SDHA Germline Mutations in SDH-Deficient GISTs: A Current Update
Abstract
1. Introduction
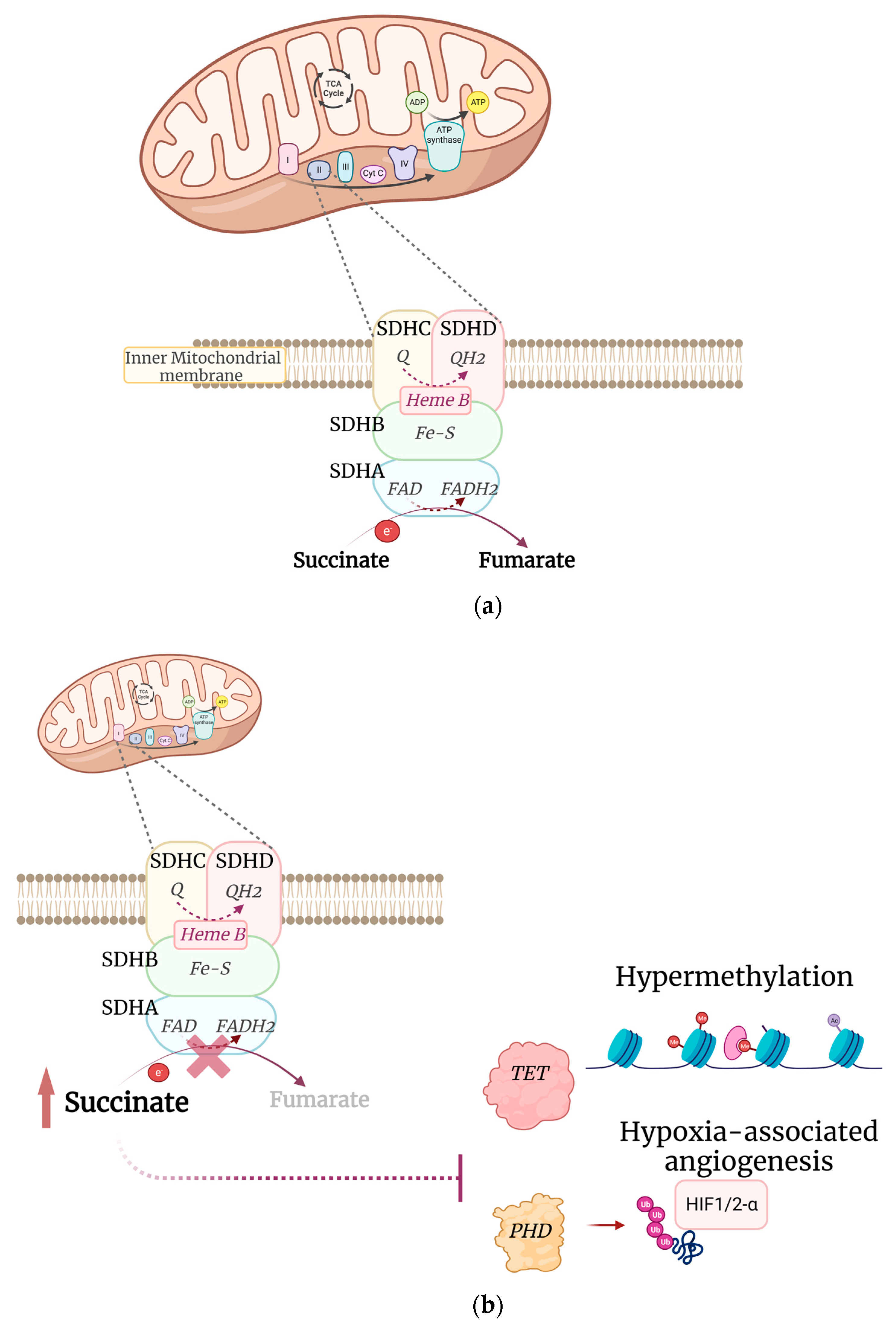
2. SDH Complex in Cancers
3. SDHA Alterations in GIST
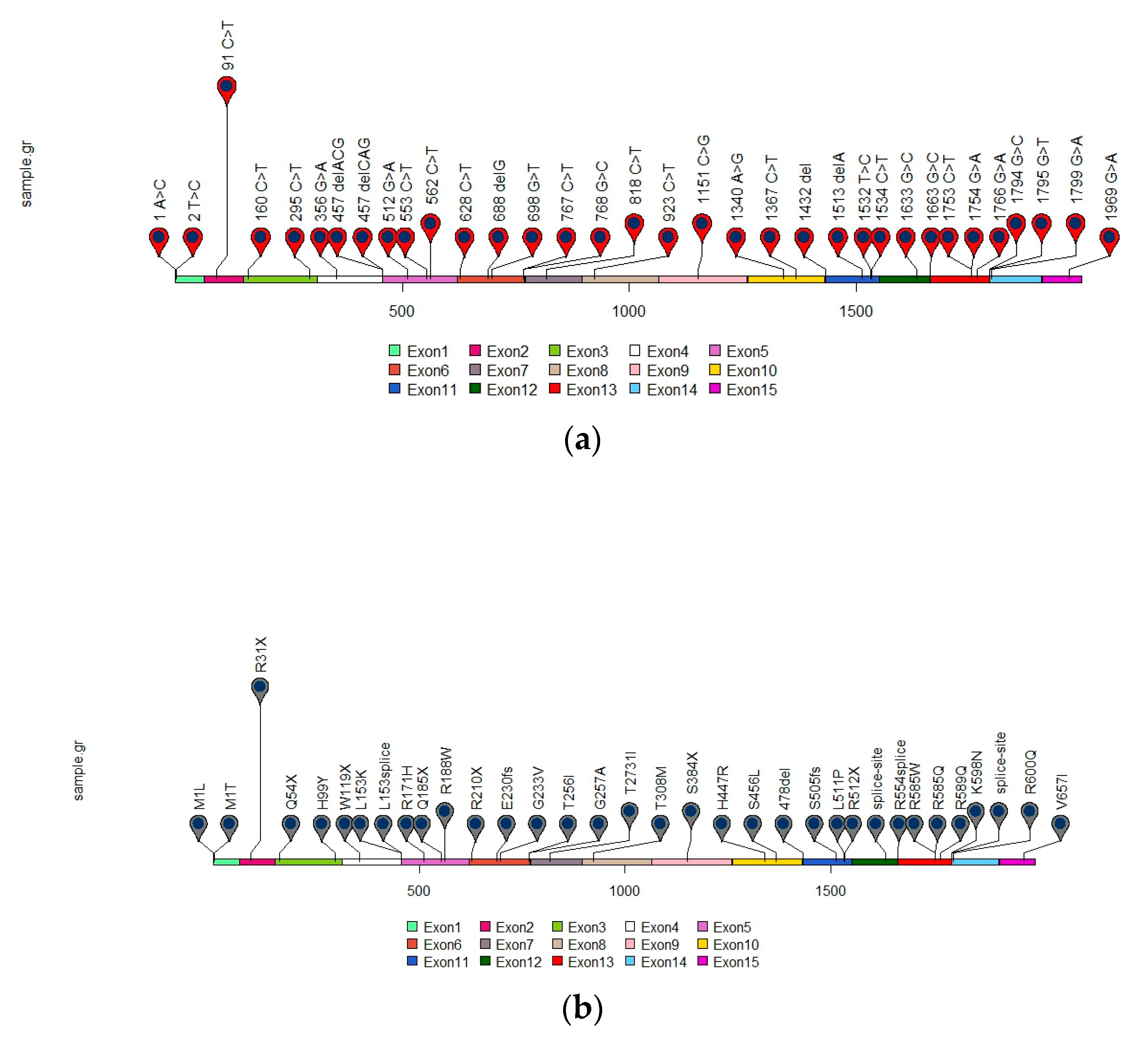
4. Germline SDH Mutations in GIST
SDHA Germline Mutations
| ID | Age | Sex | Cytology | Site | Associated Tumor | ICH SDHB | IHC SDHA | Exon | Germline Mutation (cDNA, Protein) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 22 | M | - | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Italiano et al., 2012 [37] |
| #2 | 31 | M | mixed | stomach | - | neg | neg | 5 | c.553C>T; p.Q185X | Wagner et al., 2013 [31] |
| #3 | 38 | F | epithelioid | stomach | - | neg | neg | 11 | c.1534C>T; p.R512X | Wagner et al., 2013 |
| #4 | 39 | F | mixed | stomach | - | neg | neg | 8 | c.688delG; Frameshift | Wagner et al., 2013 |
| #5 | 22 | M | mixed | stomach | - | neg | neg | 2 | c.91C>T; p.R31X | Wagner et al., 2013 |
| #6 | 53 | F | mixed | stomach | - | neg | neg | 2 | c.91C>T; p.R31X | Wagner et al., 2013 |
| #7 | 19 | M | mixed | stomach | - | neg | neg | 2 | c.91C>T; p.R31X | Wagner et al., 2013 |
| #8 | 45 | F | epithelioid | stomach | no | neg | neg | 15 | c.1969G>A; p.V657I | Dwight et al., 2013 [28] |
| #9 | 25 | F | epithelioid | stomach | no | neg | neg | 3 | c.160C>T; p.Q54X | Dwight et al., 2013 |
| #10 | 41 | M | epithelioid | stomach | no | neg | neg | 12 | c.1633+3G>C; splice-site | Dwight et al., 2013 |
| #11 | 39 | F | epitheliod/spindle | stomach | no | neg | - | 7 | c.818C>T; p.T2731I | Belinsky et al.,2013 [30] |
| #12 | 52 | F | epitheliod/spindle | stomach | no | neg | - | IVS4/ex5 | c.457-2_c457delAGC; p.L153Kfs*71 | Belinsky et al.,2013 |
| #13 | 33 | F | epitheliod/spindle | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Belinsky et al.,2013 |
| #14 | 41 | F | epithelioid | stomach | MTC | neg | neg | 2 | c.91C>T; p.R31X | Oudijk et al., 2013 [29] |
| #15 | 53 | F | spindle cell | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Oudijk et al., 2013 |
| #16 | 47 | F | mixed | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Oudijk et al., 2013 |
| #17 | 14 | M | mixed | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Oudijk et al., 2013 |
| #18 | - | - | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Miettinen et al., 2013 [11] | |
| #19 | - | - | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Miettinen et al., 2013 | |
| #20 | - | - | stomach | no | neg | neg | 2 | c.91C>T; p.R31X | Miettinen et al., 2013 | |
| #21 | - | - | stomach | no | neg | neg | 6 | c.767C>T; p.T256I | Miettinen et al., 2013 | |
| #22 | - | - | stomach | no | neg | neg | 13 | c.1794G>C; p.K598N | Miettinen et al., 2013 | |
| #23 | - | - | stomach | no | neg | neg | 14 | c.1795–1G>T; Ex 14 5’ splicing | Miettinen et al., 2013 | |
| #24 | - | - | stomach | no | neg | pos | 5 | c.562C>T; p.R188W | Miettinen et al., 2013 | |
| #25 | 23 | M | epitheliod | stomach | RCC | neg | pos | 1 | c.2T>C; p.M1T | Jiang et al., 2015 [37] |
| #26 | - | - | - | stomach | no | neg | 10 | c.1432_1432del; p.478_478del | Boikos et al., 2016 [32] | |
| #27 | - | - | - | stomach | no | neg | - | 11 | c.1513delA; p.S505fs | Boikos et al., 2016 |
| #28 | - | - | - | stomach | no | neg | - | 14 | c.1795-1G>T | Boikos et al., 2016 |
| #29 | - | - | - | stomach | no | neg | - | 10 | c.1340°>G; p.H447R | Boikos et al., 2016 |
| #30 | - | - | - | stomach | no | neg | - | 10 | c.1367C>T; p.S456L | Boikos et al., 2016 |
| #31 | - | - | - | stomach | no | neg | - | 13 | c.1753C>T; p.R585W | Boikos et al., 2016 |
| #32 | 23 | F | epitheliod | stomach | CHO | neg | - | 3 | c.295C>T; p.H99Y | Boikos et al., 2016 |
| #33 | - | - | - | stomach | no | neg | - | 5 | c.562C>T; p.R188W | Boikos et al., 2016 |
| #34 | - | - | - | stomach | no | neg | - | 7 | c.818C>T; p.T273I | Boikos et al., 2016 |
| #35 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #36 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #37 | 21 | M | epitheliod/spindle | stomach | CHO | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #38 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #39 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #40 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #41 | - | - | - | stomach | no | neg | - | 2 | c.91C>T; p.R31X | Boikos et al., 2016 |
| #42 | - | - | - | stomach | no | neg | - | 8 | c.923C>T; p.T308M | Boikos et al., 2016 |
| #43 | - | - | - | stomach | no | neg | - | 13 | c.1794G>C; p.K598N | Boikos et al., 2016 |
| #44 | 30 | M | epitheliod/spindle | stomach | PGL, CHO | neg | - | 11 | c.1532T>C; p.L511P | Boikos et al., 2016 |
| #45 | - | - | - | stomach | - | neg | - | SDHA deletion | Boikos et al.,2016 | |
| #46 | 21 | - | - | - | neg | neg | 2 | c.91C>T; p.R31X | Gault et al., 2018 [38] | |
| #47 | 27 | - | - | - | neg | neg | 2 | c.91C>T; p.R31X | Gault et al., 2018 | |
| #48 | 20 | F | epithelioid | stomach | PGL | - | - | 1 | c.1A > C; p.(Met1?) | Carrera et al., 2019 [33] |
| #49 | 28 | F | stomach | no | 9 | c.1151 C>G; p.S384X | Pantaleo et al., 2011 [26] | |||
| #50 | 30 | M | stomach | no | 2 | c.91 C>T; p.R31X | Pantaleo et al., 2011 | |||
| #51 | 39 | F | stomach | no | 13 | c.1766 G>A; p.R589Q | Pantaleo et al., 2011 [34] | |||
| #52 | 37 | F | stomach | no | 5 | c.457-3_457-1 delCAG; p.L153splice | Pantaleo et al., 2014 [35] | |||
| #53 | 31 | F | stomach | no | 9 | c.1151 C>G; p.S384X | Pantaleo et al., 2022 [36] | |||
| #54 | 61 | M | stomach | PGL | 6 | c.698G>T; p.G233V | Pantaleo et al., 2022 | |||
| #55 | 21 | F | stomach | no | 5 | c.512G>A; p.R171H | Pantaleo et al., 2022 | |||
| #56 | 38 | M | stomach | no | 6 | c.768G>C; p.G257A | Pantaleo et al., 2022 | |||
| #57 | 70 | F | stomach | no | 4 | c.356G>A; p.W119X | Pantaleo et al., 2022 | |||
| #58 | 66 | F | stomach | no | 14 | c.1799G>A; p.R600Q | Pantaleo et al., 2022 | |||
| #59 | 17 | M | stomach | no | 12 | c.1663+3 G>C; p.R554splice | Pantaleo et al., 2022 | |||
| #60 | 55 | F | stomach | no | 14 | c.1799G>A; p.R600Q | Pantaleo et al., 2022 | |||
| #61 | 50 | M | stomach | no | 13 | c.1754G>A; p.R585Q | Pantaleo et al., 2022 | |||
| #62 | 17 | M | stomach | no | 6 | c.628C>T; p.R210X | Pantaleo et al., 2022 |
5. SDHA Germline Consideration
6. Other SDH Subunit Mutations
7. Current Therapies of SDH-Deficient GISTs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kindblom, L.G.; Remotti, H.E.; Aldenborg, F.; Meis-Kindblom, J.M. Gastrointestinal Pacemaker Cell Tumor (GIPACT): Gastrointestinal Stromal Tumors Show Phenotypic Characteristics of the Interstitial Cells of Cajal. Am. J. Pathol. 1998, 152, 1259. [Google Scholar] [PubMed]
- Isozaki, K.; Hirota, S. Gain-of-Function Mutations of Receptor Tyrosine Kinases in Gastrointestinal Stromal Tumors. Curr. Genomics 2006, 7, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, D.; Rossi, S.; Polano, M.; Tamborini, E.; Lorenzetto, E.; Sbaraglia, M.; Mondello, A.; Massani, M.; Lamon, S.; Bracci, R.; et al. Quadruple-Negative GIST Is a Sentinel for Unrecognized Neurofibromatosis Type 1 Syndrome. Clin. Cancer Res. 2017, 23, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Nannini, M.; Astolfi, A.; Urbini, M.; Indio, V.; Santini, D.; Heinrich, M.C.; Corless, C.L.; Ceccarelli, C.; Saponara, M.; Mandrioli, A.; et al. Integrated Genomic Study of Quadruple-WT GIST (KIT/PDGFRA/SDH/RAS Pathway Wild-Type GIST). BMC Cancer 2014, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Nannini, M.; Urbini, M.; Astolfi, A.; Biasco, G.; Pantaleo, M.A. The Progressive Fragmentation of the KIT/PDGFRA Wild-Type (WT) Gastrointestinal Stromal Tumors (GIST). J. Transl. Med. 2017, 15, 113. [Google Scholar] [CrossRef]
- Carney, J.A.; Stratakis, C.A. Familial Paraganglioma and Gastric Stromal Sarcoma: A New Syndrome Distinct from the Carney Triad. Am. J. Med. Genet. 2002, 108, 132–139. [Google Scholar] [CrossRef]
- Recht, H.S.; Fishman, E.K. Carney-Stratakis Syndrome: A Dyad of Familial Paraganglioma and Gastrointestinal Stromal Tumor. Radiol. Case Rep. 2020, 15, 2071–2075. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Succinate Dehydrogenase Deficient Gastrointestinal Stromal Tumors (GISTs)—A Review. Int. J. Biochem. Cell Biol. 2014, 53, 514–519. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Lolli, C.; Nannini, M.; Astolfi, A.; Indio, V.; Saponara, M.; Urbini, M.; La Rovere, S.; Gill, A.; Goldstein, D.; et al. Good Survival Outcome of Metastatic SDH-Deficient Gastrointestinal Stromal Tumors Harboring SDHA Mutations. Genet. Med. 2015, 17, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Makhlouf, H.; Sobin, L.H.; Lasota, J. Gastrointestinal Stromal Tumors of the Jejunum and Ileum: A Clinicopathologic, Immunohistochemical, and Molecular Genetic Study of 906 Cases before Imatinib with Long-Term Follow-Up. Am. J. Surg. Pathol. 2006, 30, 477–489. [Google Scholar] [CrossRef]
- Miettinen, M.; Killian, J.K.; Wang, Z.F.; Lasota, J.; Lau, C.; Jones, L.; Walker, R.; Pineda, M.; Zhu, Y.J.; Kim, S.Y.; et al. Immunohistochemical Loss of Succinate Dehydrogenase Subunit a (SDHA) in Gastrointestinal Stromal Tumors (GISTs) Signals SDHA Germline Mutation. Am. J. Surg. Pathol. 2013, 37, 234–240. [Google Scholar] [CrossRef]
- Murray, M.; Hatcher, H.; Jessop, F.; Williams, D.; Carroll, N.; Bulusu, R.; Judson, I. Treatment of Wild-Type Gastrointestinal Stromal Tumor (WT-GIST) with Imatinib and Sunitinib. Pediatr. Blood Cancer 2008, 50, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Indio, V.; Schipani, A.; Nannini, M.; Urbini, M.; Rizzo, A.; De Leo, A.; Altimari, A.; Di Scioscio, V.; Messelodi, D.; Tarantino, G.; et al. Gene Expression Landscape of Sdh-Deficient Gastrointestinal Stromal Tumors. J. Clin. Med. 2021, 10, 1057. [Google Scholar] [CrossRef]
- Bannon, A.E.; Kent, J.; Forquer, I.; Town, A.; Klug, L.R.; McCann, K.; Beadling, C.; Harismendy, O.; Sicklick, J.K.; Corless, C.; et al. Biochemical, Molecular, and Clinical Characterization of Succinate Dehydrogenase Subunit A Variants of Unknown Significance. Clin. Cancer Res. 2017, 23, 6733–6743. [Google Scholar] [CrossRef]
- Killian, J.K.; Kim, S.Y.; Miettinen, M.; Smith, C.; Merino, M.; Tsokos, M.; Quezado, M.; Smith, W.I.; Jahromi, M.S.; Xekouki, P.; et al. Succinate Dehydrogenase Mutation Underlies Global Epigenomic Divergence in Gastrointestinal Stromal Tumor. Cancer Discov. 2013, 3, 648–657. [Google Scholar] [CrossRef]
- Urbini, M.; Astolfi, A.; Indio, V.; Heinrich, M.C.; Corless, C.L.; Nannini, M.; Ravegnini, G.; Biasco, G.; Pantaleo, M.A. SDHC Methylation in Gastrointestinal Stromal Tumors (GIST): A Case Report. BMC Med. Genet. 2015, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Barletta, J.A.; Hornick, J.L. Succinate Dehydrogenase-Deficient Tumors: Diagnostic Advances and Clinical Implications. Adv. Anat. Pathol. 2012, 19, 193–203. [Google Scholar] [CrossRef]
- Janeway, K.A.; Kim, S.Y.; Lodish, M.; Nosé, V.; Rustin, P.; Gaal, J.; Dahia, P.L.M.; Liegl, B.; Ball, E.R.; Raygada, M.; et al. Defects in Succinate Dehydrogenase in Gastrointestinal Stromal Tumors Lacking KIT and PDGFRA Mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Baysal, B.E. A Recurrent Stop-Codon Mutation in Succinate Dehydrogenase Subunit B Gene in Normal Peripheral Blood and Childhood T-Cell Acute Leukemia. PLoS ONE 2007, 2, e436. [Google Scholar] [CrossRef] [PubMed]
- Beamer, L.C. Cowden Syndrome: What Oncology Nurses Need to Know about Increased Risk of Developing Certain Cancers. Oncol. Nurs. Forum 2014, 41, 555–557. [Google Scholar] [CrossRef]
- Niemeijer, N.D.; Papathomas, T.G.; Korpershoek, E.; De Krijger, R.R.; Oudijk, L.; Morreau, H.; Bayley, J.P.; Hes, F.J.; Jansen, J.C.; Dinjens, W.N.M.; et al. Succinate Dehydrogenase (SDH)-Deficient Pancreatic Neuroendocrine Tumor Expands the SDH-Related Tumor Spectrum. J. Clin. Endocrinol. Metab. 2015, 19, E1386–E1393. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Astolfi, A.; Di Battista, M.; Heinrich, M.C.; Paterini, P.; Scotlandi, K.; Santini, D.; Catena, F.; Manara, M.C.; Nannini, M.; et al. Insulin-like Growth Factor 1 Receptor Expression in Wild-Type GISTs: A Potential Novel Therapeutic Target. Int. J. Cancer 2009, 125, 2991–2994. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Goncalves, J.; Moog, S.; Castro-Vega, L.J.; Job, S.; Buffet, A.; Fontenille, M.J.; Woszczyk, J.; Gimenez-Roqueplo, A.P.; Letouzé, E.; et al. TET-Mediated Hypermethylation Primes SDH-Deficient Cells for HIF2α-Driven Mesenchymal Transition. Cell Rep. 2020, 30, 4551–4566.e7. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Drier, Y.; Johnstone, S.E.; Hemming, M.L.; Tarjan, D.R.; Hegazi, E.; Shareef, S.J.; Javed, N.M.; Raut, C.P.; Eschle, B.K.; et al. Altered Chromosomal Topology Drives Oncogenic Programs in SDH-Deficient GISTs. Nature 2019, 575, 229–233. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Astolfi, A.; Indio, V.; Moore, R.; Thiessen, N.; Heinrich, M.C.; Gnocchi, C.; Santini, D.; Catena, F.; Formica, S.; et al. SDHA Loss-of-Function Mutations in KIT-PDGFRA Wild-Type Gastrointestinal Stromal Tumors Identified by Massively Parallel Sequencing. J. Natl. Cancer Inst. 2011, 103, 983–987. [Google Scholar] [CrossRef]
- Matyakhina, L.; Bei, T.A.; McWhinney, S.R.; Pasini, B.; Cameron, S.; Gunawan, B.; Stergiopoulos, S.G.; Boikos, S.; Muchow, M.; Dutra, A.; et al. Genetics of Carney Triad: Recurrent Losses at Chromosome 1 but Lack of Germline Mutations in Genes Associated with Paragangliomas and Gastrointestinal Stromal Tumors. J. Clin. Endocrinol. Metab. 2007, 92, 2938–2943. [Google Scholar] [CrossRef]
- Dwight, T.; Benn, D.E.; Clarkson, A.; Vilain, R.; Lipton, L.; Robinson, B.G.; Clifton-Bligh, R.J.; Gill, A.J. Loss of SDHA Expression Identifies SDHA Mutations in Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumors. Am. J. Surg. Pathol. 2013, 37, 226–233. [Google Scholar] [CrossRef]
- Oudijk, L.; Gaal, J.; Korpershoek, E.; Van Nederveen, F.H.; Kelly, L.; Schiavon, G.; Verweij, J.; Mathijssen, R.H.J.; Den Bakker, M.A.; Oldenburg, R.A.; et al. SDHA Mutations in Adult and Pediatric Wild-Type Gastrointestinal Stromal Tumors. Mod. Pathol. 2013, 26, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Belinsky, M.G.; Rink, L.; von Mehren, M. Succinate Dehydrogenase Deficiency in Pediatric and Adult Gastrointestinal Stromal Tumors. Front. Oncol. 2013, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Remillard, S.P.; Zhang, Y.X.; Doyle, L.A.; George, S.; Hornick, J.L. Loss of Expression of SDHA Predicts SDHA Mutations in Gastrointestinal Stromal Tumors. Mod. Pathol. 2013, 26, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors. JAMA Oncol. 2016, 2, 922–928. [Google Scholar] [CrossRef]
- Carrera, S.; Beristain, E.; Sancho, A.; Iruarrizaga, E.; Rivero, P.; Mañe, J.M.; López Vivanco, G. Germline c.1A>C Heterozygous Pathogenic Variant in SDHA Reported for the First Time in a Young Adult with a Gastric Gastrointestinal Stromal Tumour (GIST): A Case Report. Hered. Cancer Clin. Pract. 2019, 17, 23. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Astolfi, A.; Urbini, M.; Nannini, M.; Paterini, P.; Indio, V.; Saponara, M.; Formica, S.; Ceccarelli, C.; Casadio, R.; et al. Analysis of All Subunits, SDHA, SDHB, SDHC, SDHD, of the Succinate Dehydrogenase Complex in KIT/PDGFRA Wild-Type GIST. Eur. J. Hum. Genet. 2014, 22, 32–39. [Google Scholar] [CrossRef]
- Pantaleo, M.A.; Nannini, M.; Astolfi, A.; Biasco, G. A Distinct Pediatric-Type Gastrointestinal Stromal Tumor in Adults: Potential Role of Succinate Dehydrogenase Subunit a Mutations. Am. J. Surg. Pathol. 2011, 35, 1750–1752. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.A.; Urbini, M.; Schipani, A.; Nannini, M.; Indio, V.; De Leo, A.; Vincenzi, B.; Brunello, A.; Grignani, G.; Casagrande, M.; et al. SDHA Germline Variants in Adult Patients With SDHA-Mutant Gastrointestinal Stromal Tumor. Front. Oncol. 2022, 11, 5400. [Google Scholar] [CrossRef]
- Italiano, A.; Chen, C.L.; Sung, Y.S.; Singer, S.; DeMatteo, R.P.; LaQuaglia, M.P.; Besmer, P.; Socci, N.; Antonescu, C.R. SDHA Loss of Function Mutations in a Subset of Young Adult Wild-Type Gastrointestinal Stromal Tumors. BMC Cancer 2012, 12, 408. [Google Scholar] [CrossRef]
- Gault, M.D.; Mandelker, D.; Delair, D.; Stewart, C.R.; Kemel, Y.; Sheehan, M.R.; Siegel, B.; Kennedy, J.; Marcell, V.; Arnold, A.; et al. Germline SDHA Mutations in Children and Adults with Cancer. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002584. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Y.; Zhou, Y.H.; Hou, Y.Y.; Wang, J.Y.; Li, J.L.; Li, M.; Tong, H.X.; Lu, W.Q. A Novel Germline Mutation in SDHA Identified in a Rare Case of Gastrointestinal Stromal Tumor Complicated with Renal Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12188–12197. [Google Scholar]
- Pasini, B.; McWhinney, S.R.; Bei, T.; Matyakhina, L.; Stergiopoulos, S.; Muchow, M.; Boikos, S.A.; Ferrando, B.; Pacak, K.; Assie, G.; et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur. J. Hum. Genet. 2008, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, C.A.; Carney, J.A. The Triad of Paragangliomas, Gastric Stromal Tumours and Pulmonary Chondromas (Carney Triad), and the Dyad of Paragangliomas and Gastric Stromal Sarcomas (Carney-Stratakis Syndrome): Molecular Genetics and Clinical Implications. Proc. J. Intern. Med. 2009, 266, 43–52. [Google Scholar] [CrossRef]
- Miettinen, M.; Wang, Z.F.; Sarlomo-Rikala, M.; Osuch, C.; Rutkowski, P.; Lasota, J. Succinate Dehydrogenase-Deficient GISTs: A Clinicopathologic, Immunohistochemical, and Molecular Genetic Study of 66 Gastric GISTs with Predilection to Young Age. Am. J. Surg. Pathol. 2011, 35, 1712–1721. [Google Scholar] [CrossRef]
- McWhinney, S.R.; Pasini, B.; Stratakis, C.A. Familial Gastrointestinal Stromal Tumors and Germ-Line Mutations. N. Engl. J. Med. 2007, 357, 1054–1056. [Google Scholar] [CrossRef]
- Nannini, M.; Rizzo, A.; Indio, V.; Schipani, A.; Astolfi, A.; Pantaleo, M.A. Targeted Therapy in SDH-Deficient GIST. Ther. Adv. Med. Oncol. 2021, 13, 17588359211023278. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; et al. Gastrointestinal Stromal Tumours: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, X.; Wu, X.; He, J.; Gao, J.; Shuai, X.; Wang, G.; Zhang, P.; Tao, K. Clinicopathologic Study of Succinate-Dehydrogenase-Deficient Gastrointestinal Stromal Tumors: A Single-Institutional Experience in China. Medicine 2017, 96, e7668. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Barysauskas, C.M.; von Mehren, M.; Heinrich, M.C.; Corless, C.L.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; Choy, E.; Yap, J.T.; et al. Long-Term Follow-up Results of the Multicenter Phase II Trial of Regorafenib in Patients with Metastatic and/or Unresectable GI Stromal Tumor after Failure of Standard Tyrosine Kinase Inhibitor Therapy. Ann. Oncol. 2016, 27, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, K.N.; Villalobos, V.M.; Kamaya, A.; Fisher, G.A.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; D’Adamo, D.; McMillan, A.; Demetri, G.D.; et al. A Multicenter Phase II Study of Pazopanib in Patients with Advanced Gastrointestinal Stromal Tumors (GIST) Following Failure of at Least Imatinib and Sunitinib. Ann. Oncol. 2014, 25, 236–240. [Google Scholar] [CrossRef]
- Von Mehren, M.; George, S.; Heinrich, M.C.; Schuetze, S.M.; Yap, J.T.; Yu, J.Q.; Abbott, A.; Litwin, S.; Crowley, J.; Belinsky, M.; et al. Linsitinib (OSI-906) for the Treatment of Adult and Pediatric Wild-Type Gastrointestinal Stromal Tumors, a SARC Phase II Study. Clin. Cancer Res. 2020, 26, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Zhang, W.; Li, J.; Wang, Y. Abnormal Mgmt Promoter Methylation in Gastrointestinal Stromal Tumors: Genetic Susceptibility and Association with Clinical Outcome. Cancer Manag. Res. 2020, 12, 9941–9952. [Google Scholar] [CrossRef]
- De Silva, M.; Rastogi, S.; Chan, D.; Angel, C.; Prall, O.; Gill, A.; Guminski, A. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor: From Diagnostic Dilemma to Novel Personalised Therapy in 2 Case Reports. Transl. Cancer Res. 2021, 10, 3588. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.; Qin, L.-X.; D’Angelo, S.P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Shoushtari, A.N.; Condy, M.M.; Konen, T.; Fruauff, A.; et al. A Phase Ib/II Study of MEK162 (Binimetinib [BINI]) in Combination with Imatinib in Patients with Advanced Gastrointestinal Stromal Tumor (GIST). J. Clin. Oncol. 2015, 33, 10507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipani, A.; Nannini, M.; Astolfi, A.; Pantaleo, M.A. SDHA Germline Mutations in SDH-Deficient GISTs: A Current Update. Genes 2023, 14, 646. https://doi.org/10.3390/genes14030646
Schipani A, Nannini M, Astolfi A, Pantaleo MA. SDHA Germline Mutations in SDH-Deficient GISTs: A Current Update. Genes. 2023; 14(3):646. https://doi.org/10.3390/genes14030646
Chicago/Turabian StyleSchipani, Angela, Margherita Nannini, Annalisa Astolfi, and Maria A. Pantaleo. 2023. "SDHA Germline Mutations in SDH-Deficient GISTs: A Current Update" Genes 14, no. 3: 646. https://doi.org/10.3390/genes14030646
APA StyleSchipani, A., Nannini, M., Astolfi, A., & Pantaleo, M. A. (2023). SDHA Germline Mutations in SDH-Deficient GISTs: A Current Update. Genes, 14(3), 646. https://doi.org/10.3390/genes14030646







