, E.; Gromoll, J.; Wistuba, J.; Simoni, M. Intratesticular testosterone is increased in men with Klinefelter syndrome and may not be released into the bloodstream owing to altered testicular vascularization– a preliminary report. Andrology 2014, 2, 275–281. [Google Scholar] [CrossRef]
- Giudice, M.G.; Vermeulen, M.; Wyns, C. Blood Testis Barrier and Somatic Cells Impairment in a Series of 35 Adult Klinefelter Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 5717. [Google Scholar] [CrossRef] [PubMed]
- Deebel, N.A.; Galdon, G.; Zarandi, N.P.; Stogner-Underwood, K.; Howards, S.; Lovato, J.; Kogan, S.; Atala, A.; Lue, Y.; Sadri-Ardekani, H. Age-related presence of spermatogonia in patients with Klinefelter syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 58–72. [Google Scholar] [CrossRef]
- Heckmann, L.; Langenstroth-Röwer, D.; Pock, T.; Wistuba, J.; Stukenborg, J.B.; Zitzmann, M.; Kliesch, S.; Schlatt, S.; Neuhaus, N. A diagnostic germ cell score for immature testicular tissue at risk of germ cell loss. Hum. Reprod. 2018, 33, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Vloeberghs, V.; Gies, I.; Mateizel, I.; Sermon, K.; De Schepper, J.; Tournaye, H.; Goossens, E. When does germ cell loss and fibrosis occur in patients with Klinefelter syndrome? Hum. Reprod. 2018, 33, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Winge, S.B.; Dalgaard, M.D.; Belling, K.G.; Jensen, J.M.; Nielsen, J.E.; Aksglaede, L.; Schierup, M.H.; Brunak, S.; Skakkebæk, N.E.; Juul, A.; et al. Transcriptome analysis of the adult human Klinefelter testis and cellularity-matched controls reveals disturbed differentiation of Sertoli- and Leydig cells. Cell Death Dis. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Coerdt, W.; Rehder, H.; Gausmann, I.; Johannisson, R.; Gropp, A. Quantitative histology of human fetal testes in chromosomal disease. Pediatr. Pathol. 1985, 3, 245–259. [Google Scholar] [CrossRef]
- Murken, J.D.; Stengel-Rutkowski, S.; Walther, J.U.; Westenfelder, S.R.; Remberger, K.H.; Zimmer, F. Letter: Klinefelter’s syndrome in a fetus. Lancet 1974, 2, 171. [Google Scholar] [CrossRef]
- Lahlou, N.; Fennoy, I.; Carel, J.C.; Roger, M. Inhibin B and anti-Müllerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Wikström, A.M.; Raivio, T.; Hadziselimovic, F.; Wikström, S.; Tuuri, T.; Dunkel, L. Klinefelter syndrome in adolescence: Onset of puberty is associated with accelerated germ cell depletion. J. Clin. Endocrinol. Metab. 2004, 89, 2263–2270. [Google Scholar] [CrossRef]
- D’Aurora, M.; Ferlin, A.; Garolla, A.; Franchi, S.; D’Onofrio, L.; Trubiani, O.; Palka, G.; Foresta, C.; Stuppia, L.; Gatta, V. Testis Transcriptome Modulation in Klinefelter Patients with Hypospermatogenesis. Sci. Rep. 2017, 7, 45729. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Van Steirteghem, A.C.; Liu, J.; Joris, H.; Nagy, Z.; Janssenswillen, C.; Tournaye, H.; Derde, M.P.; Van Assche, E.; Devroey, P. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum. Reprod. 1993, 8, 1055–1060. [Google Scholar] [CrossRef]
- Van Steirteghem, A.C.; Nagy, Z.; Joris, H.; Liu, J.; Staessen, C.; Smitz, J.; Wisanto, A.; Devroey, P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum. Reprod. 1993, 8, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Devroey, P.; Liu, J.; Nagy, Z.; Goossens, A.; Tournaye, H.; Camus, M.; Van Steirteghem, A.; Silber, S. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum. Reprod. 1995, 10, 1457–1460. [Google Scholar] [CrossRef]
- Devroey, P.; Nagy, P.; Tournaye, H.; Liu, J.; Silber, S.; Van Steirteghem, A. Outcome of intracytoplasmic sperm injection with testicular spermatozoa in obstructive and non-obstructive azoospermia. Hum. Reprod. 1996, 11, 1015–1018. [Google Scholar] [CrossRef]
- Tournaye, H.; Liu, J.; Nagy, P.Z.; Camus, M.; Goossens, A.; Silber, S.; Van Steirteghem, A.C.; Devroey, P. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum. Reprod. 1996, 11, 127–132. [Google Scholar] [CrossRef]
- Tournaye, H.; Verheyen, G.; Nagy, P.; Ubaldi, F.; Goossens, A.; Silber, S.; Van Steirteghem, A.C.; Devroey, P. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum. Reprod. 1997, 12, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H. Surgical sperm recovery for intracytoplasmic sperm injection: Which method is to be preferred? Hum. Reprod. 1999, 14 (Suppl. 1), 71–81. [Google Scholar] [CrossRef]
- Tournaye, H.; Staessen, C.; Liebaers, I.; Van Assche, E.; Devroey, P.; Bonduelle, M.; Van Steirteghem, A. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum. Reprod. 1996, 11, 1644–1649. [Google Scholar] [CrossRef]
- Tournaye, H.; Camus, M.; Vandervorst, M.; Nagy, Z.; Joris, H.; Van Steirteghem, A.; Devroey, P. Surgical Sperm Retrieval for Intracytoplasmic Sperm Injection. Int. J. Androl. 1997, 20 (Suppl. 3), 69–73. [Google Scholar] [PubMed]
- Palermo, G.D.; Schlegel, P.N.; Sills, E.S.; Veeck, L.L.; Zaninovic, N.; Menendez, S.; Rosenwaks, Z. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N. Engl. J. Med. 1998, 338, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Plotton, I.; Giscard d’Estaing, S.; Cuzin, B.; Brosse, A.; Benchaib, M.; Lornage, J.; Ecochard, R.; Dijoud, F.; Lejeune, H.; FERTIPRESERVE group. Preliminary results of a prospective study of testicular sperm extraction in young versus adult patients with nonmosaic 47,XXY Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.W.; Cremers, J.F.; Zitzmann, M.; Nieschlag, E.; Kliesch, S.; Rohayem, J. A history of undescended testes in young men with Klinefelter syndrome does not reduce the chances for successful microsurgical testicular sperm extraction. Andrology 2018, 6, 525–531. [Google Scholar] [CrossRef]
- Vernaeve, V.; Staessen, C.; Verheyen, G.; Van Steirteghem, A.; Devroey, P.; Tournaye, H. Can biological or clinical parameters predict testicular sperm recovery in 47,XXY Klinefelter’s syndrome patients? Hum. Reprod. 2004, 19, 1135–1139. [Google Scholar] [CrossRef]
- Schiff, J.D.; Palermo, G.D.; Veeck, L.L.; Goldstein, M.; Rosenwaks, Z.; Schlegel, P.N. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 6263–6267. [Google Scholar] [CrossRef] [PubMed]
- Bakircioglu, E.M.; Erden, H.F.; Kaplancan, T.; Ciray, N.; Bener, F.; Bahceci, M. Aging may adversely affect testicular sperm recovery in patients with Klinefelter syndrome. Urology 2006, 68, 1082–1086. [Google Scholar] [CrossRef]
- Kyono, K.; Uto, H.; Nakajo, Y.; Kumagai, S.; Araki, Y.; Kanto, S. Seven pregnancies and deliveries from non-mosaic Klinefelter syndrome patients using fresh and frozen testicular sperm. J. Assist. Reprod. Genet. 2007, 24, 47–51. [Google Scholar] [CrossRef]
- Ozveri, H.; Kayabasoglu, F.; Demirel, C.; Donmez, E. Outcomes of Micro-Dissection TESE in Patients with Non-Mosaic Klinefelter’s Syndrome without Hormonal Treatment. Int. J. Fertil. Steril. 2015, 8, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kumanov, P. Increased prolactin secretion and thyrotrophin response to thyrotrophin releasing hormone in Klinefelter’s syndrome. Andrologia 1995, 27, 41–45. [Google Scholar] [CrossRef]
- Wikström, A.M.; Dunkel, L. Testicular function in Klinefelter syndrome. Horm. Res. 2008, 69, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.A.; Bolyakov, A.; Cohen, P.; Travis, A. Reproduction in men with Klinefelter syndrome: The past, the present, and the future. Semin. Reprod. Med. 2009, 27, 137–148. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Jensen, A.S.; Høst, C.; Bojesen, A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta. Paediatr. 2011, 100, 871–877. [Google Scholar] [CrossRef]
- Sokol, R.Z. It’s not all about the testes: Medical issues in Klinefelter patients. Fertil. Steril. 2012, 98, 261–265. [Google Scholar] [CrossRef]
- Okada, H.; Goda, K.; Yamamoto, Y.; Sofikitis, N.; Miyagawa, I.; Mio, Y.; Koshida, M.; Horie, S. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter’s syndrome. Fertil. Steril. 2005, 84, 1662–1664. [Google Scholar] [CrossRef]
- Bakircioglu, M.E.; Ulug, U.; Erden, H.F.; Tosun, S.; Bayram, A.; Ciray, N.; Bahceci, M. Klinefelter syndrome: Does it confer a bad prognosis in treatment of nonobstructive azoospermia? Fertil. Steril. 2011, 95, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Ricci, J.A.; Palermo, G.D.; Gosden, L.V.; Rosenwaks, Z.; Schlegel, P.N. Successful fertility treatment for Klinefelter’s syndrome. J. Urol. 2009, 182, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Ferhi, K.; Avakian, R.; Griveau, J.F.; Guille, F. Age as only predictive factor for successful sperm recovery in patients with Klinefelter’s syndrome. Andrologia 2009, 41, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Yarali, H.; Polat, M.; Bozdag, G.; Gunel, M.; Alpas, I.; Esinler, I.; Dogan, U.; Tiras, B. TESE-ICSI in patients with non-mosaic Klinefelter syndrome: A comparative study. Reprod. Biomed. Online 2009, 18, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghian, M.; Modarresi, T.; Hosseinifar, H.; Hosseini, J.; Farrahi, F.; Dadkhah, F.; Chehrazi, M.; Khalili, G.; Sadighi Gilani, M.A. Comparison of sperm retrieval and intracytoplasmic sperm injection outcome in patients with and without Klinefelter syndrome. Urology 2014, 83, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Rohayem, J.; Fricke, R.; Czeloth, K.; Mallidis, C.; Wistuba, J.; Krallmann, C.; Zitzmann, M.; Kliesch, S. Age and markers of Leydig cell function, but not of Sertoli cell function predict the success of sperm retrieval in adolescents and adults with Klinefelter’s syndrome. Andrology 2015, 3, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Ozer, C.; Caglar Aytac, P.; Goren, M.R.; Toksoz, S.; Gul, U.; Turunc, T. Sperm retrieval by microdissection testicular sperm extraction and intracytoplasmic sperm injection outcomes in nonobstructive azoospermic patients with Klinefelter syndrome. Andrologia 2018, 50, e12983. [Google Scholar] [CrossRef] [PubMed]
- Kailash, Y.; Raheem, A.A.; Homa, S.T. How Successful Is Surgical Sperm Retrieval in Klinefelter Syndrome? Front. Reprod. Health 2021, 3, 636629. [Google Scholar] [CrossRef]
- Madgar, I.; Dor, J.; Weissenberg, R.; Raviv, G.; Menashe, Y.; Levron, J. Prognostic value of the clinical and laboratory evaluation in patients with nonmosaic Klinefelter syndrome who are receiving assisted reproductive therapy. Fertil. Steril. 2002, 77, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Fang, A.; Fan, Y.; Fu, X.; Lan, Y.; Liu, M.; Cao, S.; An, G. Role of treatment with human chorionic gonadotropin and clinical parameters on testicular sperm recovery with microdissection testicular sperm extraction and intracytoplasmic sperm injection outcomes in 184 Klinefelter syndrome patients. Fertil. Steril. 2020, 114, 997–1005. [Google Scholar] [CrossRef]
- Majzoub, A.; Arafa, M.; Al Said, S.; Agarwal, A.; Seif, A.; Al Naimi, A.; El Bardisi, H. Outcome of testicular sperm extraction in nonmosaic Klinefelter syndrome patients: What is the best approach? Andrologia 2016, 48, 171–176. [Google Scholar] [CrossRef]
- Vicdan, K.; Akarsu, C.; Sözen, E.; Buluç, B.; Vicdan, A.; Yılmaz, Y.; Biberoğlu, K. Outcome of intracytoplasmic sperm injection using fresh and cryopreserved-thawed testıcular spermatozoa in 83 azoospermic men with Klinefelter syndrome. J. Obstet. Gynaecol. Res. 2016, 42, 1558–1566. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Lanfranco, F.; Garolla, A.; Pelliccione, F.; Vignozzi, L.; Ferlin, A.; Foresta, C.; Jannini, E.A.; Maggi, M.; et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 265–275. [Google Scholar] [CrossRef]
- Seo, J.T.; Park, Y.S.; Lee, J.S. Successful testicular sperm extraction in Korean Klinefelter syndrome. Urology 2004, 64, 1208–1211. [Google Scholar] [CrossRef]
- Ramasamy, R.; Lin, K.; Gosden, L.V.; Rosenwaks, Z.; Palermo, G.D.; Schlegel, P.N. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil. Steril. 2009, 92, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, A.H.; Tangal, S.; Gulpinar, O.; Onal, K.; Pabuccu, R. Should repeated TESE be performed following a failed TESE in men with Klinefelter Syndrome? Andrology 2014, 2, 42–44. [Google Scholar] [CrossRef]
- Koga, M.; Tsujimura, A.; Takeyama, M.; Kiuchi, H.; Takao, T.; Miyagawa, Y.; Takada, S.; Matsumiya, K.; Fujioka, H.; Okamoto, Y.; et al. Clinical comparison of successful and failed microdissection testicular sperm extraction in patients with nonmosaic Klinefelter syndrome. Urology 2007, 70, 341–345. [Google Scholar] [CrossRef]
- Greco, E.; Scarselli, F.; Minasi, M.G.; Casciani, V.; Zavaglia, D.; Dente, D.; Tesarik, J.; Franco, G. Birth of 16 healthy children after ICSI in cases of nonmosaic Klinefelter syndrome. Hum. Reprod. 2013, 28, 1155–1160. [Google Scholar] [CrossRef]
- Westlander, G.; Ekerhovd, E.; Bergh, C. Low levels of serum inhibin B do not exclude successful sperm recovery in men with nonmosaic Klinefelter syndrome. Fertil. Steril. 2003, 79 (Suppl. 3), 1680–1682. [Google Scholar] [CrossRef]
- Aksglaede, L.; Jørgensen, N.; Skakkebaek, N.E.; Juul, A. Low semen volume in 47 adolescents and adults with 47,XXY Klinefelter or 46,XX male syndrome. Int. J. Androl. 2009, 32, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Rosenlund, B.; Hreinsson, J.G.; Hovatta, O. Birth of a healthy male after frozen thawed blastocyst transfer following intracytoplasmic injection of frozen thawed testicular spermatozoa from a man with nonmosaic Klinefelter’s syndrome. J. Assist. Reprod. Genet. 2002, 19, 149–151. [Google Scholar] [CrossRef]
- Yarali, H.; Bozdag, G. An ongoing pregnancy after frozen thawed embryo transfer in a patient with Klinefelter’s syndrome. Gynecol. Obstet. Investig. 2006, 62, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Vicdan, K.; Akarsu, C.; Vicdan, A.; Sözen, E.; Buluç, B.; Biberoğlu, K.; Ozoğul, C. Birth of a healthy boy using fresh testicular sperm in a patient with Klinefelter syndrome combined with Kartagener syndrome. Fertil. Steril. 2011, 96, 577–579. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Abeliovich, D.; Werner, M.; Schenker, J.G.; Safran, A.; Lewin, A. A birth in non-mosaic Klinefelter’s syndrome after testicular fine needle aspiration, intracytoplasmic sperm injection and preimplantation genetic diagnosis. Hum. Reprod. 1998, 13, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Vicdan, K.; Akarsu, C.; Vicdan, A.; Dingiloglu, B.; Sözen, E.; Isik, A.Z.; Coskun, Z. Births using frozen-thawed testicular spermatozoa and frozen-thawed embryos in two azoospermic patients with non-mosaic Klinefelter’s Syndrome. J. Turk. Ger. Gynecol. Assoc. 2007, 8, 424–427. [Google Scholar]
- Nodar, F.; De Vincentiis, S.; Olmedo, S.B.; Papier, S.; Urrutia, F.; Acosta, A.A. Birth of twin males with normal karyotype after intracytoplasmic sperm injection with use of testicular spermatozoa from a nonmosaic patient with Klinefelter’s syndrome. Fertil. Steril. 1999, 71, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Ron-el, R.; Friedler, S.; Strassburger, D.; Komarovsky, D.; Schachter, M.; Raziel, A. Birth of a healthy neonate following the intracytoplasmic injection of testicular spermatozoa from a patient with Klinefelter’s syndrome. Hum. Reprod. 1999, 14, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Rienzi, L.; Ubaldi, F.; Tesarik, J. Klinefelter’s syndrome and assisted reproduction. Fertil. Steril. 2001, 76, 1068–1069. [Google Scholar] [CrossRef]
- Poulakis, V.; Witzsch, U.; Diehl, W.; de Vries, R.; Becht, E.; Trotnow, S. Birth of two infants with normal karyotype after intracytoplasmic injection of sperm obtained by testicular extraction from two men with nonmosaic Klinefelter’s syndrome. Fertil. Steril. 2001, 76, 1060–1062. [Google Scholar] [CrossRef]
- Kyono, K.; Fukunaga, N.; Haigo, K.; Chiba, S.; Araki, Y. Pregnancy achieved following ICSI from a man with Klinefelter’s syndrome and spinal cord injury. Hum. Reprod. 2001, 16, 2347–2349. [Google Scholar] [CrossRef]
- Mehta, A.; Paduch, D.A. Klinefelter syndrome: An argument for early aggressive hormonal and fertility management. Fertil. Steril. 2012, 98, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef]
- Okada, H.; Goda, K.; Muto, S.; Maruyama, O.; Koshida, M.; Horie, S. Four pregnancies in nonmosaic Klinefelter’s syndrome using cryopreserved-thawed testicular spermatozoa. Fertil. Steril. 2005, 84, 1508. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Zou, S.; Wang, S.; Qiu, J.; Xiao, Q.; Zhou, L.; Ping, P. Comparison and outcomes of nonobstructive azoospermia patients with different etiology undergoing MicroTESE and ICSI treatments. Transl. Androl. Urol. 2019, 8, 366–373. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Schlegel, P.N. Microdissection testicular sperm extraction: An update. Asian J. Androl. 2013, 15, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Staessen, C.; Coonen, E.; Van Assche, E.; Tournaye, H.; Joris, H.; Devroey, P.; Van Steirteghem, A.C.; Liebaers, I. Preimplantation diagnosis for X and Y normality in embryos from three Klinefelter patients. Hum. Reprod. 1996, 11, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Ron-El, R.; Strassburger, D.; Gelman-Kohan, S.; Friedler, S.; Raziel, A.; Appelman, Z. A 47,XXY fetus conceived after ICSI of spermatozoa from a patient with non-mosaic Klinefelter’s syndrome: Case report. Hum. Reprod. 2000, 15, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sofikitis, N.; Kaponis, A.; Georgiou, J.; Giannakis, D.; Mamoulakis, C.; Loutradis, D.; Yiannakopoulos, X.; Mio, Y.; Miyagawa, I.; et al. Use of a highly sensitive quantitative telomerase assay in intracytoplasmic sperm injection programmes for the treatment of 47,XXY non-mosaic Klinefelter men. Andrologia 2002, 34, 218–226. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Lin, C.-J.; Lee, L.-M.; Chen, S.-M. Outcome of Intracytoplasmic Injection of Sperm Obtained by Testicular Sperm Extraction from 14 Azoospermic Men Suffering from 47,XXY Non-mosaic Klinefelter’s Syndrome. Taiwan. J. Obstet. Gynecol. 2004, 43, 88–96. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Fabris, G.F.; Tesorio, N.; Tesarik, J. Birth of a healthy boy after fertilization of cryopreserved oocytes with cryopreserved testicular spermatozoa from a man with nonmosaic Klinefelter syndrome. Fertil. Steril. 2008, 89, 991.e5–991.e7. [Google Scholar] [CrossRef]
- Takada, S.; Tsujimura, A.; Ueda, T.; Matsuoka, Y.; Takao, T.; Miyagawa, Y.; Koga, M.; Takeyama, M.; Okamoto, Y.; Matsumiya, K.; et al. Androgen decline in patients with nonobstructive azoospemia after microdissection testicular sperm extraction. Urology 2008, 72, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Bolyakov, A.; Roosma, J.; Schlegel, P.N.; Paduch, D.A. Successful testicular sperm retrieval in adolescents with Klinefelter syndrome treated with at least 1 year of topical testosterone and aromatase inhibitor. Fertil. Steril. 2013, 100, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Rives, N.; Milazzo, J.P.; Perdrix, A.; Castanet, M.; Joly-Hélas, G.; Sibert, L.; Bironneau, A.; Way, A.; Macé, B. The feasibility of fertility preservation in adolescents with Klinefelter syndrome. Hum. Reprod. 2013, 28, 1468–1479. [Google Scholar] [CrossRef]
- Nahata, L.; Yu, R.N.; Paltiel, H.J.; Chow, J.S.; Logvinenko, T.; Rosoklija, I.; Cohen, L.E. Sperm Retrieval in Adolescents and Young Adults with Klinefelter Syndrome: A Prospective, Pilot Study. J. Pediatr. 2016, 170, 260–265.e1-2. [Google Scholar] [CrossRef]
- Chihara, M.; Ogi, K.; Ishiguro, T.; Yoshida, K.; Godo, C.; Takakuwa, K.; Enomoto, T. Microdissection testicular sperm extraction in five Japanese patients with non-mosaic Klinefelter’s syndrome. Reprod. Med. Biol. 2018, 17, 209–216. [Google Scholar] [CrossRef]
- Chen, W.; Bai, M.Z.; Yang, Y.; Sun, D.; Wu, S.; Sun, J.; Wu, Y.; Feng, Y.; Wei, Y.; Chen, Z.; et al. ART strategies in Klinefelter syndrome. J. Assist. Reprod. Genet. 2020, 37, 2053–2079. [Google Scholar] [CrossRef]
- Ohlander, S.; Hotaling, J.; Kirshenbaum, E.; Niederberger, C.; Eisenberg, M.L. Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction: A meta-analysis. Fertil. Steril. 2014, 101, 344–349. [Google Scholar] [CrossRef]
- Guttenbach, M.; Martinez-Expósito, M.J.; Michelmann, H.W.; Engel, W.; Schmid, M. Incidence of diploid and disomic sperm nuclei in 45 infertile men. Hum. Reprod. 1997, 12, 468–473. [Google Scholar] [CrossRef]
- Guttenbach, M.; Michelmann, H.W.; Hinney, B.; Engel, W.; Schmid, M. Segregation of sex chromosomes into sperm nuclei in a man with 47,XXY Klinefelter’s karyotype: A FISH analysis. Hum. Genet. 1997, 99, 474–477. [Google Scholar] [CrossRef]
- Okada, H.; Fujioka, H.; Tatsumi, N.; Kanzaki, M.; Okuda, Y.; Fujisawa, M.; Hazama, M.; Matsumoto, O.; Gohji, K.; Arakawa, S.; et al. Klinefelter’s syndrome in the male infertility clinic. Hum. Reprod. 1999, 14, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Hennebicq, S.; Pelletier, R.; Bergues, U.; Rousseaux, S. Risk of trisomy 21 in offspring of patients with Klinefelter’s syndrome. Lancet 2001, 357, 2104–2105. [Google Scholar] [CrossRef]
- Morel, F.; Bernicot, I.; Herry, A.; Le Bris, M.J.; Amice, V.; De Braekeleer, M. An increased incidence of autosomal aneuploidies in spermatozoa from a patient with Klinefelter’s syndrome. Fertil. Steril. 2003, 79 (Suppl. 3), 1644–1646. [Google Scholar] [CrossRef]
- Ferlin, A.; Garolla, A.; Foresta, C. Chromosome abnormalities in sperm of individuals with constitutional sex chromosomal abnormalities. Cytogenet. Genome Res. 2005, 111, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Nagayoshi, M.; Takemoto, Y.; Yamaguchi, T.; Takeda, S.; Watanabe, S.; Tanaka, A. Genetic risk of Klinefelter’s syndrome in assisted reproductive technology. Reprod. Med. Biol. 2017, 16, 188–195. [Google Scholar] [CrossRef]
- Turnpenny, P.D.; Ellard, S. Emery’s Elements of Medical Genetics, 14th ed.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2012. [Google Scholar]
- Forabosco, A.; Percesepe, A.; Santucci, S. Incidence of non-age-dependent chromosomal abnormalities: A population-based study on 88965 amniocenteses. Eur. J. Hum. Genet. 2009, 17, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Abramsky, L.; Chapple, J. 47,XXY (Klinefelter syndrome) and 47,XYY: Estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat. Diagn. 1997, 17, 363–368. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Schoemaker, M.J.; Wright, A.F.; Jacobs, P.A.; United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: A cohort study. J. Clin. Endocrinol. Metab. 2005, 90, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, A.S.; Halliday, J.L.; Cock, M.L.; McLachlan, R.I. The prevalence and diagnosis rates of Klinefelter syndrome: An Australian comparison. Med. J. Aust. 2011, 194, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Pook, C.J.; Cocca, A.; Grandone, A.; Al-Hussini, M.; Lam, W. The Evidence for Fertility Preservation in Pediatric Klinefelter Syndrome. Front. Reprod. Health. 2021, 3, 629179. [Google Scholar] [CrossRef]
- Hawksworth, D.J.; Szafran, A.A.; Jordan, P.W.; Dobs, A.S.; Herati, A.S. Infertility in Patients With Klinefelter Syndrome: Optimal Timing for Sperm and Testicular Tissue Cryopreservation. Rev. Urol. 2018, 20, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Franik, S.; Hoeijmakers, Y.; D’Hauwers, K.; Braat, D.D.M.; Nelen, W.L.M.; Smeets, D.; Claahsen-van der Grinten, H.L.; Ramos, L.; Fleischer, K. Klinefelter syndrome and fertility: Sperm preservation should not be offered to children with Klinefelter syndrome. Hum. Reprod. 2016, 31, 1952–1959. [Google Scholar] [CrossRef]
- Ly, A.; Sermondade, N.; Brioude, F.; Berthaut, I.; Bachelot, A.; Hamid, R.H.; Khattabi, L.E.; Prades, M.; Lévy, R.; Dupont, C. Fertility preservation in young men with Klinefelter syndrome: A systematic review. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102177. [Google Scholar] [CrossRef]
- Rohayem, J.; Nieschlag, E.; Zitzmann, M.; Kliesch, S. Testicular function during puberty and young adulthood in patients with Klinefelter’s syndrome with and without spermatozoa in seminal fluid. Andrology 2016, 4, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
| References | Pacients (n) | SSR (n) | SRR (%) | ETC (n) | CP (n) | NB (n) | Technique |
|---|---|---|---|---|---|---|---|
| [211] | 3 | 3 | 100 | 4 | 0 | 0 | cTESE |
| [157] | 1 | 1 | 100 | 1 | 0 | 0 | cTESE |
| [160] | 9 | 4 | 44 | 3 | 0 | 0 | cTESE |
| [161] | 15 | 8 | 53 | 7 | 2 | 2 | cTESE |
| [162] | 2 | 2 | 100 | 3 | 3 | 3 | cTESE |
| [199] | 7 | 4 | 57 | 4 | 4 | 1 | FNA |
| [101] | 10 | 2 | 20 | DTB | FNA | ||
| [201] | 1 | 1 | 100 | 1 | 1 | 2 | cTESE |
| [202] | 1 | 1 | 100 | 2 | 1 | 1 | cTESE |
| [105] | 20 | 8 | 40 | 8 | 4 | 7 | cTESE |
| [120] | 1 | 1 | 100 | 1 | 1 | 2 | cTESE |
| [212] | 1 | 1 | 100 | 4 | 3 | 3 | cTESE |
| [115] | 12 | 5 | 42 | 10 | 5 | 6 | cTESE |
| [203] | 1 | 1 | 100 | 1 | 1 | 2 | cTESE |
| [205] | 1 | 1 | 100 | 1 | 1 | 1 | cTESE |
| [204] | 2 | 2 | 100 | 2 | 2 | 2 | cTESE |
| [94] | 19 | 4 | 21 | 4 | 4 | 3 | cTESE |
| [95] | 4 | 3 | 75 | 4 | 1 | 1 | cTESE |
| [184] | 20 | 9 | 45 | DTB | cTESE | ||
| [196] | 1 | 1 | 100 | 2 | 1 | 1 | TESA |
| [103,213] | 24 | 12 | 50 | 4 | 4 | 5 | cTESE |
| [114] | 20 | 10 | 50 | 26 | 8 | 5 | cTESE |
| [118] | 11 | 6 | 55 | 15 | 2 | 1 | cTESE |
| [194] | 18 | 5 | 28 | 5 | 2 | 2 | cTESE |
| [214] | 14 | 8 | 57 | 8 | 6 | 9 | cTESE |
| [123] | 1 | 1 | 100 | 3 | 1 | 1 | cTESE |
| [189] | 25 | 4 | 16 | 2 | 2 | 1 | cTESE |
| [165] | 50 | 24 | 48 | DTB | cTESE | ||
| [104] | 4 | 4 | 100 | 3 | 3 | 6 | cTESE |
| [175] | 51 | 26 | 51 | 26 | 12 | 12 | mixed |
| [208] | 10 | 6 | 60 | 8 | 6 | 3 | mTESE |
| [166] | 42 | 29 | 69 | 29 | 18 | 22 | mTESE |
| [167] | 74 | 42 | 57 | DTB | mTESE | ||
| [197] | 1 | 1 | 100 | 2 | 1 | 1 | cTESE |
| [192] | 26 | 13 | 50 | 13 | 4 | 2 | mTESE |
| [168] | 17 | 6 | 35 | 9 | 7 | 8 | cTESE |
| [200] | 2 | 2 | 100 | 3 | 2 | 3 | mTESE |
| [215] | 2 | 2 | 100 | 2 | 2 | 1 | cTESE |
| [216] | 9 | 9 | 100 | DTB | mTESE | ||
| [178] | 27 | 8 | 30 | 8 | 4 | 7 | cTESE |
| [177] | 68 | 45 | 66 | 62 | 33 | 28 | mTESE |
| [179] | 39 | 22 | 56 | 18 | 7 | 2 | mTESE |
| [176] | 106 | 50 | 47 | 49 | 26 | 29 | mTESE |
| [198] | 1 | 1 | 100 | 1 | 1 | 1 | cTESE |
| [93] | 69 | 33 | 48 | DTB | mixed | ||
| [193] | 38 | 15 | 39 | 23 | 15 | 16 | mixed |
| 28 | 14 | 50 | (cTESE) | ||||
| 10 | 1 | 10 | (mTSE) | ||||
| [217] | 10 | 7 | 70 | DTB | mTESE | ||
| [218] | 5 | 1 | 20 | DTB | cTESE | ||
| [191] | 18 | 3 | 17 | 3 | 1 | 1 | mTESE |
| [29] | 65 | 25 | 38 | 36 | 16 | 17 | cTESE |
| [180] | 134 | 38 | 28 | 18 | 4 | 5 | mTESE |
| [169] | 9 | 6 | 67 | 6 | 1 | 1 | mTESE |
| [163] | 41 | 23 | 56 | DTB | cTESE | ||
| 25 | 13 | 52 | (15–22 y) | ||||
| 16 | 10 | 63 | 10 | 4 | 3 | (≥23 y) | |
| [181] | 135 | 46 | 33 | DTB | mTESE | ||
| 10 | 1 | 10 | (13–14 y) | ||||
| 50 | 19 | 38 | (13–19 y) | ||||
| 85 | 26 | 31 | (20–61 y) | ||||
| [186] | 43 | 6 | 14 | 6 | 3 | 5 | mixed |
| 23 | 0 | 0 | (cTESE) | ||||
| 20 | 6 | 30 | (mTSE) | ||||
| [219] | 10 | 5 | 50 | DTB | mTESE | ||
| [187] | 83 | 35 | 42 | 41 | 22 | 25 | mixed |
| 43 | 22 | 51 | (cTESE) | ||||
| 40 | 13 | 33 | (mTSE) | ||||
| [220] | 5 | 2 | 40 | 2 | 1 | 1 | mTESE |
| [182] | 110 | 22 | 20 | 31 | 11 | 9 | mTESE |
| [88] | 1 | 1 | 100 | 1 | 1 | 1 | cTESE |
| [185] | 184 | 80 | 43 | 90 | 31 | 24 | mTESE |
| [31] | 76 | 31 | 40 | 44 | 18 | 21 | cTESE |
| Totals | 1809 | 777 | 669 | 313 | 315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, R.; Ferraz, L.; Barros, A.; Sousa, M. The Klinefelter Syndrome and Testicular Sperm Retrieval Outcomes. Genes 2023, 14, 647. https://doi.org/10.3390/genes14030647
Sá R, Ferraz L, Barros A, Sousa M. The Klinefelter Syndrome and Testicular Sperm Retrieval Outcomes. Genes. 2023; 14(3):647. https://doi.org/10.3390/genes14030647
Chicago/Turabian StyleSá, Rosália, Luís Ferraz, Alberto Barros, and Mário Sousa. 2023. "The Klinefelter Syndrome and Testicular Sperm Retrieval Outcomes" Genes 14, no. 3: 647. https://doi.org/10.3390/genes14030647
APA StyleSá, R., Ferraz, L., Barros, A., & Sousa, M. (2023). The Klinefelter Syndrome and Testicular Sperm Retrieval Outcomes. Genes, 14(3), 647. https://doi.org/10.3390/genes14030647







