Competition for Light Interception in Different Plant Canopy Characteristics of Diverse Cotton Cultivars
Abstract
1. Introduction
- (1)
- Evaluate the developing features of different canopy structures throughout the growth period;
- (2)
- Determine the cumulative changes in leaf area index, associated with radiation interception in various cotton cultivars and biomass.
2. Materials and Methods
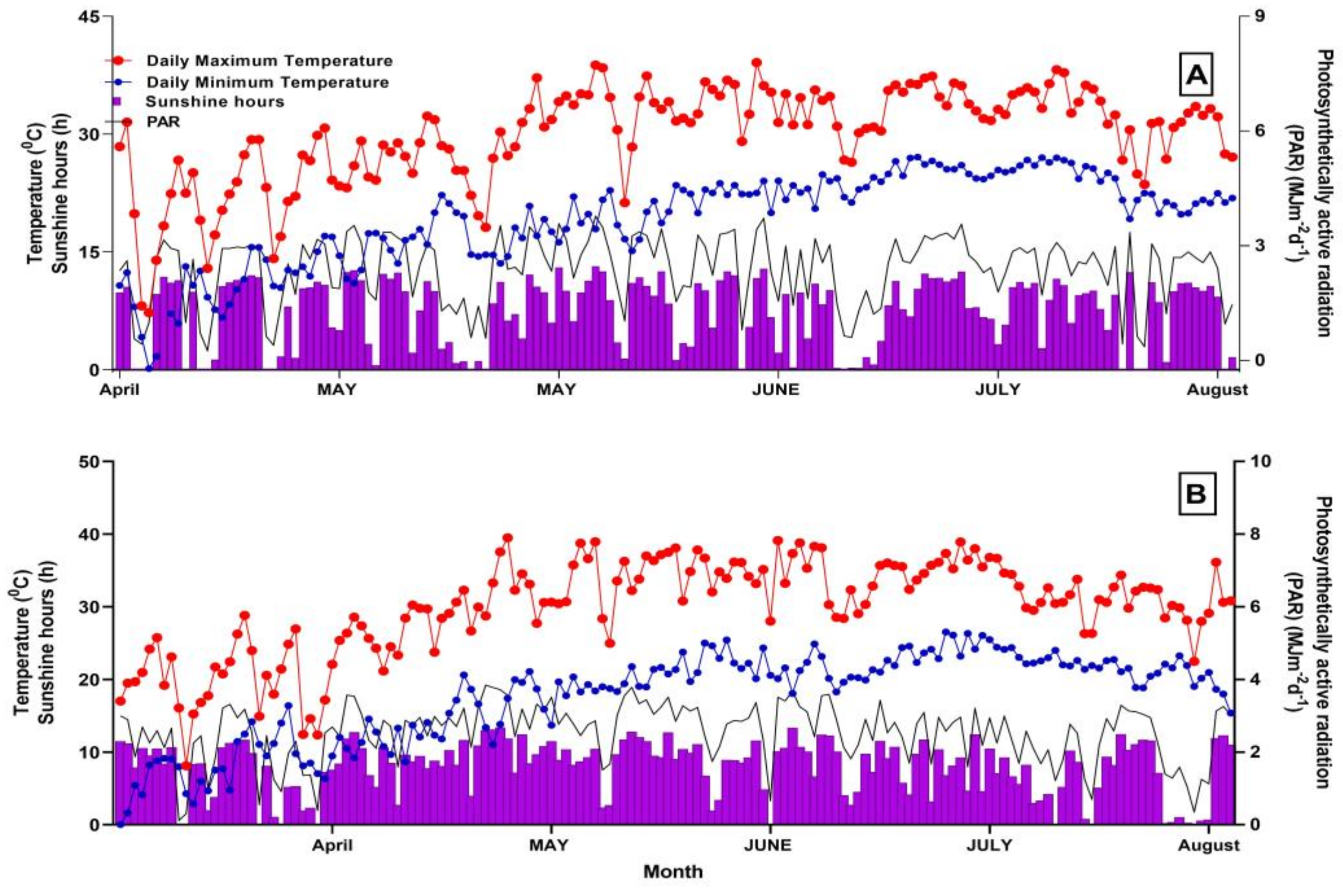
2.1. Experimental Site
2.2. Experimental Design
2.3. Data Collection
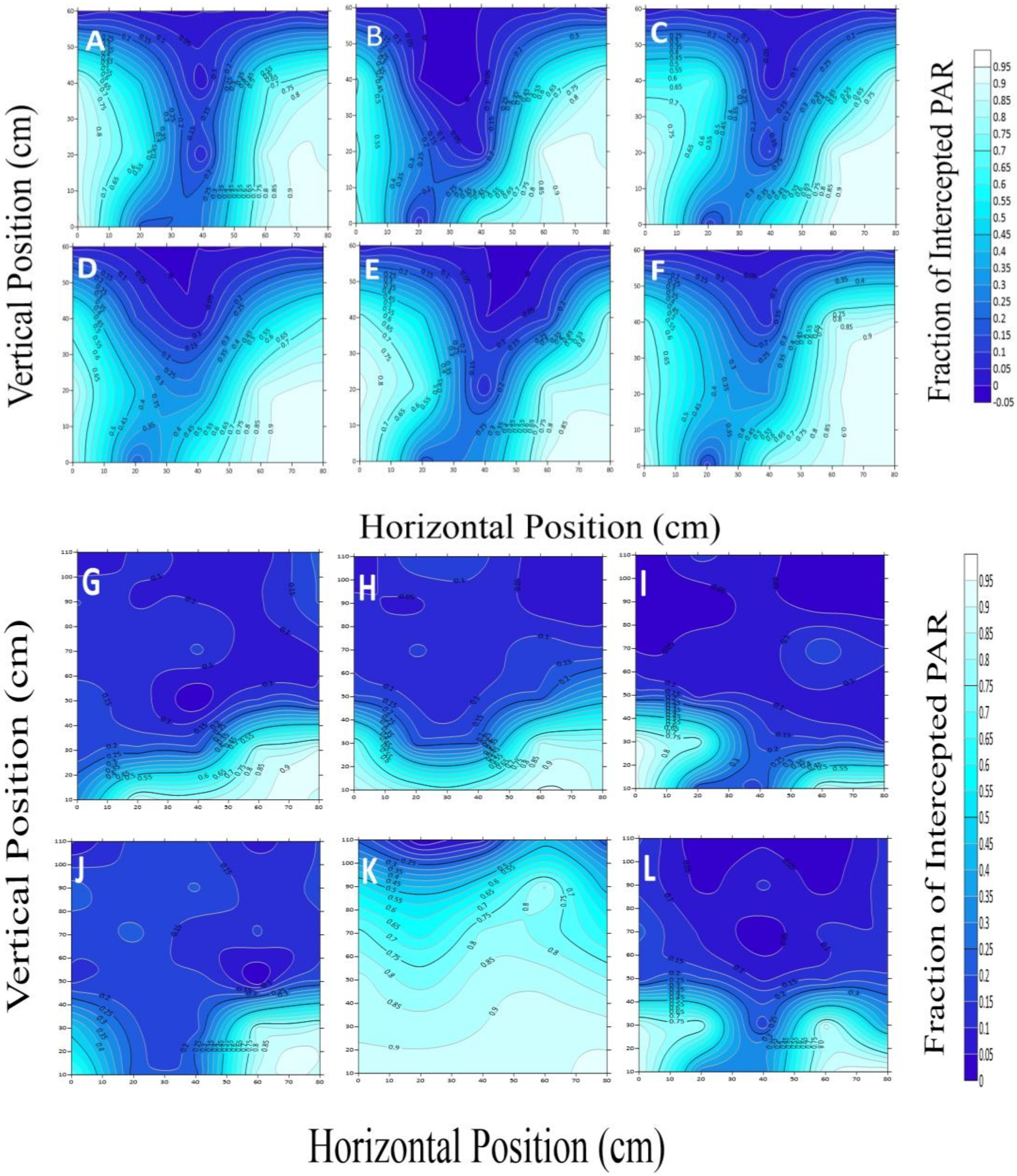
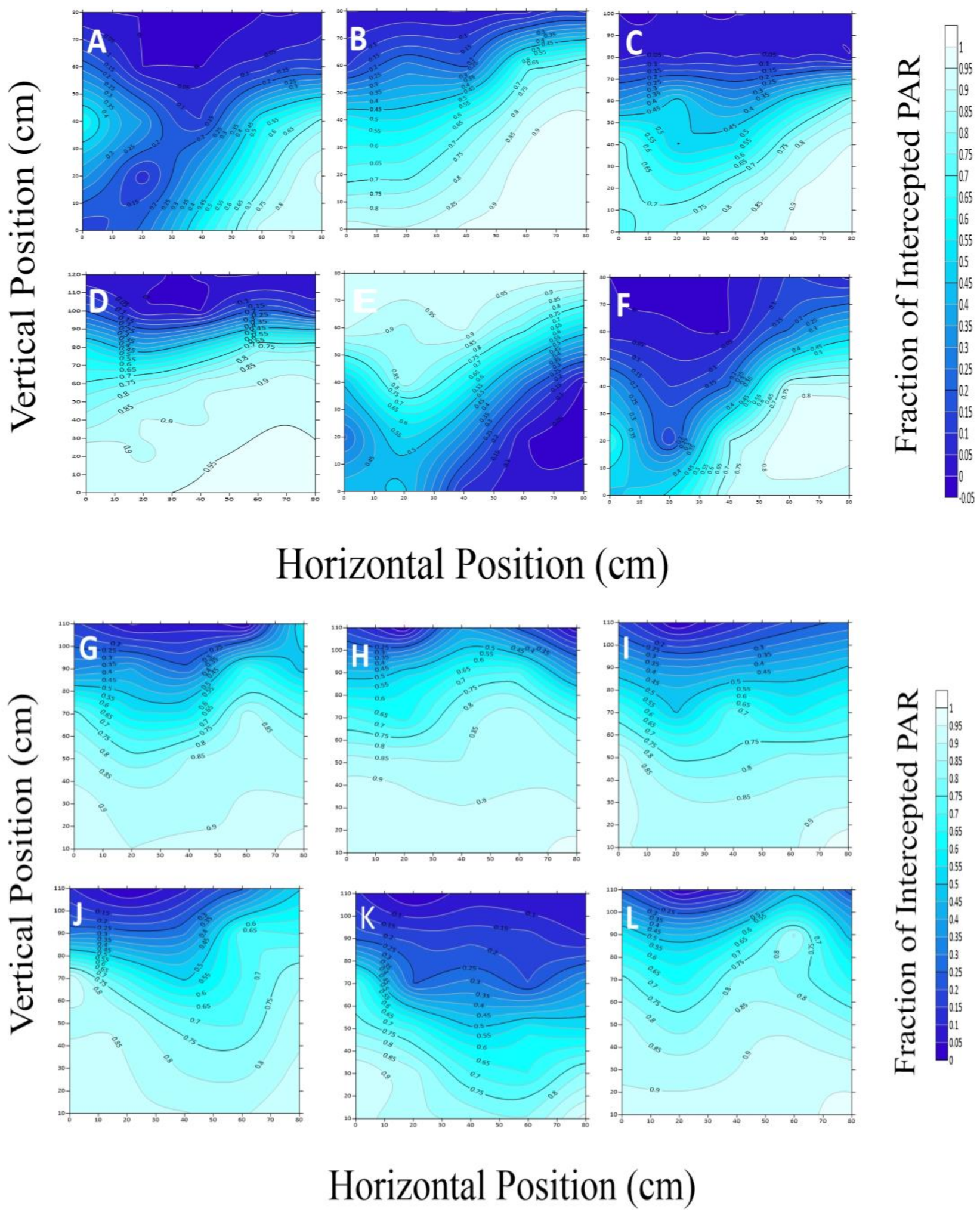
2.3.1. PAR Interception and Transmission in the Canopy
2.3.2. Estimation of PAR Distribution in the Canopy
2.3.3. Calculation of Accumulated tPAR within the Whole Canopy
2.3.4. Leaf Area Index (LAI), Biomass Accumulation and Duration of Growth Period
2.4. Yield and Yield Component Determination
2.5. Statistical Analysis
3. Results
3.1. The Terrestrial Distribution Features of Canopy PAR
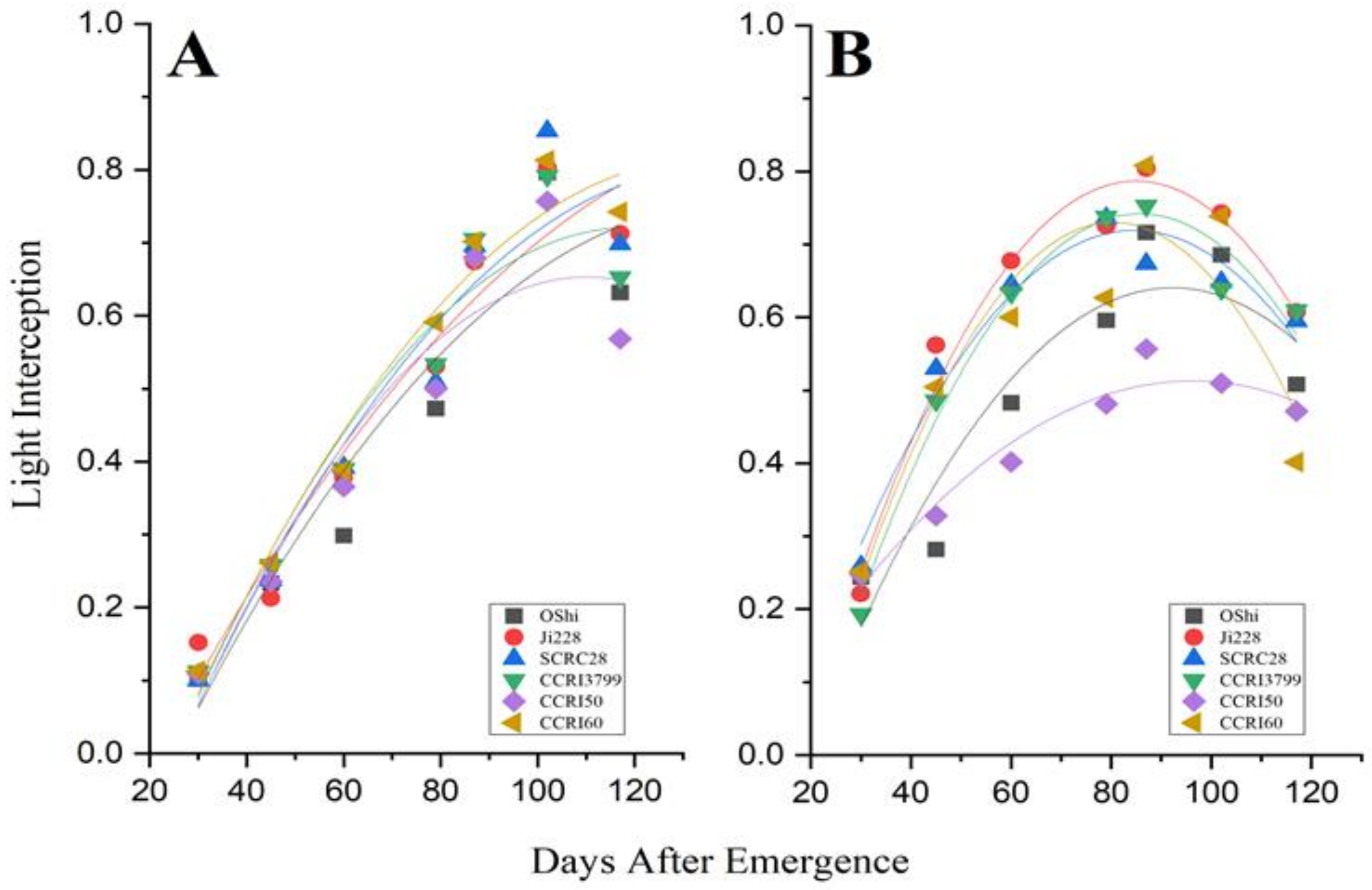
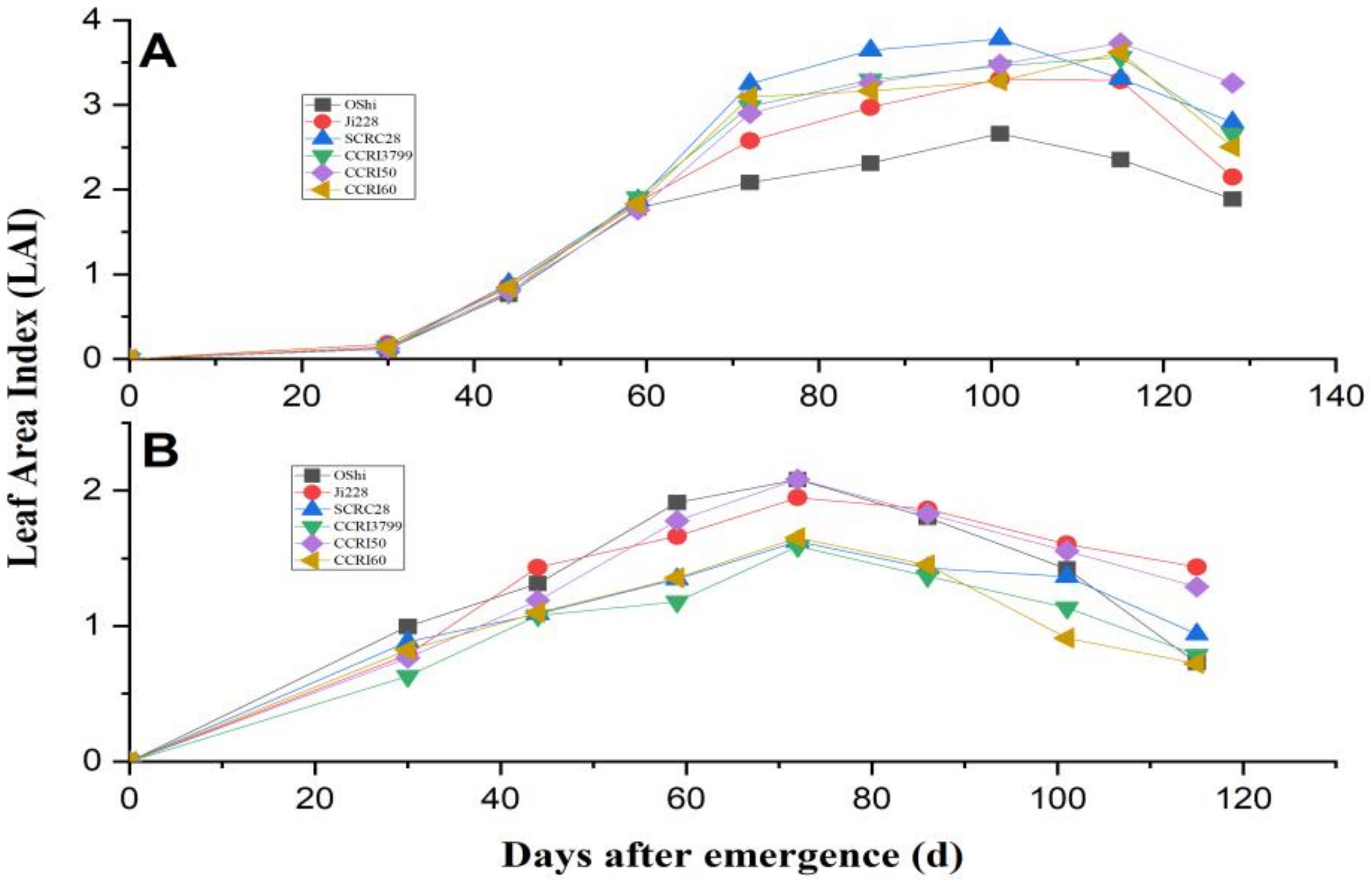
3.2. LAI Development and Biomass Accumulation in Various Cultivars
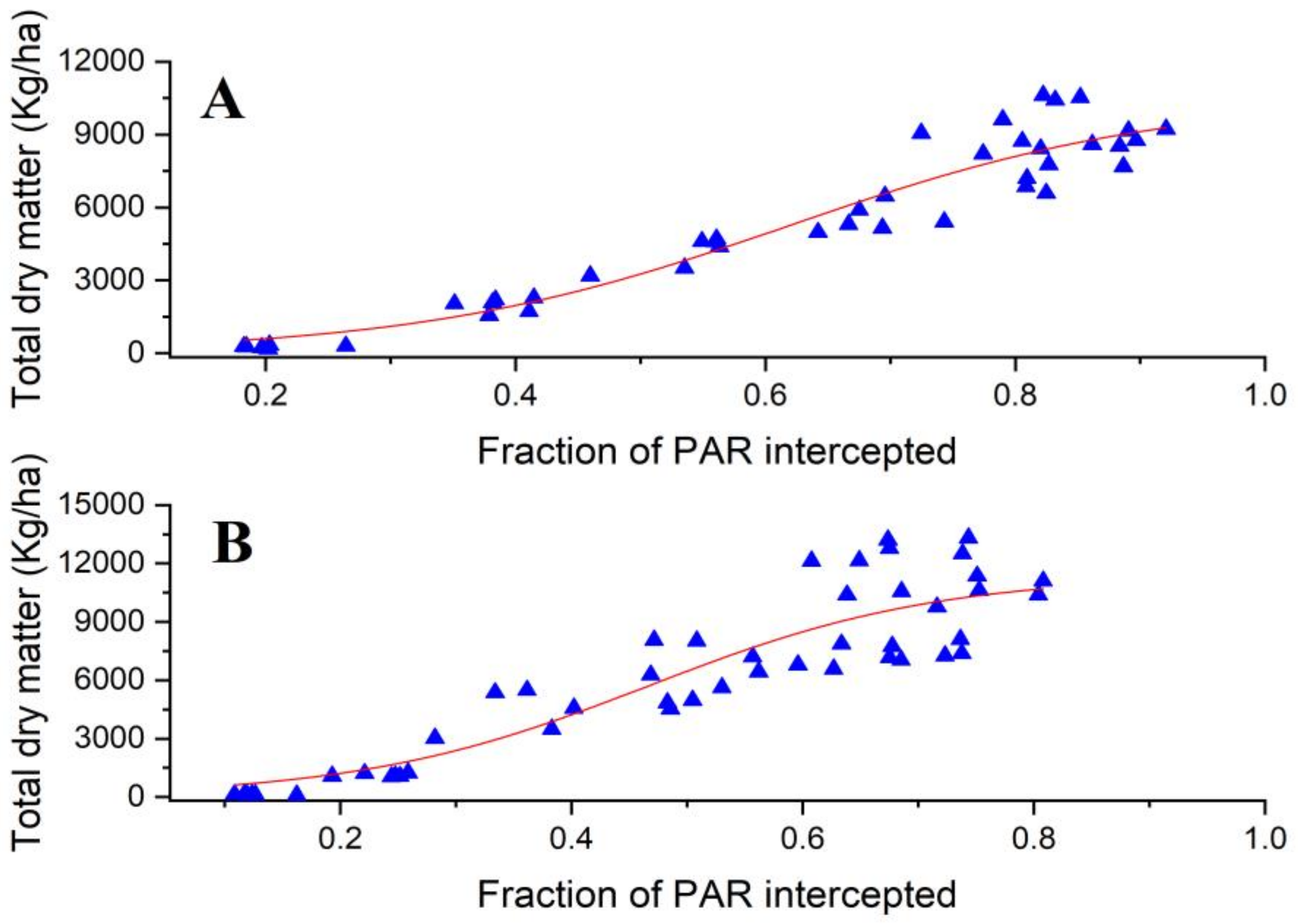
3.3. The Relationships and Fitted Models for Leaf Area Index, Total Dry Matter and Fraction of Intercepted PAR
3.4. Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, U.F.; Khalid, M.N.; Aziz, S.; Amjad, I.; Khalid, A.; Noor, H.; Sajid, H.B. Genetic studies in different F2 segregating population for yield and fiber quality traits in cotton (Gossypium hirsutum L.). Int. J. Agri. Biosci. 2022, 11, 11. [Google Scholar]
- Zafar, M.M.; Manan, A.; Razzaq, A.; Zulfqar, M.; Saeed, A.; Kashif, M.; Khan, A.I.; Sarfraz, Z.; Mo, H.; Iqbal, M.S.J.A. Exploiting Agronomic and Biochemical Traits to Develop Heat Resilient Cotton Cultivars under Climate Change Scenarios. Agronomy 2021, 11, 1885. [Google Scholar] [CrossRef]
- Zafar, M.M.; Zhang, Y.; Farooq, M.A.; Ali, A.; Firdous, H.; Haseeb, M.; Fiaz, S.; Shakeel, A.; Razzaq, A.; Ren, M.J.A. Biochemical and Associated Agronomic Traits in Gossypium hirsutum L. under High Temperature Stress. Agronomy 2022, 12, 1310. [Google Scholar] [CrossRef]
- Bashir, M.H.; Nawaz, M.S.; Khan, A.Z.; Aziz, S.; Ullah, F.; Qasim, M. Characterization and advancement of microsatellite (SSR) markers for various stresses in wheat. Int. J. Agri. Biosci. 2022, 11, 8. [Google Scholar]
- Khan, M.M.; Shakeel Nawaz, M.; Saeed, A.; Khan, M.A. Combining ability estimates of various morphological and quality traits of okra. Int. J. Agri. Biosci. 2022, 11, 9. [Google Scholar]
- Hassan, A.; Nasser, A.; Shahani, A.A.A.; Aziz, S.; Khalid, M.N.; Mushtaq, N.; Munir, M.A. Assessment of fiber and yield related traits in mutant population of cotton. Int. J. Agri. Biosci. 2022, 11, 8. [Google Scholar]
- Zafar, M.M.; Jia, X.; Shakeel, A.; Sarfraz, Z.; Manan, A.; Imran, A.; Mo, H.; Ali, A.; Youlu, Y.; Razzaq, A.J.F.i.P.S. Unraveling Heat Tolerance in Upland Cotton (Gossypium hirsutum L.) Using Univariate and Multivariate Analysis. Front. Plant Sci. 2021, 12, 727835. [Google Scholar] [CrossRef] [PubMed]
- Acreche, M.M.; Briceño-Félix, G.; Sánchez, J.A.M.; Slafer, G. Radiation interception and use efficiency as affected by breeding in Mediterranean wheat. Field Crop. Res. 2009, 110, 91–97. [Google Scholar] [CrossRef]
- Reta-Sánchez, D.G.; Fowler, J.L. Canopy Light Environment and Yield of Narrow-Row Cotton as Affected by Canopy Architecture. Agron. J. 2002, 94, 1317–1323. [Google Scholar] [CrossRef]
- Bai, Z.; Mao, S.; Han, Y.; Feng, L.; Wang, G.; Yang, B.; Zhi, X.; Fan, Z.; Lei, Y.; Du, W.; et al. Study on Light Interception and Biomass Production of Different Cotton Cultivars. PLoS ONE 2016, 11, e0156335. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Paterson, A.H. High throughput phenotyping of cotton plant height using depth images under field conditions. Comput. Electron. Agric. 2016, 130, 57–68. [Google Scholar] [CrossRef]
- Feng, G.; Luo, H.; Zhang, Y.; Gou, L.; Yao, Y.; Lin, Y.; Zhang, W. Relationship between plant canopy characteristics and photosynthetic productivity in diverse cultivars of cotton (Gossypium hirsutum L.). Crop. J. 2016, 4, 499–508. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N.; Robinson, T.L.; Lenz, F.; Denning, S.S. The Bases of Productivity in Apple Production Systems: The Role of Light Interception by Different Shoot Types. J. Am. Soc. Hortic. Sci. 1996, 121, 886–893. [Google Scholar] [CrossRef]
- Louarn, G.; Lecoeur, J.; Lebon, E. A Three-dimensional Statistical Reconstruction Model of Grapevine (Vitis vinifera) Simulating Canopy Structure Variability within and between Cultivar/Training System Pairs. Ann. Bot. 2007, 101, 1167–1184. [Google Scholar] [CrossRef]
- Monteith, J.L. Climate and the efficiency of crop production in Britain. Philos Trans. R. Soc. Lond. B Biol. Sci. 1977, 281, 277–294. [Google Scholar] [CrossRef]
- Vos, J.; Evers, J.; Buck-Sorlin, G.H.; Andrieu, B.; Chelle, M.; De Visser, P.H.B. Functional–structural plant modelling: A new versatile tool in crop science. J. Exp. Bot. 2010, 61, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- Rey, H.; Dauzat, J.; Chenu, K.; Barczi, J.-F.; Dosio, G.A.A.; Lecoeur, J. Using a 3-D Virtual Sunflower to Simulate Light Capture at Organ, Plant and Plot Levels: Contribution of Organ Interception, Impact of Heliotropism and Analysis of Genotypic Differences. Ann. Bot. 2008, 101, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Sinoquet, H.; Thanisawanyangkura, S.; Mabrouk, H.; Kasemsap, P. Characterization of the Light Environment in Canopies Using 3D Digitising and Image Processing. Ann. Bot. 1998, 82, 203–212. [Google Scholar] [CrossRef]
- Melo, J.D.; Carreno, E.M.; Calviño, A.; Padilha-Feltrin, A. Determining spatial resolution in spatial load forecasting using a grid-based model. Electr. Power Syst. Res. 2014, 111, 177–184. [Google Scholar] [CrossRef]
- Li, Y.; Mao, S.; Feng, L.; Han, Y.; Wang, G.; Fan, Z.; Sun, E. Spatial distribution characteristics of photosynthetic active radiation in cotton canopy based on geo-statistics. Trans. Chin. Soc. Agric. Eng. 2012, 28, 200–206. [Google Scholar]
- Zhi, X.; Han, Y.; Mao, S.; Wang, G.; Feng, L.; Yang, B.; Fan, Z.; Du, W.; Lü, J.; Li, Y. Light Spatial Distribution in the Canopy and Crop Development in Cotton. PLoS ONE 2014, 9, e113409. [Google Scholar] [CrossRef]
- Mariscal, M.; Orgaz, F.; Villalobos, F. Modelling and measurement of radiation interception by olive canopies. Agric. For. Meteorol. 2000, 100, 183–197. [Google Scholar] [CrossRef]
- Xing, F.; Han, Y.; Feng, L.; Zhi, X.; Wang, G.; Yang, B.; Fan, Z.; Lei, Y.; DU, W.; Wang, Z.; et al. Genotypic variation in spatiotemporal distribution of canopy light interception in relation to yield formation in cotton. J. Cotton Res. 2018, 1, 13. [Google Scholar] [CrossRef]
- Reynolds, M.P.; B, M.D.; Gutiérrez-Rodríguez, M.; Saavedra, A.L. Photosynthesis of wheat in a warm, irrigated environment: I: Genetic diversity and crop productivity. Field Crop. Res. 2000, 66, 37–50. [Google Scholar] [CrossRef]
- Chenu, K.; Franck, N.; Dauzat, J.; Barczi, J.-F.; Rey, H.; Lecoeur, J. Integrated responses of rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Funct. Plant Biol. 2005, 32, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Gutiérrez, A.J.; Combes, D.; Rakocevic, M.; de Berranger, C.; Eprinchard-Ciesla, A.; Sinoquet, H.; Varlet-Grancher, C. Functional relationships to estimate Morphogenetically Active Radiation (MAR) from PAR and solar broadband irradiance measurements: The case of a sorghum crop. Agric. For. Meteorol. 2009, 149, 1244–1253. [Google Scholar] [CrossRef]
- Fila, G.; Sartorato, I. Using Leaf Mass per Area as predictor of light interception and absorption in crop/weed monoculture or mixed stands. Agric. For. Meteorol. 2011, 151, 575–584. [Google Scholar] [CrossRef]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. Light interception and radiation use efficiency of okra and normal leaf cotton isolines. Environ. Exp. Bot. 2011, 72, 217–222. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Paterson, A.H.; Sun, S.; Xu, R.; Robertson, J. Quantitative Analysis of Cotton Canopy Size in Field Condi-tions Using a Consumer-Grade RGB-D Camera. Front. Plant Sci. 2018, 8, 2233. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hanan, J.S.; Room, P.M.; Hasegawa, T.; Nakagawa, H.; Takahashi, W. Rice Morphogenesis and Plant Architecture: Measurement, Specification and the Reconstruction of Structural Development by 3D Architectural Modelling. Ann. Bot. 2005, 95, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Han, Y.; Li, Y.; Wang, G.; Feng, L.; Fan, Z.; Du, W.; Yang, B.; Cao, C.; Mao, S. Spatial distribution of light interception by different plant population densities and its relationship with yield. Field Crop. Res. 2015, 184, 17–27. [Google Scholar] [CrossRef]
- Zarate-Valdez, J.L.; Whiting, M.L.; Lampinen, B.D.; Metcalf, S.; Ustin, S.; Brown, P. Prediction of leaf area index in almonds by vegetation indexes. Comput. Electron. Agric. 2012, 85, 24–32. [Google Scholar] [CrossRef]
- Vargas, L.; Andersen, M.; Jensen, C.; Jørgensen, U. Estimation of leaf area index, light interception and biomass accumulation of Miscanthus sinensis ‘Goliath’ from radiation measurements. Biomass Bioenergy 2002, 22, 1–14. [Google Scholar] [CrossRef]
- Maddonni, G.; Otegui, M. Leaf area, light interception, and crop development in maize. Field Crop. Res. 1996, 48, 81–87. [Google Scholar] [CrossRef]
- Ruiz, R.; Bertero, H. Light interception and radiation use efficiency in temperate quinoa (Chenopodium quinoa Willd) cultivars . Eur. J. Agron. 2008, 29, 144–152. [Google Scholar] [CrossRef]
- Girma, K.; Teal, R.K.; Freeman, K.W.; Boman, R.K.; Raun, W.R. Cotton lint yield and quality as affected by applications of N, P, and K fertilizers. J. Cotton Sci. 2007, 11, 12–19. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201300769319 (accessed on 20 December 2022).
- Wang, Y.; Ye, G.; Luan, N.; Xiao, J.; Chen, Y.; Chen, D. Boll size affects the insecticidal protein content in Bacillus thuringiensis (Bt) cotton. Field Crop. Res. 2009, 110, 106–110. [Google Scholar] [CrossRef]

| Year 2018 | |||||
|---|---|---|---|---|---|
| Seeding Date | Squaring Stage | Flowering Stage | Boll-Opening Stage | Duration (Days) | |
| OShi | 20-Apr | 29-May | 26-Jun | 6-Aug | 108 |
| Ji228 | 20-Apr | 31-May | 26-Jun | 11-Aug | 113 |
| SCRC28 | 20-Apr | 29-May | 25-Jun | 7-Aug | 109 |
| CCRI3799 | 20-Apr | 31-May | 26-Jun | 8-Aug | 110 |
| CCRI50 | 20-Apr | 26-May | 22-Jun | 1-Aug | 103 |
| CCRI60 | 20-Apr | 31-May | 25-Jun | 6-Aug | 108 |
| Year 2019 | |||||
| OShi | 18-Apr | 1-Jun | 27-Jun | 16-Aug | 120 |
| Ji228 | 18-Apr | 2-Jun | 29-Jun | 20-Aug | 124 |
| SCRC28 | 18-Apr | 31-May | 25-Jun | 20-Aug | 124 |
| CCRI3799 | 18-Apr | 31-May | 29-Jun | 19-Aug | 123 |
| CCRI50 | 18-Apr | 22-May | 15-Jun | 7-Aug | 111 |
| CCRI60 | 18-Apr | 2-Jun | 27-Jun | 22-Aug | 126 |
| Year 2018, N = 7 | R2 | Year 2019, N = 7 | R2 | |
|---|---|---|---|---|
| Equation | Equation | |||
| OShi | Y= −0.000057X2 + 0.01602X − 0.03672 | 0.88 | Y= −0.00012X2 + 0.02215X − 0.38134 | 0.86 |
| Ji228 | Y= −0.000045X2 + 0.01443X − 0.29045 | 0.93 | Y= −0.00017X2 + 0.03004X − 0.48937 | 0.96 |
| SCRC28 | Y= −0.000065X2 + 0.01777X − 0.40792 | 0.92 | Y= −0.00014X2 + 0.02443X − 0.31470 | 0.95 |
| CCRI3799 | Y= −0.000081X2 + 0.01942X − 0.43106 | 0.93 | Y= −0.00016X2 + 0.02909X − 0.50655 | 0.97 |
| CCRI50 | Y= −0.000091X2 + 0.02017X − 0.45775 | 0.89 | Y= −0.00064X2 + 0.01244X − 0.08539 | 0.94 |
| CCRI60 | Y= −0.000067X2 + 0.01806X − 0.39894 | 0.96 | Y= −0.00019X2 + 0.03127X − 0.52644 | 0.84 |
| Year 2018 | R2 | Year 2019 | R2 | |
|---|---|---|---|---|
| Equation | Equation | |||
| OShi | Y = −0.00032X2 + 0.04392X − 0.08444 | 0.92 | Y = −0.00068X2 + 0.13385X − 3.48763 | 0.94 |
| Ji228 | Y = −0.00029X2 + 0.04712X − 0.10468 | 0.95 | Y = −0.00054X2 + 0.12217X − 3.27755 | 0.97 |
| SCRC28 | Y = −0.00043X2 + 0.05881X − 0.1593 | 0.92 | Y = −0.00070X2 + 0.14243 − 3.79326 | 0.96 |
| CCRI3799 | Y = −0.00026X2 + 0.0404X − 0.05105 | 0.96 | Y = −0.00070X2 + 0.14147 − 3.7355 | 0.94 |
| CCRI50 | Y = −0.00026X2 + 0.03937X − 0.10683 | 0.91 | Y = −0.00054X2 + 0.1063X − 2.68308 | 0.98 |
| CCRI60 | Y = −0.00031X2 + 0.04948X − 0.16102 | 0.91 | Y = −0.00080X2 + 0.15809 − 4.21312 | 0.95 |
| Year 2018 | Year 2019 | |||
|---|---|---|---|---|
| Equation | R2 | Equation | R2 | |
| OShi | Y = 8442.43475/(1 + 212.37688e−0.07082X) | 0.99 | Y = 11166.84/(1 + 147.69962e−0.06458X) | 0.98 |
| Ji228 | Y = 10932.71558/(1 + 111.96196e−0.06114X) | 0.98 | Y = 24945.82/(1 + 135.04495e−0.04143X) | 0.98 |
| SCRC28 | Y = 10887.38529/(1 + 115.46423e−0.06098X) | 0.97 | Y = 13404.76/(1 + 1485.80753e−0.08578X) | 0.96 |
| CCRI3799 | Y = 9238.54557/(1 + 249.42951e−0.07848X) | 0.98 | Y = 14920.98/(1 + 126.1678e−0.04995X) | 0.96 |
| CCRI50 | Y = 9593.09873/(1 + 127.34178e−0.06266X) | 0.99 | Y = 10283.46/(1 + 538.99072e−0.07553X) | 0.97 |
| CCRI60 | Y = 12613.49407/(1 + 43.72154e−0.04241X) | 0.97 | y = 16753.47/(1 + 123.76809e−0.04891X) | 0.97 |
| Treatment | Seed Cotton Yield | Lint Yield | Lint Percentage |
|---|---|---|---|
| Year 2018 | |||
| OShi | 2929.8 ± 51.7e | 1187.0 ± 43.4b | 39.3 ± 0.47abc |
| Ji228 | 3282.3 ± 32.0c | 1219.4 ± 43.4ab | 40.2 ± 0.41ab |
| SCRC28 | 3889.1 ± 87.5a | 1289.4 ± 94.1ab | 38.3 ± 2.10c |
| CCRI3799 | 3115.9 ± 70.3d | 1067.9 ± 61.2c | 39.5 ± 0.73abc |
| CCRI50 | 2506.1 ± 75.9f | 1037.8 ± 56.6c | 40.9 ± 0.19a |
| CCRI60 | 3696.1 ± 19.0b | 1325.4 ± 78.9a | 38.7 ± 1.01bc |
| Year 2019 | |||
| OShi | 3786.1 ± 18.1c | 1513.4 ± 56.4b | 41.4 ± 0.42ab |
| Ji228 | 4318.4 ± 57.2b | 1627.1 ± 63.8a | 42.1 ± 0.62ab |
| SCRC28 | 4474.6 ± 83.5a | 1565.9 ± 80.6ab | 42.1 ± 0.81ab |
| CCRI3799 | 3808.3 ± 54.1c | 1513.9 ± 40.7b | 41.7 ± 0.78ab |
| CCRI50 | 3086.3 ± 48.9d | 1249.3 ± 78.7c | 42.6 ± 0.25a |
| CCRI60 | 4295.1 ± 77.6b | 1595.9 ± 53.6ab | 40.9 ± 1.41b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, F.; Dev, W.; Xin, M.; Han, Y.; Feng, L.; Lei, Y.; Yang, B.; Wang, G.; Li, X.; Wang, Z.; et al. Competition for Light Interception in Different Plant Canopy Characteristics of Diverse Cotton Cultivars. Genes 2023, 14, 364. https://doi.org/10.3390/genes14020364
Sultana F, Dev W, Xin M, Han Y, Feng L, Lei Y, Yang B, Wang G, Li X, Wang Z, et al. Competition for Light Interception in Different Plant Canopy Characteristics of Diverse Cotton Cultivars. Genes. 2023; 14(2):364. https://doi.org/10.3390/genes14020364
Chicago/Turabian StyleSultana, Fahmida, Washu Dev, Minghua Xin, Yingchun Han, Lu Feng, Yaping Lei, Beifang Yang, Guoping Wang, Xiaofei Li, Zhanbiao Wang, and et al. 2023. "Competition for Light Interception in Different Plant Canopy Characteristics of Diverse Cotton Cultivars" Genes 14, no. 2: 364. https://doi.org/10.3390/genes14020364
APA StyleSultana, F., Dev, W., Xin, M., Han, Y., Feng, L., Lei, Y., Yang, B., Wang, G., Li, X., Wang, Z., Xing, F., Xiong, S., & Li, Y. (2023). Competition for Light Interception in Different Plant Canopy Characteristics of Diverse Cotton Cultivars. Genes, 14(2), 364. https://doi.org/10.3390/genes14020364






