The Inhibitory Effects of RNA-Interference-Mediated Guanylate Cyclase Knockdown on Larval Metamorphosis and Early Progeny Growth of Razor Clam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals and Sample Preparation
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Verification of cGMPase Sequences
2.4. Sequencing and Phylogenetic Analyses
2.5. qRT-PCR of cGMPase mRNA
2.6. Synthesizing and Screening Effective dsRNA against cGMPase
2.6.1. Processing PCR Products
2.6.2. dsRNA Purification
2.7. Interference Studies during Larval Development
2.8. Adult Growth Test of dsRNA
2.9. Statistical Analysis
3. Results
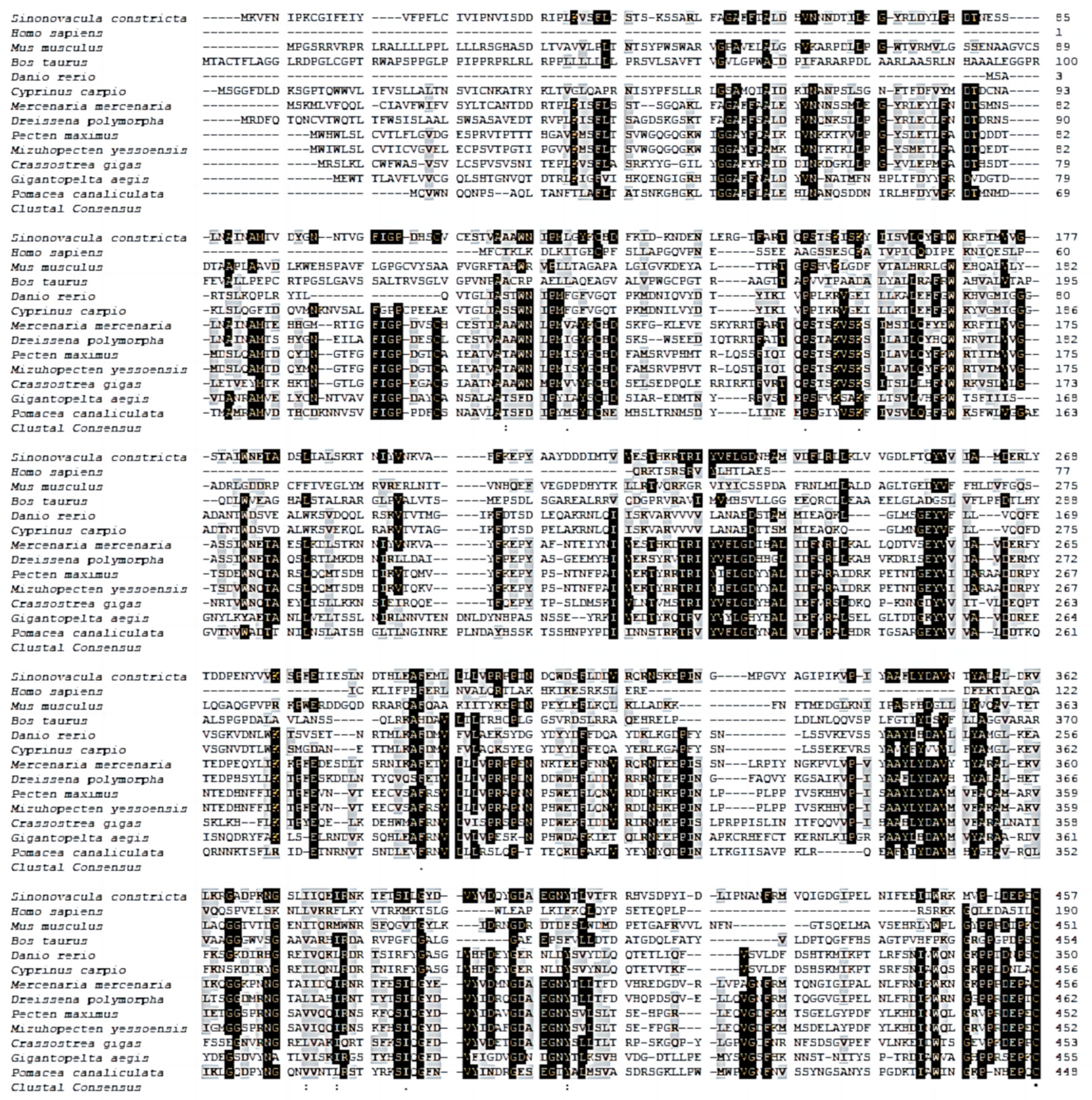
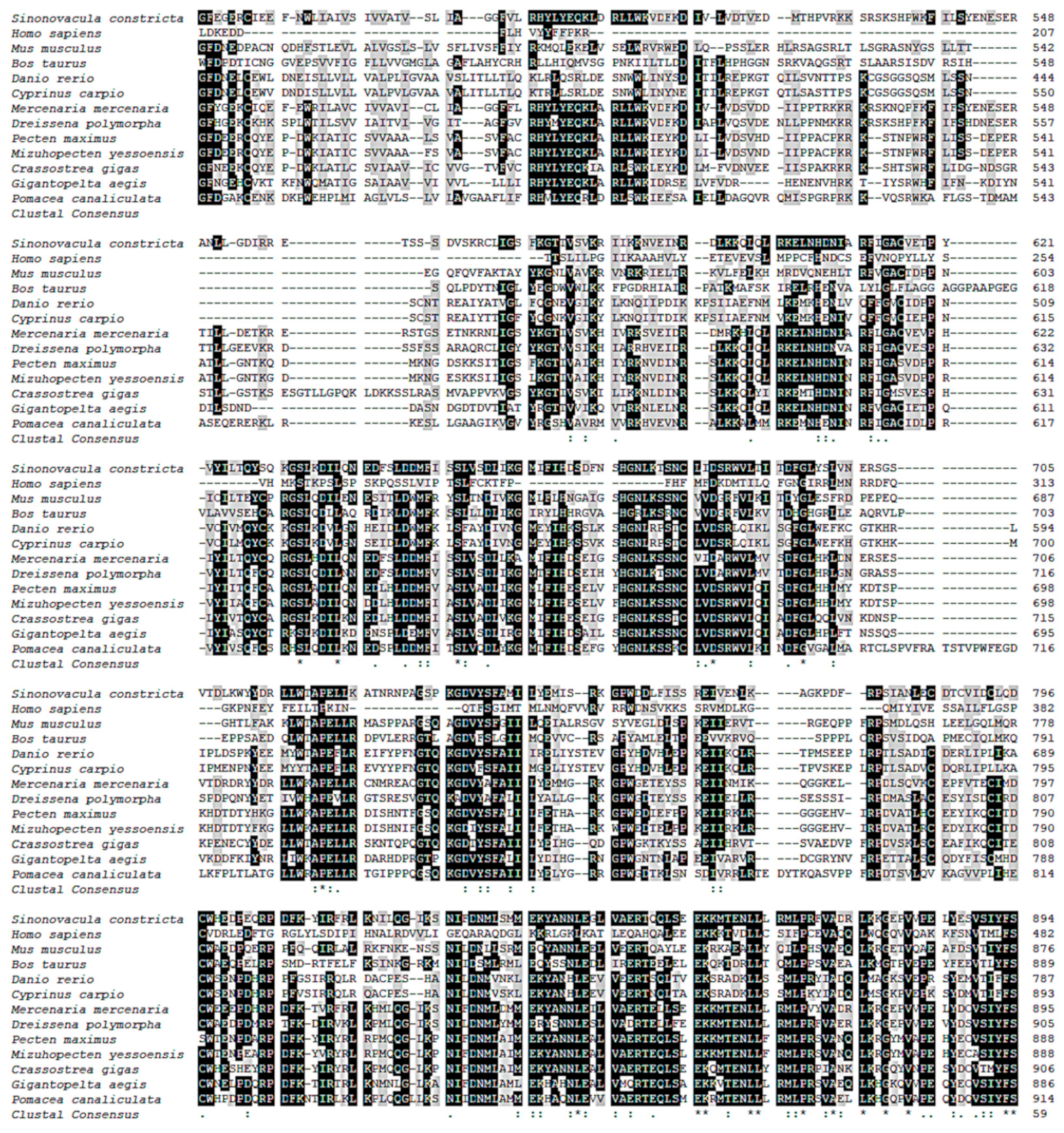
3.1. cGMPase Sequence Analysis
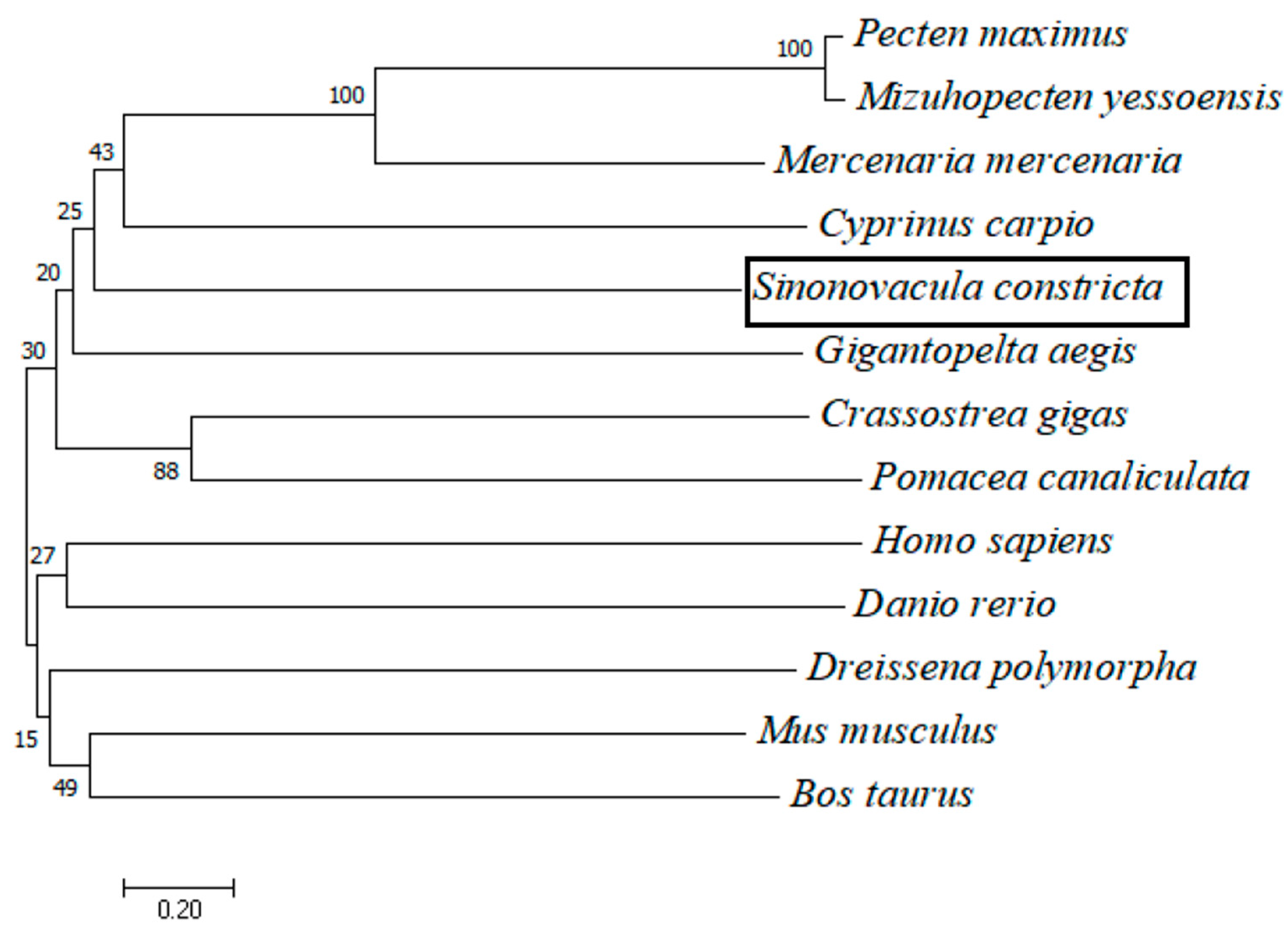
3.2. cGMPase Amino Acid Sequence Homology and Phylogenetic Analysis
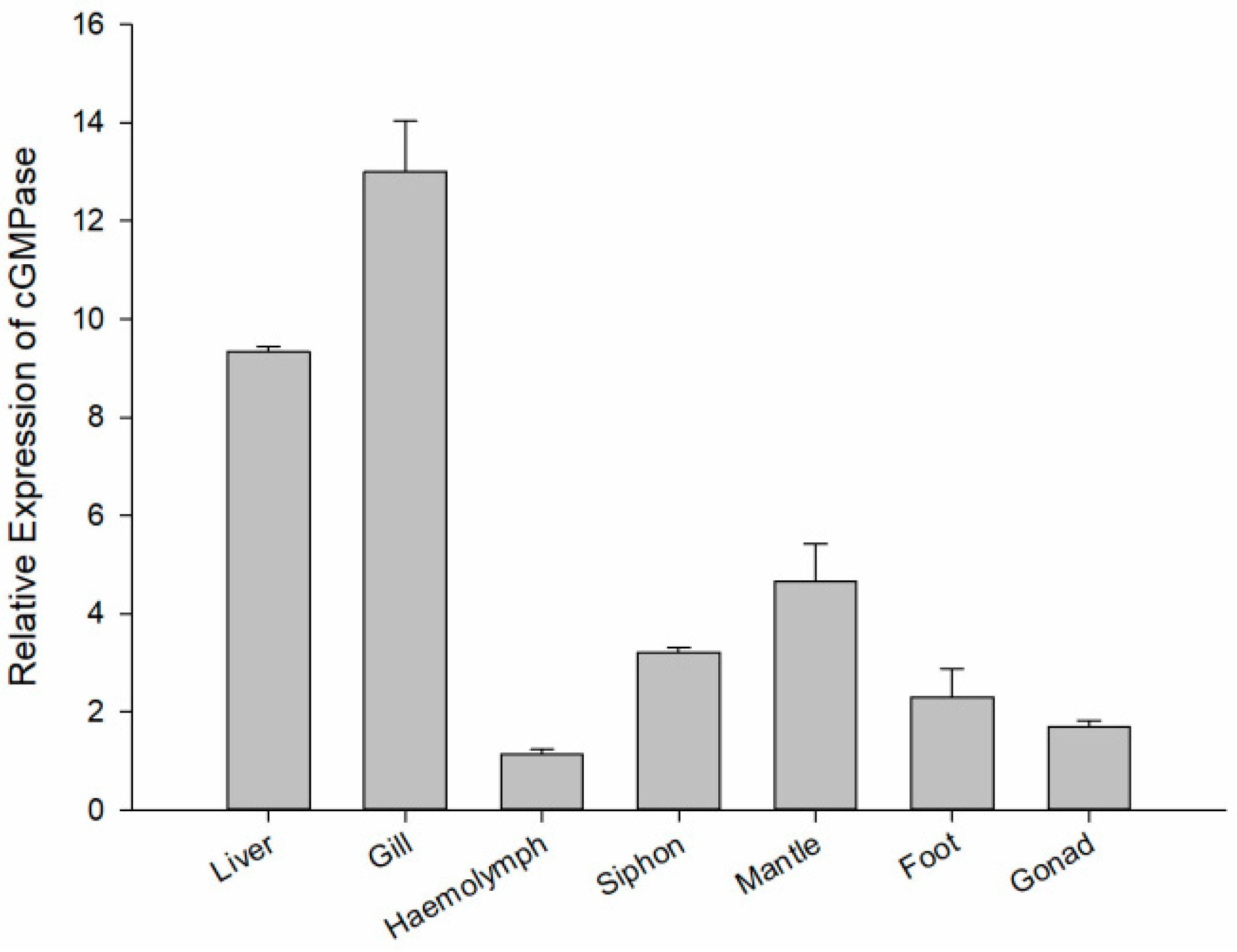
3.3. cGMPase Expression Analysis in Different Tissues
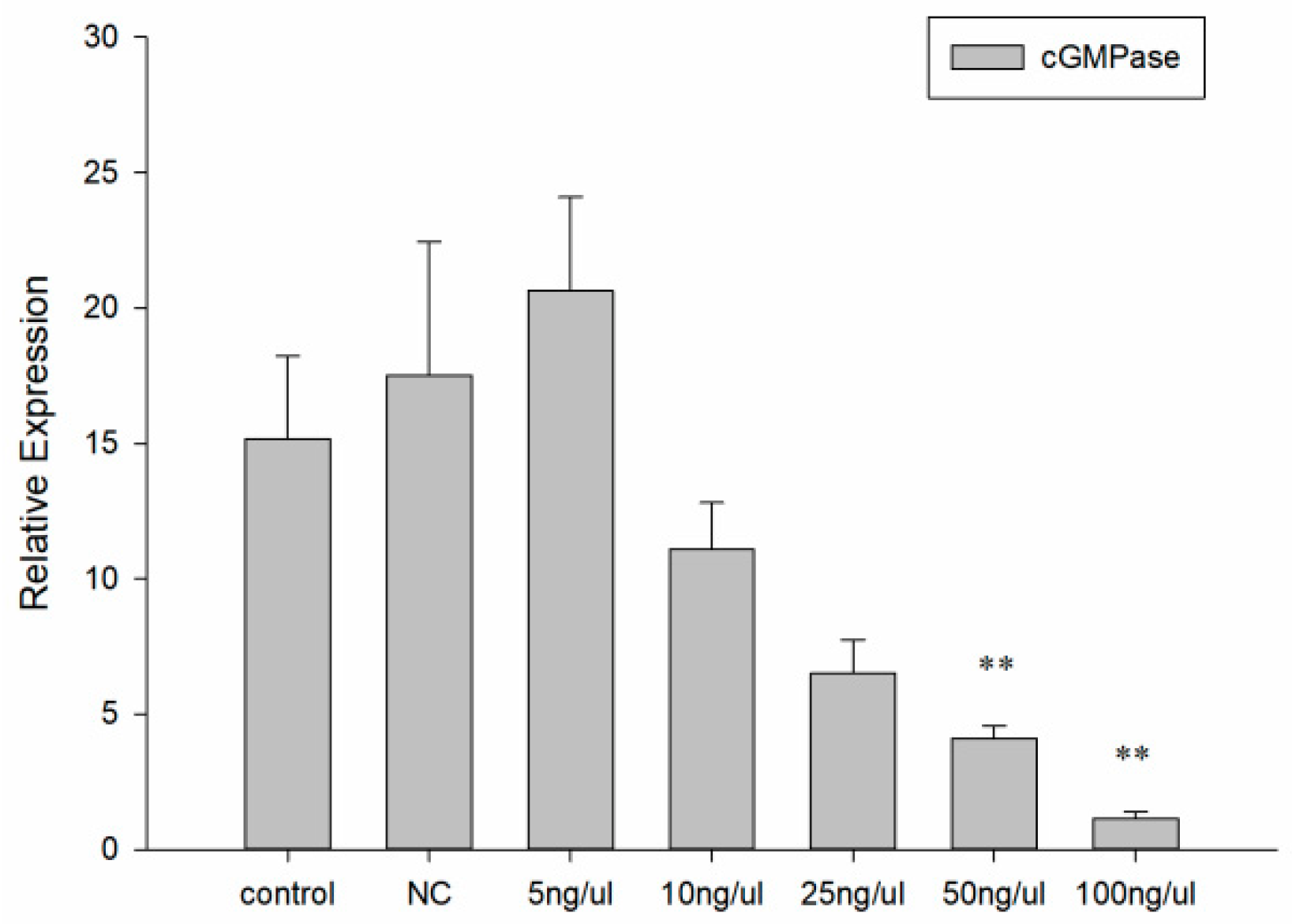
3.4. dsRNA Effective Sequence and Concentration
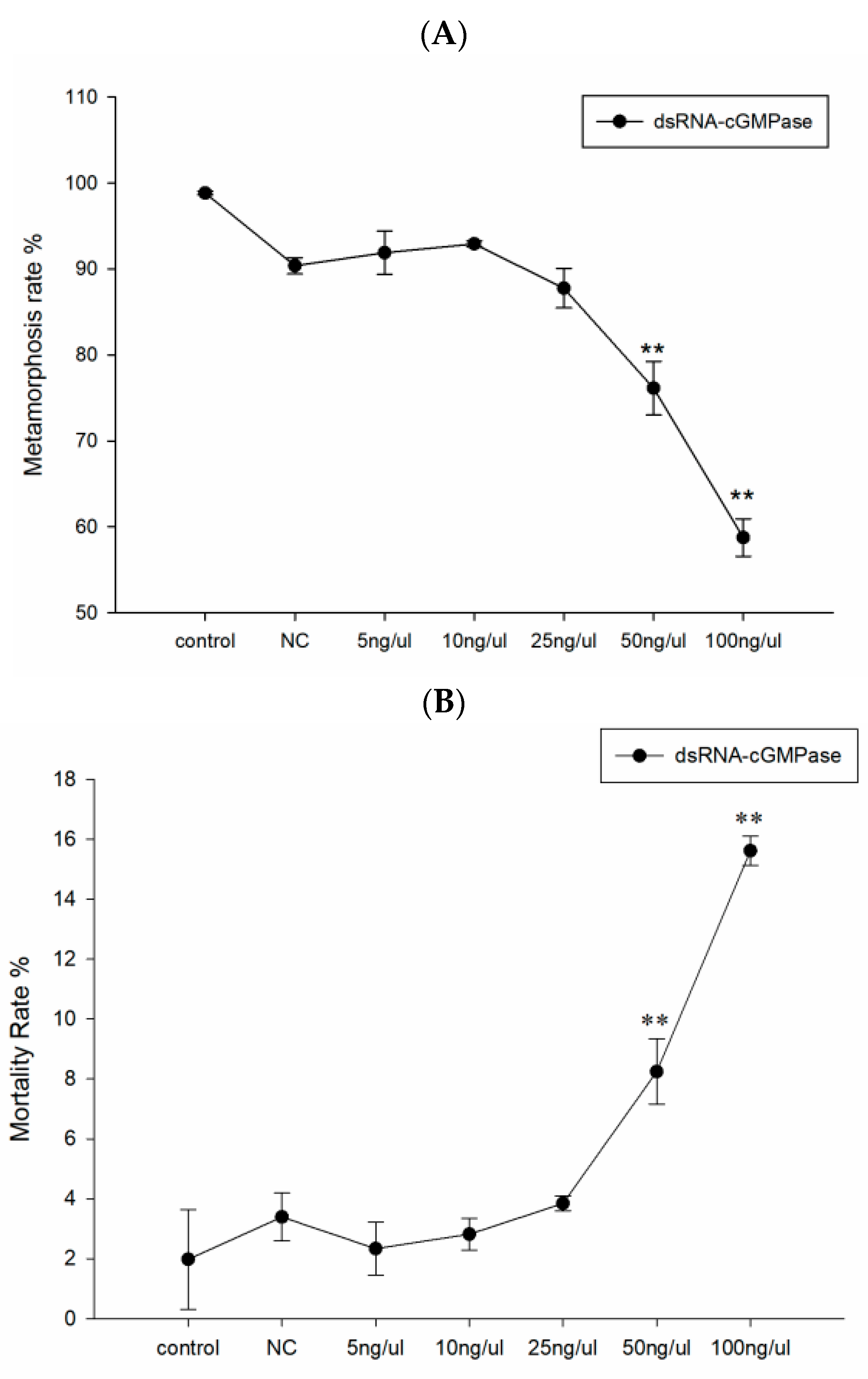
3.5. The Effects of dsRNA on Metamorphosis
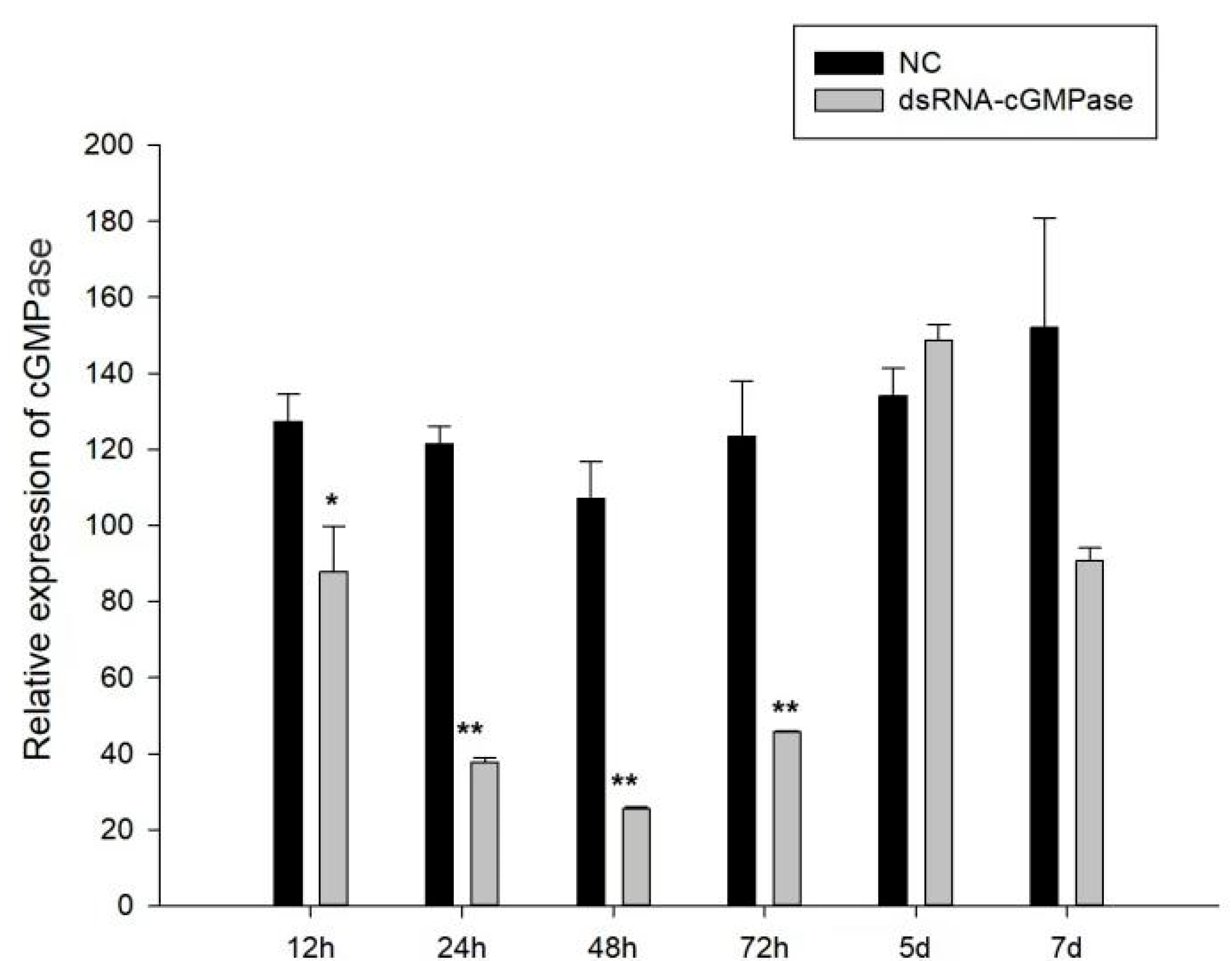
3.6. The Effects of dsRNA on S. constricta Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, H. RNA Interference to Knock Down Gene Expression. Methods Mol. Biol. 2018, 1706, 293–302. [Google Scholar]
- Dzitoyeva, S.; Dimitrijevic, N.; Manev, H. Intra-abdominal injection of double-stranded RNA into anesthetized adult Drosophila triggers RNA interference in the central nervous system. Mol. Psychiatry 2001, 6, 665–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, N.; Zhang, Y.; Duan, X.L.; Li, J.H.; Huang, W.F.; Evans, J.D.; DeGrandi-Hoffman, G.; Chen, Y.P.; Huang, S.K. RNA Interference-Mediated Knockdown of Genes Encoding Spore Wall Proteins Confers Protection against Nosema ceranae Infection in the European Honey Bee, Apis mellifera. Microorganisms 2021, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.J.; Gondhalekar, A.D.; Fardisi, M.; Pluchar, K.D.; Saltzmann, K.D.; Bennett, G.W.; Scharf, M.E. RNA interference and functional characterization of a tergal gland α amylase in the German cockroach, Blattella germanica L. Insect Mol. Biol. 2018, 27, 143–153. [Google Scholar] [CrossRef]
- Sun, G.; Dong, Y.; Sun, C.; Yao, H.; Lin, Z. Vital Role of Glutamate Dehydrogenase Gene in Ammonia Detoxification and the Association Between its SNPs and Ammonia Tolerance in Sinonovacula constricta. Front. Physiol. 2021, 12, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Fabioux, C.; Corporeau, C.; Quillien, V.; Favrel, P.; Huvet, A. Invivo RNA interference in oyster—Vasa silencing inhibits germ cell development. FEBS J. 2009, 276, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.G.; Sutherland, E.W. Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3′,5′-monophosphate from guanosine triphosphate. J. Biol. Chem. 1969, 244, 6363–6370. [Google Scholar] [CrossRef]
- Schultz, G.; Bohme, E.; Munske, K. Guanyl cyclase. Determination of enzyme activity. Life Sci. 1969, 15, 3–7. [Google Scholar]
- Follmann, M.; Ackerstaff, J.; Redlich, G.; Wunder, F.; Lang, D.; Kern, A.; Fey, P.; Griebenow, N.; Kroh, W.; Becker-Pelster, E.-M.; et al. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J. Med. Chem. 2017, 6, 134–136. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.R.; Gao, W.; Guo, X.P.; Ding, D.W.; Yoshida, A.; Osatomi, K.; Yang, J.L. Effects on larval metamorphosis in the mussel Mytilus coruscus of compounds that act on downstream effectors of G-protein-coupled receptors. J. Mar. Biol. Assoc. 2018, 98, 333–339. [Google Scholar] [CrossRef]
- Ashman, D.F.; Lipton, R.; Melicow, M.M.; Price, T.D. Isolation of adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate from rat urine. Biochem. Biophys. Res. Commun. 2003, 11, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Hao-Chuan, L.I.; Liu, S. Activation of cGMP-PKG signaling pathway contributes to neuronal hyperexcitability and hyperalgesia after in vivo prolonged compression or in vitro acute dissociation of dorsal root ganglion in rats. Acta Physiol. Sin. 2012, 64, 563–576. [Google Scholar]
- Seya, K.; Ono, K.; Fujisawa, S.; Motomura, S.; Furukawa, K.I. Intracellular calcium ion regulates myocardial apoptosis induced by mitochondrial NO-cGMP pathway. J. Mol. Cell. Cardiol. 2008, 45, S30–S31. [Google Scholar] [CrossRef]
- Bowler, C.; Yamagata, H.; Neuhaus, G.; Chua, N.H. Phytochrome signal transduction pathways are regulated by reciprocal control mechanisms. Genes Dev. 1994, 12, 2188–2202. [Google Scholar] [CrossRef] [PubMed]
- Borgs, M.; Bollen, M.; Keppens, S.; Yap, S.H.; Stalmans, W.; Vanstapel, F. Modulation of basal hepatic glycogenolysis by nitric oxide. Hepatology 2016, 23, 77–81. [Google Scholar] [CrossRef]
- Manoury, B.; Idres, S.; Leblais, V.; Fischmeister, R. Ion channels as effectors of cyclic nucleotide pathways: Functional relevance for arterial tone regulation. Pharmacol. Ther. 2020, 209, 107–109. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Xiang, Y.; Lin, M.; Gao, J. Ghrelin protects human umbilical vein endothelial cells against advanced glycation end products-induced apoptosis via NO/cGMP signaling. Int. J. Clin. Exp. Med. 2015, 8, 15269–15275. [Google Scholar]
- Zhenyu, W.; Shengsheng, Z.; Peicai, L.; Xiaofang, L.; Jiajia, W.; Luqing, Z.; Yueqi, W. Effect of Aurantii Fructus Immaturus Flavonoid on the Contraction of Isolated Gastric Smooth Muscle Strips in Rats. Evid.-Based Complement. Altern. Med. 2016, 6, 5616905. [Google Scholar]
- Niu, D.; Li, B.; Xie, S.; Dong, Z.; Li, J. Integrated mRNA and Small RNA Sequencing Reveals Regulatory Expression of Larval Metamorphosis of the Razor Clam. Mar. Biotechnol. 2020, 22, 696–705. [Google Scholar] [CrossRef]
- RamSonneveld, R.; Hoenderop, J.G.; Isidori, A.M.; Henique, C.; Dijkman, H.B.; Berden, J.H.; Tharaux, P.-L.; van der Vlag, J.; Nijenhuis, T. Sildenafil Prevents Podocyte Injury via PPAR-γ-Mediated TRPC6 Inhibition. J. Am. Soc. Nephrol. JASN 2017, 28, 1491–1505. [Google Scholar] [CrossRef]
- Romero, M.R.; Phuong, M.A.; Bishop, C.; Krug, P.J. Nitric oxide signaling differentially affects habitat choice by two larval morphs of the sea slug Alderia willowi: Mechanistic insight into evolutionary transitions in dispersal strategies. J. Exp. Biol. 2012, 216, 1114–1125. [Google Scholar] [PubMed]
- Yang, X.X.; Wong, Y.H.; Zhang, Y.; Zhang, G.; Qian, P.Y. Exploring the regulatory role of nitric oxide (NO) and the NO-p38MAPK/cGMP pathway in larval settlement of the bryozoan Bugula neritina. Biofouling 2018, 34, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Jin, K.; Wang, L.; Feng, B.; Li, J. Molecular characterization and expression analysis of four cathepsin L genes in the razor clam, Sinonovacula constricta. Fish Shellfish. Immunol. 2013, 35, 581–588. [Google Scholar] [CrossRef]
- Niu, D.; Xie, S.; Bai, Z.; Wang, L.; Jin, K.; Li, J. Identification, expression, and responses to bacterial challenge of the cathepsin C gene from the razor clam Sinonovacula constricta. Dev. Comp. Immunol. 2014, 46, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Clare, A.S.; Fusetani, N.; Jones, M.B. Settlement and metamorphosis of marine invertebrate larvae—Introduction. Biofouling 2008, 12, 1–2. [Google Scholar] [CrossRef]
- Huan, P.; Wang, H.; Liu, B. Transcriptomic analysis of the clam Meretrix meretrix on different larval stages. Mar. Biotechnol. 2012, 14, 69–78. [Google Scholar] [CrossRef]
- Satuito, C.G.; Natoyama, K.; Yamazaki, M.; Shimizu, K.; Fusetani, N. Induction of metamorphosis in the pediveliger larvae of the mussel Mytilus galloprovincialis by neuroactive compounds. Fish. Sci. 2010, 65, 384–389. [Google Scholar] [CrossRef]
- Sanchez-Lazo, C.; Martinez-Pita, I.; Young, T.; Alfaro, A.C. Induction of settlement in larvae of the mussel Mytilus galloprovincialis using neuroactive compounds. Aquaculture 2012, 344, 210–215. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, S.H.; Li, Y.F.; Liu, Z.W.; Liang, X.; Bao, W.Y.; Li, J.L. Effects of neuroactive compounds, ions and organic solvents on larval metamorphosis of the mussel Mytilus coruscus. Aquaculture 2013, 396, 106–112. [Google Scholar] [CrossRef]
- Li, Z.; Niu, D.H.; Peng, M.X.; Xiong, Y.; Ji, J.; Dong, Z.G.; Li, J.L. Dopamine β-hydroxylase and its role in regulating the growth and larval metamorphosis in Sinonovacula constricta. Gene 2020, 737, 144418–144419. [Google Scholar] [CrossRef]
- Feil, R.; Kemp-Harper, B. cGMP signalling: From bench to bedside. Conference on cGMP generators, effectors and therapeutic implications. EMBO Rep. 2006, 6, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P. Regulation of metabolism by cGMP. Pharmacol. Ther. 2013, 4, 75–80. [Google Scholar]
- Mao, J.H. Regulation of cell function by cGMP. Adv. Biochem. Biophys. 1995, 6, 511–516. [Google Scholar]
- Bruckdorfer, R. The basics about nitric oxide. Mol. Asp. Med. 2005, 26, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A. Nitric oxide in marine invertebrates: A comparative perspective. Comp. Biochem. Physiol. 2005, 142A, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.A.; Pitari, G.M.; Kazerounian, S.; Ruiz-Stewart, I.; Park, J.; Schulz, S.; Chepenik, K.P.; Waldman, S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000, 52, 375–414. [Google Scholar]
- Zhang, Y.; He, L.-S.; Zhang, G.; Xu, Y.; Lee, O.-O.; Matsumura, K.; Qian, P.-Y. The regulatory role of the NO/cGMP signal transduction cascade during larval attachment and metamorphosis of the barnacle Balanus (=Amphibalanus) amphitrite. J. Exp. Biol. 2012, 215, 3813–3822. [Google Scholar]
- Comes, S.; Locascio, A.; Silvestre, F.; d’Ischia, M.; Russo, G.L.; Tosti, E.; Branno, M.; Palumbo, A. Regulatory roles of nitric oxide during larval development and metamorphosis in Ciona intestinalis. Dev. Biol. 2007, 306, 772–784. [Google Scholar] [CrossRef]
- Pechenik, J.A.; Cochrane, D.E.; Li, W.; West, E.T.; Pires, A.; Leppo, M. Nitric oxide inhibits metamorphosis in larvae of Crepidula fornicata, the slippershell snail. Biol. Bull. 2007, 213, 160–171. [Google Scholar] [CrossRef]
- Leise, E.M.; Thavaradhara, K.; Durham, N.R.; Turner, B.E. Serotonin and nitric oxide regulate metamorphosis in the marine snail Ilyanassa obsoleta. Am. Zool. 2001, 41, 258–267. [Google Scholar]
- Bishop, C.D.; Brandhorst, B.P. NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus. Biol. Bull. 2001, 201, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D.; Pires, A.; Norby, S.W.; Boudko, D.; Moroz, L.L.; Hadfield, M.G. Analysis of nitric oxide-cyclic guanosine monophosphate signaling during metamorphosis of the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia). Evol. Dev. 2008, 10, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D.; Brandhorst, B.P. On nitric oxide signaling, metamorphosis, and the evolution of biphasic life cycles. Evol. Dev. 2003, 5, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Leise, E.M.; Kempf, S.; Durham, N.; Gifondorwa, D.J. Induction of metamorphosis in the marine gastropod Ilyanassa obsoleta: 5HT, NO and programmed cell death. Acta Biol. Hung. 2004, 55, 293–300. [Google Scholar] [CrossRef]
- Ueda, N.; Richards, G.S.; Degnan, B.M.; Kranz, A.; Adamska, M.; Croll, R.P.; Degnan, S.M. An ancient role for nitric oxide in regulating the animal pelagobenthic life cycle: Evidence from a marine sponge. Sci. Rep. 2016, 6, 37546. [Google Scholar] [CrossRef]
- Castellano, I.; Ercolesi, E.; Palumbo, A. Nitric oxide affects ERK signaling through down-regulation of MAP kinase phosphatase levels during larval development of the ascidian Ciona intestinalis. PLoS ONE 2014, 9, 102907. [Google Scholar] [CrossRef]
- Furuya, M.; Yoshida, M.; Hayashi, Y.; Ohnuma, N.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type Natriuretic Peptide is a Growth Inhibitor of Rat Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 1991, 177, 927–931. [Google Scholar] [CrossRef]




| Primers Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| M13-F | AGGGTTTTCCCAGTCACG | Sequence verification of cGMPase |
| M13-R | AGCGGATAACAATTTCACAC | |
| cGMPase F1 | TGCCTTCCTGTATGATGCT | |
| cGMPase R1 | TTTCAGTAACTCAGGTGCC | |
| cGMPase F2 | CTGTTAGGAAAAAGAGTCG | |
| cGMPase R2 | GCTACAGATCCTGCTTATT | |
| cGMPase F3 | ATGATGAGTAGATGACCCC | |
| cGMPase R3 | CATCATACAGGAAGGCAGC | |
| 18S-F | TCGGTTCTATTGCGTTGGTTTT | qRT-PCR of control |
| 18S-R | CAGTTGGCATCGTTTATGGTCA |
| Name of Primer | Specific Sequence (5′–3′) |
|---|---|
| cGMPase F1 | GCGACGTGTATTCTTTCGCC |
| cGMPase R1 | CAACGCAGTGAAGCCAACAA |
| cGMPase F2 | ACTACGACCGATTGCTGTGG |
| cGMPase R2 | TGTCCTCTCCGCAACAAGAC |
| cGMPase F3 | AACCGGATTTCCGACCTAGC |
| cGMPase R3 | TTCTCCACGATGGCGTCAAA |
| cGMPase T7 F1 | TAATACGACTCACTATAGGGGCGACGTGTATTCTTTCGCC |
| cGMPase T7 R1 | TAATACGACTCACTATAGGGCAACGCAGTGAAGCCAACAA |
| cGMPase T7 F2 | TAATACGACTCACTATAGGGACTACGACCGATTGCTGTGG |
| cGMPase T7 R2 | TAATACGACTCACTATAGGGTGTCCTCTCCGCAACAAGAC |
| cGMPase T7 F3 | TAATACGACTCACTATAGGGAACCGGATTTCCGACCTAGC |
| cGMPase T7 R3 | TAATACGACTCACTATAGGGTTCTCCACGATGGCGTCAAA |
| EGFP-F | CAGTGCTTCAGCCGCTACC |
| EGFP-R | AGTTCACCTTGATGCCGTTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Li, B.; Li, Y.; Niu, D. The Inhibitory Effects of RNA-Interference-Mediated Guanylate Cyclase Knockdown on Larval Metamorphosis and Early Progeny Growth of Razor Clam. Genes 2023, 14, 459. https://doi.org/10.3390/genes14020459
Han Y, Li B, Li Y, Niu D. The Inhibitory Effects of RNA-Interference-Mediated Guanylate Cyclase Knockdown on Larval Metamorphosis and Early Progeny Growth of Razor Clam. Genes. 2023; 14(2):459. https://doi.org/10.3390/genes14020459
Chicago/Turabian StyleHan, Yuting, Beibei Li, Yifeng Li, and Donghong Niu. 2023. "The Inhibitory Effects of RNA-Interference-Mediated Guanylate Cyclase Knockdown on Larval Metamorphosis and Early Progeny Growth of Razor Clam" Genes 14, no. 2: 459. https://doi.org/10.3390/genes14020459
APA StyleHan, Y., Li, B., Li, Y., & Niu, D. (2023). The Inhibitory Effects of RNA-Interference-Mediated Guanylate Cyclase Knockdown on Larval Metamorphosis and Early Progeny Growth of Razor Clam. Genes, 14(2), 459. https://doi.org/10.3390/genes14020459





