Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Extraction of Total RNA, Construction of cDNA Library, and Transcriptome Sequencing
2.3. Quality Control, Alignment, and Quantification of RNA-Seq Data
2.4. Analysis of Differentially Expressed Transcripts
2.5. Analysis of lncRNA-Regulated Target Genes
2.6. Protein–Protein Interaction (PPI) Network Construction
2.7. GO and KEGG Pathway Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR) Validation
3. Results
3.1. Results of Sequencing and Characteristics of Transcripts
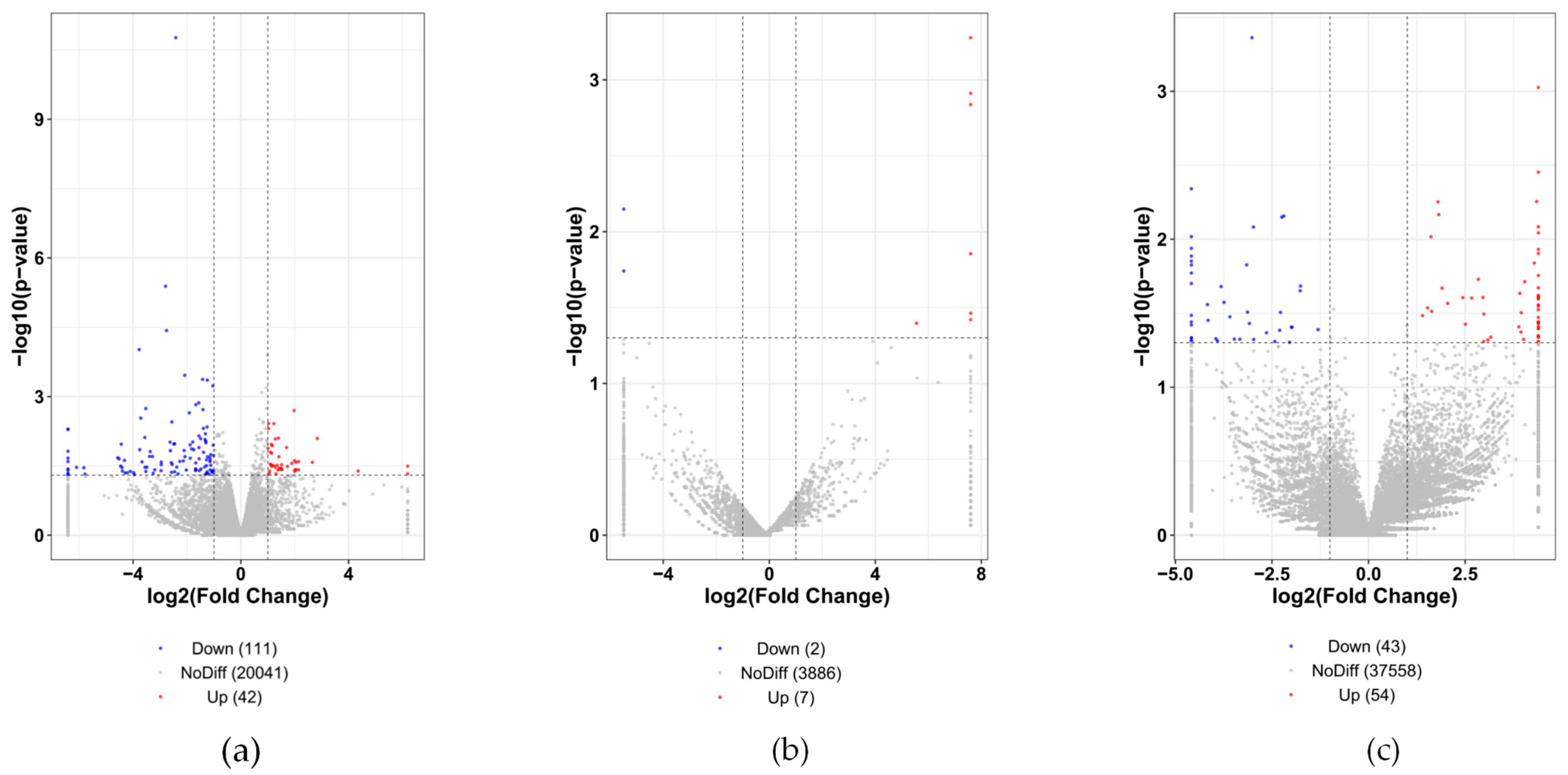
3.2. Differential Transcription Expression Profile
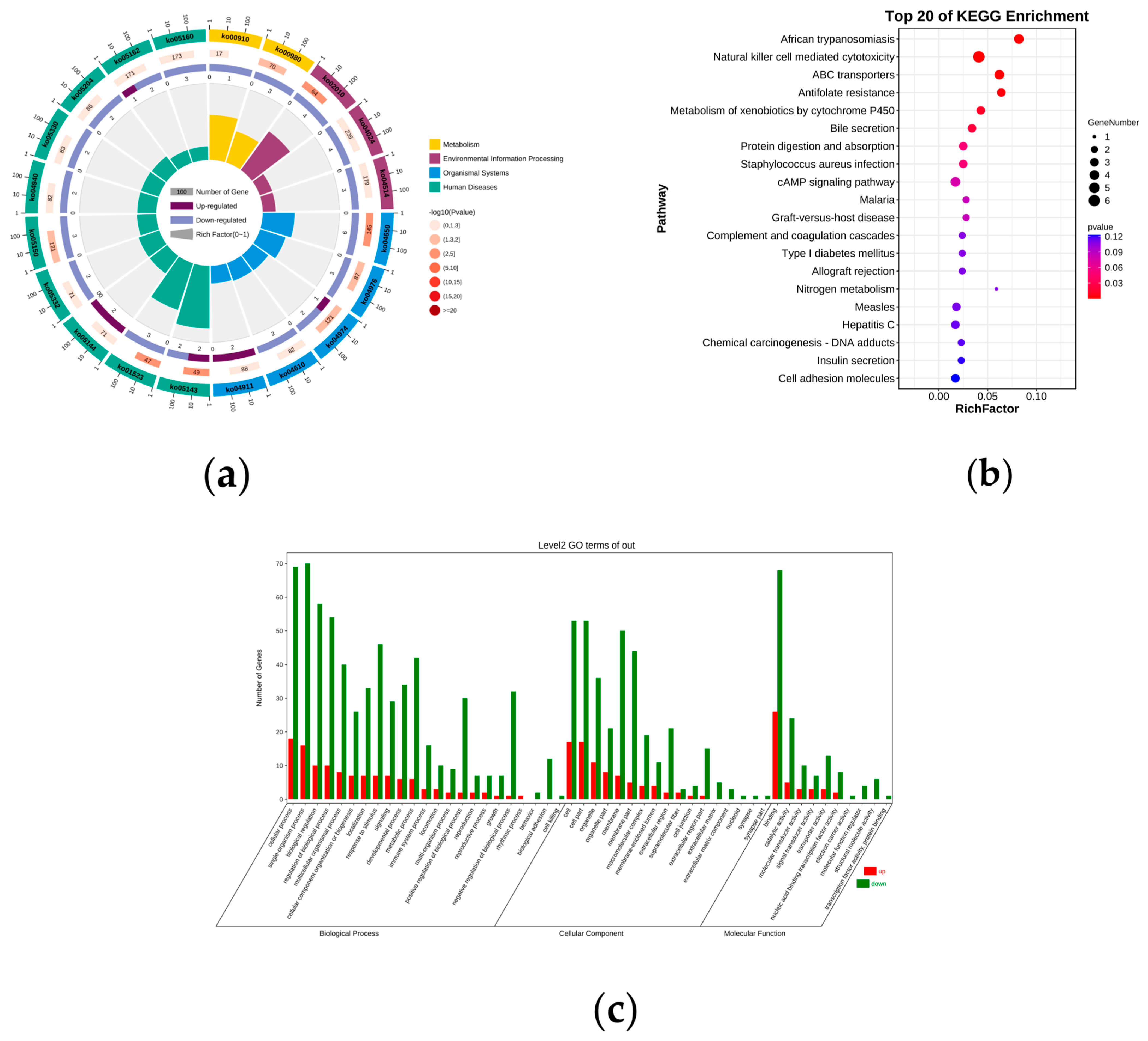
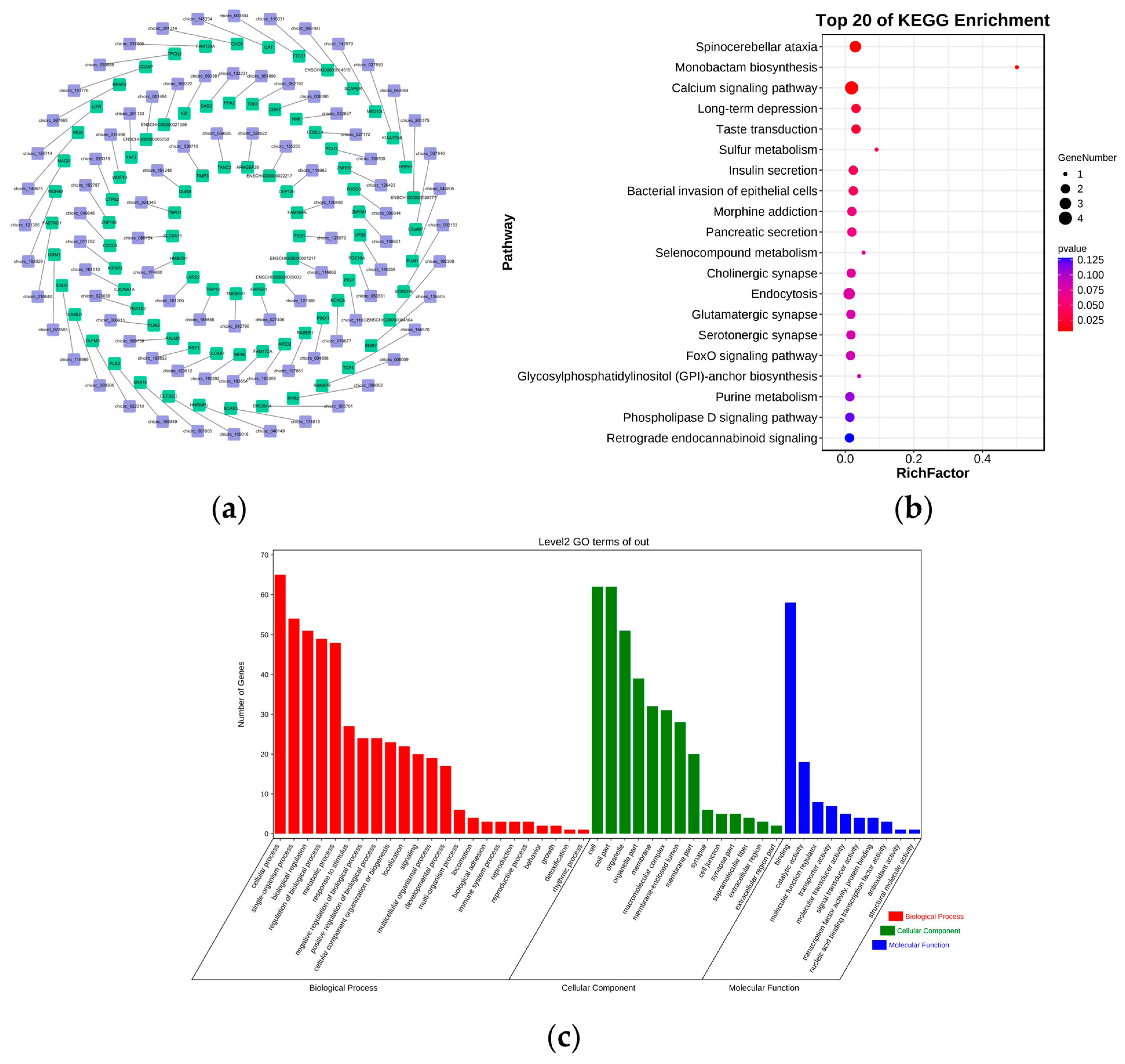
3.3. GO and KEGG Enrichment Analysis of DE mRNAs
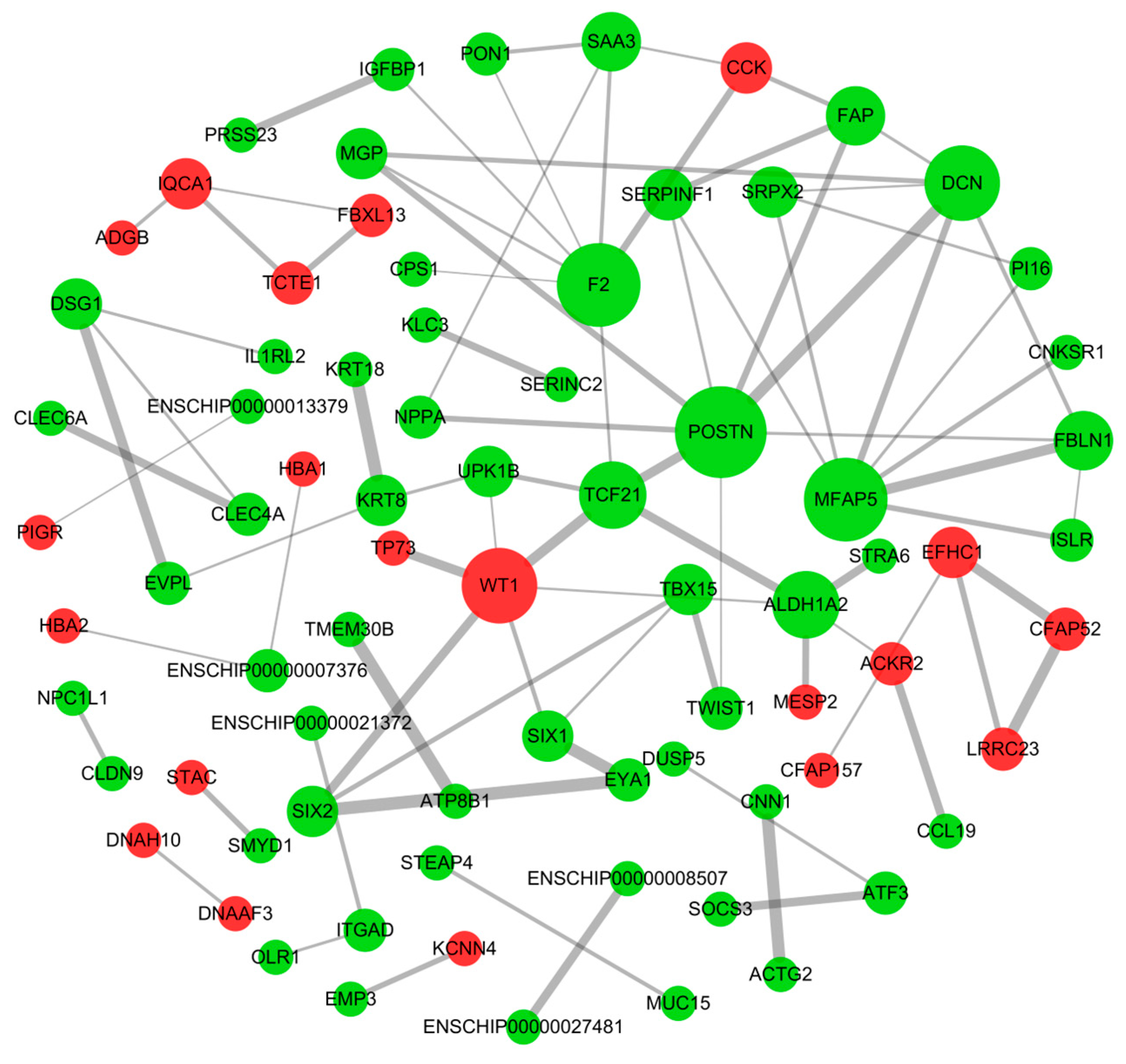
3.4. Protein–Protein Interaction Network of DE mRNAs
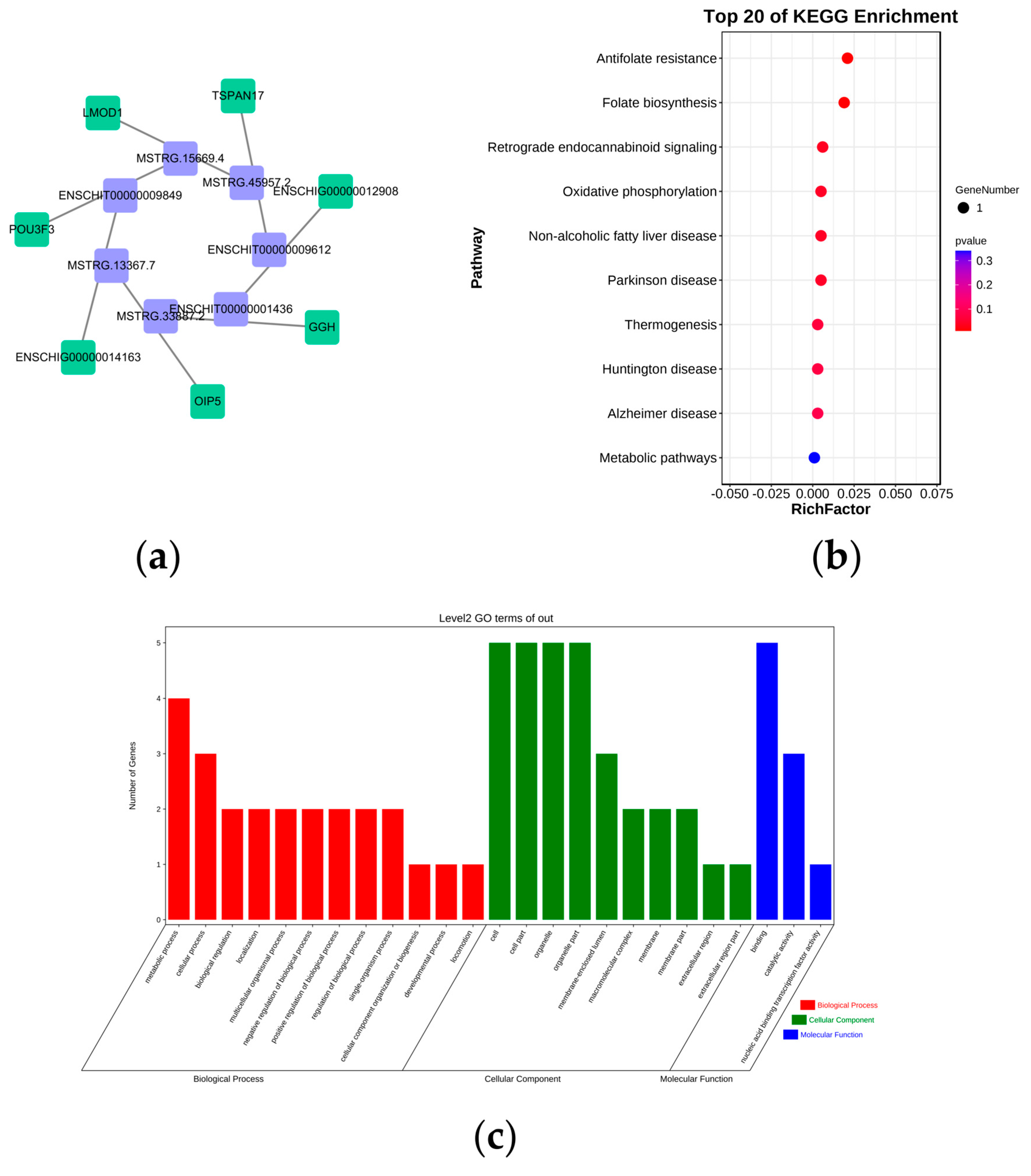
3.5. Functional and Pathway Enrichment Analyses of DE lncRNAs
3.6. Functional Identification of DE circRNAs
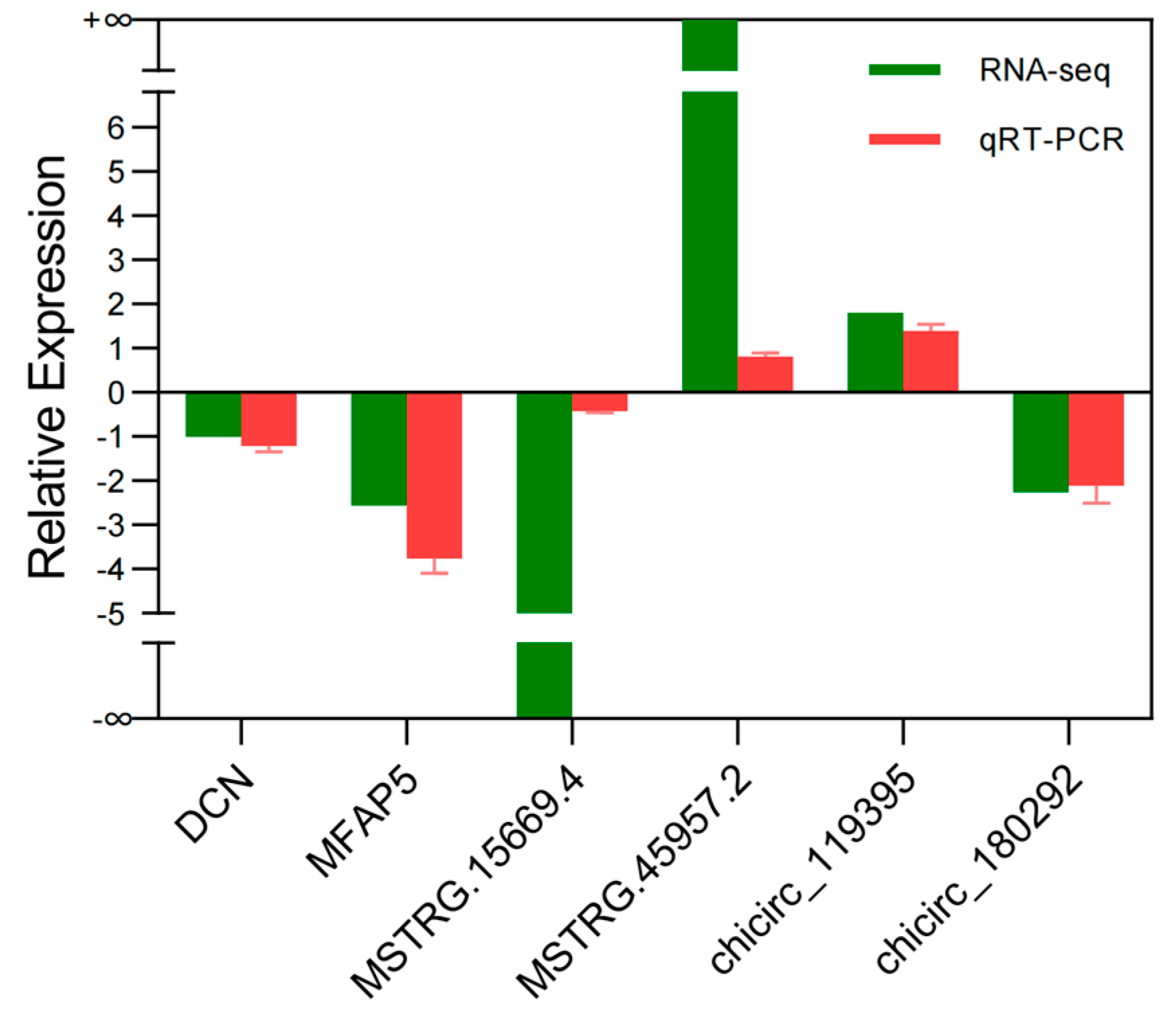
3.7. Sequencing Data Validation
4. Discussion
4.1. SOCS3 in the Hypothalamus May Affect the Fertility of Leizhou Goats
4.2. Pivot Protein Function Analysis in High- and Low-Fecundity Leizhou Goats
4.3. Functional Analysis of DE lncRNAs in High- and Low-Fecundity Leizhou Goats
4.4. Functional Analysis of DE circRNAs in High- and Low-Fecundity Leizhou Goats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Zhang, H.; Yang, H.; Zhao, Z.; Blair, H.T.; Zhai, M.; Yu, Q.; Wu, P.; Fang, C.; Xie, M. Polymorphisms and association of GRM1, GNAQ and HCRTR1 genes with seasonal reproduction and litter size in three sheep breeds. Reprod. Domest. Anim. 2022, 57, 532–540. [Google Scholar] [CrossRef]
- van Wettere, W.H.; Herde, P.; Hughes, P.E. Supplementing sow gestation diets with betaine during summer increases litter size of sows with greater numbers of parities. Anim. Reprod. Sci. 2012, 132, 44–49. [Google Scholar] [CrossRef]
- Esmaeili-Fard, S.M.; Gholizadeh, M.; Hafezian, S.H.; Abdollahi-Arpanahi, R. Genome-wide association study and pathway analysis identify NTRK2 as a novel candidate gene for litter size in sheep. PLoS ONE 2021, 16, e0244408. [Google Scholar] [CrossRef]
- Vashi, Y.; Magotra, A.; Kalita, D.; Banik, S.; Sahoo, N.R.; Gupta, S.K.; Naskar, S. Evaluation of candidate genes related to litter traits in Indian pig breeds. Reprod. Domest. Anim. 2021, 56, 577–585. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Xiang, H.; Dun, W.; Brahi, D.O.H.; Yin, T.; Zhao, X. Mitochondrial DNA T7719G in tRNA-Lys gene affects litter size in Small-tailed Han sheep. J. Anim. Sci. Biotechnol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, X.; Xue, H.; Zou, X.; Liu, G.; Deng, M.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. RNA-Seq Reveals miRNA and mRNA Co-regulate Muscle Differentiation in Fetal Leizhou Goats. Front. Vet. Sci. 2022, 9, 829769. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Wang, L.J.; Sun, X.W.; Zhang, J.J.; Zhao, Y.J.; Na, R.S.; Zhang, J.H. Transcriptome analysis of the Capra hircus ovary. PLoS ONE 2015, 10, e0121586. [Google Scholar] [CrossRef]
- Mikhael, S.; Punjala-Patel, A.; Gavrilova-Jordan, L. Hypothalamic-Pituitary-Ovarian Axis Disorders Impacting Female Fertility. Biomedicines 2019, 7, 5. [Google Scholar] [CrossRef]
- Clarke, I.J. Interface between metabolic balance and reproduction in ruminants: Focus on the hypothalamus and pituitary. Horm. Behav. 2014, 66, 15–40. [Google Scholar] [CrossRef]
- Stincic, T.L.; Kelly, M.J. Estrogenic regulation of reproduction and energy homeostasis by a triumvirate of hypothalamic arcuate neurons. J. Neuroendocrinol. 2022, 34, e13145. [Google Scholar] [CrossRef]
- Prevot, V.; Dehouck, B.; Sharif, A.; Ciofi, P.; Giacobini, P.; Clasadonte, J. The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr. Rev. 2018, 39, 333–368. [Google Scholar] [CrossRef]
- Simonneaux, V. A Kiss to drive rhythms in reproduction. Eur. J. Neurosci. 2020, 51, 509–530. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Koonin, E.V. Functional Long Non-coding RNAs Evolve from Junk Transcripts. Cell 2020, 183, 1151–1161. [Google Scholar] [CrossRef]
- Liang, C.; Han, M.; Zhou, Z.; Liu, Y.; He, X.; Jiang, Y.; Ouyang, Y.; Hong, Q.; Chu, M. Hypothalamic Transcriptome Analysis Reveals the Crucial MicroRNAs and mRNAs Affecting Litter Size in Goats. Front. Vet. Sci. 2021, 8, 747100. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881.e813. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Bouckenheimer, J.; Fauque, P.; Lecellier, C.H.; Bruno, C.; Commes, T.; Lemaître, J.M.; De Vos, J.; Assou, S. Differential long non-coding RNA expression profiles in human oocytes and cumulus cells. Sci. Rep. 2018, 8, 2202. [Google Scholar] [CrossRef]
- Wang, C.; Tan, S.; Liu, W.R.; Lei, Q.; Qiao, W.; Wu, Y.; Liu, X.; Cheng, W.; Wei, Y.Q.; Peng, Y.; et al. RNA-Seq profiling of circular RNA in human lung adenocarcinoma and squamous cell carcinoma. Mol. Cancer 2019, 18, 134. [Google Scholar] [CrossRef]
- Perera, B.M.; Bongso, T.A.; Abeynaike, P. Oestrus synchronisation in goats using cloprostenol. Vet. Rec. 1978, 102, 314. [Google Scholar] [CrossRef]
- Alavez Ramírez, A.; Arroyo Ledezma, J.; Montes Pérez, R.; Zamora Bustillos, R.; Navarrete Sierra, L.F.; Magaña Sevilla, H. Short communication: Estrus synchronization using progestogens or cloprostenol in tropical hair sheep. Trop. Anim. Health Prod. 2014, 46, 1515–1518. [Google Scholar] [CrossRef]
- Yan, S.; Yue, Y.; Sun, M.; Chen, Y.; Wang, X.; Qian, H. Comparative Transcriptome Analysis Reveals Relationship among mRNAs, lncRNAs, and circRNAs of Slow Transit Constipation. Biomed Res. Int. 2021, 2021, 6672899. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fatet, A.; Pellicer-Rubio, M.-T.; Leboeuf, B. Reproductive cycle of goats. Anim. Reprod. Sci. 2011, 124, 211–219. [Google Scholar] [CrossRef]
- Muriuki, C.; Bush, S.J.; Salavati, M.; McCulloch, M.E.B.; Lisowski, Z.M.; Agaba, M.; Djikeng, A.; Hume, D.A.; Clark, E.L. A Mini-Atlas of Gene Expression for the Domestic Goat (Capra hircus). Front. Genet. 2019, 10, 1080. [Google Scholar] [CrossRef]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef]
- Wen, Y.L.; Guo, X.F.; Ma, L.; Zhang, X.S.; Zhang, J.L.; Zhao, S.G.; Chu, M.X. The expression and mutation of BMPR1B and its association with litter size in small-tail Han sheep (Ovis aries). Arch. Anim. Breed. 2021, 64, 211–221. [Google Scholar] [CrossRef]
- Silva, J.R.; van den Hurk, R.; van Tol, H.T.; Roelen, B.A.; Figueiredo, J.R. Expression of growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15), and BMP receptors in the ovaries of goats. Mol. Reprod. Dev. 2005, 70, 11–19. [Google Scholar] [CrossRef]
- Bi, Y.; Li, J.; Wang, X.; He, L.; Lan, K.; Qu, L.; Lan, X.; Song, X.; Pan, C. Two Novel Rare Strongly Linked Missense SNPs (P27R and A85G) Within the GDF9 Gene Were Significantly Associated With Litter Size in Shaanbei White Cashmere (SBWC) Goats. Front. Vet. Sci. 2020, 7, 406. [Google Scholar] [CrossRef]
- Aboelhassan, D.M.; Darwish, A.M.; Ali, N.I.; Ghaly, I.S.; Farag, I.M. A study on mutation points of GDF9 gene and their association with prolificacy in Egyptian small ruminants. J. Genet. Eng. Biotechnol. 2021, 19, 85. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Ko, E.K.; Chorich, L.P.; Sullivan, M.E.; Cameron, R.S.; Layman, L.C. JAK/STAT signaling pathway gene expression is reduced following Nelf knockdown in GnRH neurons. Mol. Cell. Endocrinol. 2018, 470, 151–159. [Google Scholar] [CrossRef]
- Bellefontaine, N.; Chachlaki, K.; Parkash, J.; Vanacker, C.; Colledge, W.; d’Anglemont de Tassigny, X.; Garthwaite, J.; Bouret, S.G.; Prevot, V. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Investig. 2014, 124, 2550–2559. [Google Scholar] [CrossRef]
- Robertson, S.A.; Leinninger, G.M.; Myers, M.G. Molecular and neural mediators of leptin action. Physiol. Behav. 2008, 94, 637–642. [Google Scholar] [CrossRef]
- Inagaki-Ohara, K.; Mayuzumi, H.; Kato, S.; Minokoshi, Y.; Otsubo, T.; Kawamura, Y.I.; Dohi, T.; Matsuzaki, G.; Yoshimura, A. Enhancement of leptin receptor signaling by SOCS3 deficiency induces development of gastric tumors in mice. Oncogene 2014, 33, 74–84. [Google Scholar] [CrossRef]
- Guzmán, A.; Hernández-Coronado, C.G.; Rosales-Torres, A.M.; Hernández-Medrano, J.H. Leptin regulates neuropeptides associated with food intake and GnRH secretion. Ann. D’endocrinologie 2019, 80, 38–46. [Google Scholar] [CrossRef]
- Sobrino, V.; Avendaño, M.S.; Perdices-López, C.; Jimenez-Puyer, M.; Tena-Sempere, M. Kisspeptins and the neuroendocrine control of reproduction: Recent progress and new frontiers in kisspeptin research. Front. Neuroendocrinol. 2022, 65, 100977. [Google Scholar] [CrossRef]
- Araujo-Lopes, R.; Crampton, J.R.; Aquino, N.S.; Miranda, R.M.; Kokay, I.C.; Reis, A.M.; Franci, C.R.; Grattan, D.R.; Szawka, R.E. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 2014, 155, 1010–1020. [Google Scholar] [CrossRef]
- Landry, N.M.; Cohen, S.; Dixon, I.M.C. Periostin in cardiovascular disease and development: A tale of two distinct roles. Basic Res. Cardiol. 2018, 113, 1. [Google Scholar] [CrossRef]
- Monniaux, D. Driving folliculogenesis by the oocyte-somatic cell dialog: Lessons from genetic models. Theriogenology 2016, 86, 41–53. [Google Scholar] [CrossRef]
- Christoforou, E.R.; Pitman, J.L. Intrafollicular growth differentiation factor 9: Bone morphogenetic 15 ratio determines litter size in mammals. Biol. Reprod. 2019, 100, 1333–1343. [Google Scholar] [CrossRef]
- Li, C.; Cheng, D.; Xu, P.; Nie, H.; Zhang, T.; Pang, X. POSTN Promotes the Proliferation of Spermatogonial Cells by Activating the Wnt/β-Catenin Signaling Pathway. Reprod. Sci. 2021, 28, 2906–2915. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Li, K.; Ling, Y.; Kang, H. Stromal fibroblast-derived MFAP5 promotes the invasion and migration of breast cancer cells via Notch1/slug signaling. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 522–531. [Google Scholar] [CrossRef]
- Leung, C.S.; Yeung, T.L.; Yip, K.P.; Pradeep, S.; Balasubramanian, L.; Liu, J.; Wong, K.K.; Mangala, L.S.; Armaiz-Pena, G.N.; Lopez-Berestein, G.; et al. Calcium-dependent FAK/CREB/TNNC1 signalling mediates the effect of stromal MFAP5 on ovarian cancer metastatic potential. Nat. Commun. 2014, 5, 5092. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Jiang, Q. MFAP5 suppression inhibits migration/invasion, regulates cell cycle and induces apoptosis via promoting ROS production in cervical cancer. Biochem. Biophys. Res. Commun. 2018, 507, 51–58. [Google Scholar] [CrossRef]
- Neill, T.; Iozzo, R.V. The Role of Decorin Proteoglycan in Mitophagy. Cancers 2022, 14, 804. [Google Scholar] [CrossRef]
- Diehl, V.; Huber, L.S.; Trebicka, J.; Wygrecka, M.; Iozzo, R.V.; Schaefer, L. The Role of Decorin and Biglycan Signaling in Tumorigenesis. Front. Oncol. 2021, 11, 801801. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Zeng, L.; Li, J.; Klionsky, D.J.; Kroemer, G.; Jiang, J.; Tang, D.; Kang, R. DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy 2022, 18, 2036–2049. [Google Scholar] [CrossRef]
- An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [CrossRef]
- Zhang, Z.; Tang, J.; He, X.; Zhu, M.; Gan, S.; Guo, X.; Zhang, X.; Zhang, J.; Hu, W.; Chu, M. Comparative Transcriptomics Identify Key Hypothalamic Circular RNAs that Participate in Sheep (Ovis aries) Reproduction. Animals 2019, 9, 557. [Google Scholar] [CrossRef]
- Farkas, I.; Kalló, I.; Deli, L.; Vida, B.; Hrabovszky, E.; Fekete, C.; Moenter, S.M.; Watanabe, M.; Liposits, Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 5818–5829. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Fang, H.; Xu, Y.; Ding, M.; Ma, C.; Lin, Y.; Cui, Z.; Sun, H.; Niu, Q.; et al. A γ-glutamyl hydrolase lacking the signal peptide confers susceptibility to folates/antifolates in acute lymphoblastic leukemia cells. FEBS Lett. 2022, 596, 437–448. [Google Scholar] [CrossRef]
- Zhai, M.; Zhao, Z.; Yang, M.; Liang, Y.; Liang, H.; Xie, Y.; Han, J. The effect of GNAQ methylation on GnRH secretion in sheep hypothalamic neurons. J. Cell. Biochem. 2019, 120, 19396–19405. [Google Scholar] [CrossRef]
- Yang, N.V.; Pannia, E.; Chatterjee, D.; Kubant, R.; Ho, M.; Hammoud, R.; Pausova, Z.; Anderson, G.H. Gestational folic acid content alters the development and function of hypothalamic food intake regulating neurons in Wistar rat offspring post-weaning. Nutr. Neurosci. 2020, 23, 149–160. [Google Scholar] [CrossRef]
- Du, X.; He, X.; Liu, Q.; Di, R.; Liu, Q.; Chu, M. Comparative Transcriptomics Reveals the Key lncRNA and mRNA of Sunite Sheep Adrenal Gland Affecting Seasonal Reproduction. Front. Vet. Sci. 2022, 9, 816241. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. TEM 2017, 28, 399–415. [Google Scholar] [CrossRef]
- Ebbesen, K.K.; Kjems, J.; Hansen, T.B. Circular RNAs: Identification, biogenesis and function. Biochim. Biophys. Acta 2016, 1859, 163–168. [Google Scholar] [CrossRef]
- Santulli, G.; Pagano, G.; Sardu, C.; Xie, W.; Reiken, S.; D’Ascia, S.L.; Cannone, M.; Marziliano, N.; Trimarco, B.; Guise, T.A.; et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J. Clin. Investig. 2015, 125, 1968–1978. [Google Scholar] [CrossRef]
- Pal, L.; Chu, H.P.; Shu, J.; Topalli, I.; Santoro, N.; Karkanias, G. In vitro evidence of glucose-induced toxicity in GnRH secreting neurons: High glucose concentrations influence GnRH secretion, impair cell viability, and induce apoptosis in the GT1-1 neuronal cell line. Fertil. Steril. 2007, 88, 1143–1149. [Google Scholar] [CrossRef]
- Frattarelli, J.L.; Krsmanovic, L.Z.; Catt, K.J. The relationship between pulsatile GnRH secretion and cAMP production in immortalized GnRH neurons. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1022–E1030. [Google Scholar] [CrossRef]
- Vitalis, E.A.; Costantin, J.L.; Tsai, P.S.; Sakakibara, H.; Paruthiyil, S.; Iiri, T.; Martini, J.F.; Taga, M.; Choi, A.L.; Charles, A.C.; et al. Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1861–1866. [Google Scholar] [CrossRef]
- Amin, H.S.; Parikh, P.K.; Ghate, M.D. Medicinal chemistry strategies for the development of phosphodiesterase 10A (PDE10A) inhibitors—An update of recent progress. Eur. J. Med. Chem. 2021, 214, 113155. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Lomniczi, A.; Sandau, U. Contribution of glial-neuronal interactions to the neuroendocrine control of female puberty. Eur. J. Neurosci. 2010, 32, 2003–2010. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Hou, B.; Yang, C.; Li, Y.; Sun, B.; Guo, Y.; Deng, M.; Liu, D.; Liu, G. Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats. Genes 2023, 14, 444. https://doi.org/10.3390/genes14020444
Dong S, Hou B, Yang C, Li Y, Sun B, Guo Y, Deng M, Liu D, Liu G. Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats. Genes. 2023; 14(2):444. https://doi.org/10.3390/genes14020444
Chicago/Turabian StyleDong, Shucan, Biwei Hou, Chuang Yang, Yaokun Li, Baoli Sun, Yongqing Guo, Ming Deng, Dewu Liu, and Guangbin Liu. 2023. "Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats" Genes 14, no. 2: 444. https://doi.org/10.3390/genes14020444
APA StyleDong, S., Hou, B., Yang, C., Li, Y., Sun, B., Guo, Y., Deng, M., Liu, D., & Liu, G. (2023). Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats. Genes, 14(2), 444. https://doi.org/10.3390/genes14020444




