Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Case Presentation
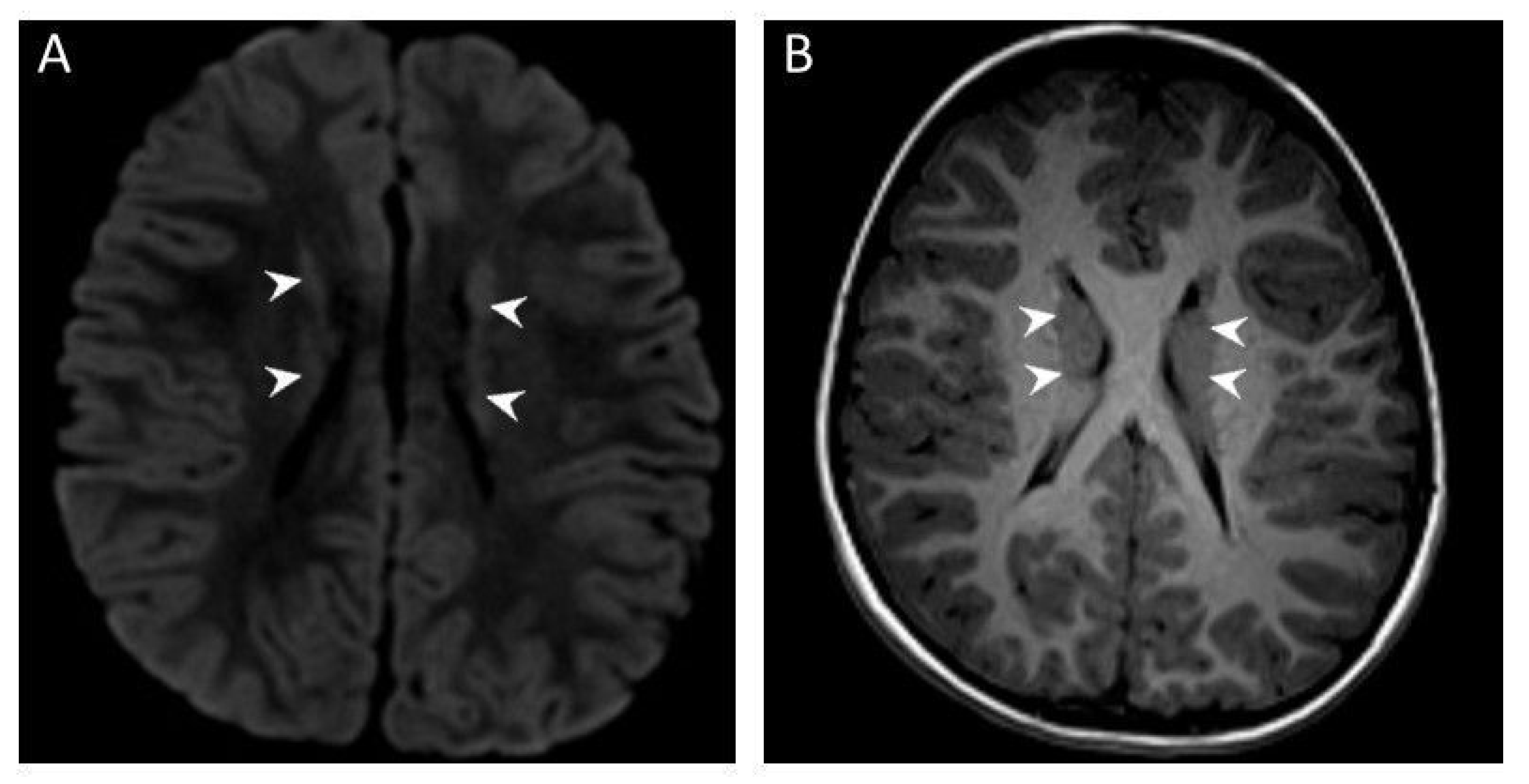
3.2. Brain MRI Results
3.3. Genetic Results
4. Discussion
| Study | Diagnosis (Patient Cohort) | Inheritance | Genomic Position (hg19) | Variant Type (NM_003611.2) | Effect (NP_003602.1) | Affected Exons |
|---|---|---|---|---|---|---|
| Krumm, 2015 [72] | ASD (SSC collection) | De novo | chrX:13774696 | c.1222-1G>T | Disruption of splice acceptor site of intron 12 | Exon 13 |
| Li, 2017 [73] | ASD (ASC/SSC collection) | Inherited | chrX:13771497 | c.1066G>C | p.Glu356Gln | Exon 11 |
| Sakakibara 2018 [56] | ASD (own cohort) | Inherited | chrX:13778441 | c.2260+2T>G | Disruption of splice donor site of intron 16 | Exons 16 and 17 |
| Tran, 2020 [74] | ASD (own cohort) | Inherited | chrX:13778788 | c.2209A>G (rs778936071) | p.Thr737Ala | Exon 16 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Online Mendelian Inherintance in Man. Available online: https://omim.org/ (accessed on 4 November 2022).
- Papillon-Leage, M.; Psaume, J. Une malformation hereditaire de la muqueuse buccale: Brides et freins anormaux. Rev. Stomatol. 1954, 55, 209–227. [Google Scholar]
- Gorlin, R.J.; Psaume, J. Orodigitofacial dysostosis—A new syndrome. J. Pediat. 1962, 61, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Donnai, D.; Kerzin-Storrar, L.; Harris, R. Familial orofaciodigital syndrome type I presenting as adult polycystic kidney disease. J. Med. Genet. 1987, 24, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.I.; Giorgio, G.; Feather, S.A.; Bulfone, A.; Wright, V.; Ghiani, M.; Selicorni, A.; Gammaro, L.; Scolari, F.; Woolf, A.S.; et al. Identification of the gene for oral-facial-digital type I syndrome. Am. J. Hum. Genet. 2001, 68, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Fauth, C.; Steindl, K.; Toutain, A.; Farrell, S.; Witsch-Baumgartner, M.; Karall, D.; Joset, P.; Böhm, S.; Baumer, A.; Maier, O.; et al. A recurrent germline mutation in the PIGA gene causes Simpson-Golabi-Behmel syndrome type 2. Am. J. Med. Genet. A 2016, 170A, 392–402. [Google Scholar] [CrossRef]
- Fauth, C.; Toutain, A. Comment on “Whole exome sequencing and array-based molecular karyotyping as aids to prenatal diagnosis in fetuses with suspected Simpson-Golabi-Behmel syndrome”. Prenat. Diagn. 2017, 37, 1055–1056. [Google Scholar] [CrossRef]
- Pezzella, N.; Bove, G.; Tammaro, R.; Franco, B. OFD1: One gene, several disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 57–71. [Google Scholar] [CrossRef]
- Ferrante, M.I.; Zullo, A.; Barra, A.; Bimonte, S.; Messaddeq, N.; Studer, M.; Dollé, P.; Franco, B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat. Genet. 2006, 38, 112–117. [Google Scholar] [CrossRef]
- Giorgio, G.; Alfieri, M.; Prattichizzo, C.; Zullo, A.; Cairo, S.; Franco, B. Functional characterization of the OFD1 protein reveals a nuclear localization and physical interaction with subunits of a chromatin remodeling complex. Mol. Biol. Cell. 2007, 18, 4397–4404. [Google Scholar] [CrossRef]
- Singla, V.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Reiter, J.F. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell. 2010, 18, 410–424. [Google Scholar] [CrossRef]
- Abramowicz, I.; Carpenter, G.; Alfieri, M.; Colnaghi, R.; Outwin, E.; Parent, P.; Thauvin-Robinet, C.; Iaconis, D.; Franco, B.; O’Driscoll, M. Oral-facial-digital syndrome type I cells exhibit impaired DNA repair; unanticipated consequences of defective OFD1 outside of the cilia network. Hum. Mol. Genet. 2017, 26, 19–32. [Google Scholar] [CrossRef]
- Guo, J.; Higginbotham, H.; Li, J.; Nichols, J.; Hirt, J.; Ghukasyan, V.; Anton, E.S. Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun. 2015, 6, 7857. [Google Scholar] [CrossRef]
- Hasenpusch-Theil, K.; Theil, T. Multifaceted roles of primary cilia in the development of the cerebral cortex. Front. Cell. Dev. Biol. 2021, 9, 630161. [Google Scholar] [CrossRef]
- Franco, B. Oral–facial–digital type I syndrome. In Ciliopathies: A Reference for Clinicians; Kenny, T.D., Beales, P.L., Eds.; Oxford Academic: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Bisschoff, I.J.; Zeschnigk, C.; Horn, D.; Wellek, B.; Rieß, A.; Wessels, M.; Willems, P.; Jensen, P.; Busche, A.; Bekkebraten, J.; et al. Novel mutations including deletions of the entire OFD1 gene in 30 families with type 1 orofaciodigital syndrome: A study of the extensive clinical variability. Hum. Mutat. 2013, 34, 237–247. [Google Scholar] [CrossRef]
- Del Giudice, E.; Macca, M.; Imperati, F.; D’Amico, A.; Parent, P.; Pasquier, L.; Layet, V.; Lyonnet, S.; Stamboul-Darmency, V.; Thauvin-Robinet, C.; et al. CNS involvement in OFD1 syndrome: A clinical, molecular, and neuroimaging study. Orphanet J. Rare Dis. 2014, 9, 74. [Google Scholar] [CrossRef]
- Odent, S.; Le Marec, B.; Toutain, A.; David, A.; Vigneron, J.; Tréguier, C.; Jouan, H.; Milon, J.; Fryns, J.P.; Verloes, A. Central nervous system malformations and early end-stage renal disease in oro-facio-digital syndrome type I: A review. Am. J. Med. Genet. 1998, 75, 389–394. [Google Scholar] [CrossRef]
- Holub, M.; Potocki, L.; Bodamer, O.A. Central nervous system malformations in oral-facial-digital syndrome, type 1. Am. J. Med. Genet. A 2005, 136, 218. [Google Scholar] [CrossRef]
- Dehghan Tezerjani, M.; Maroofian, R.; Vahidi Mehrjardi, M.Y.; Chioza, B.A.; Zamaninejad, S.; Kalantar, S.M.; Nori-Shadkam, M.; Ghadimi, H.; Baple, E.L.; Crosby, A.H.; et al. A novel mutation in the OFD1 gene in a family with oral-facial-digital syndrome type 1: A Case Report. Iran. J. Public Health 2016, 45, 1359–1366. [Google Scholar]
- Bruel, A.L.; Franco, B.; Duffourd, Y.; Thevenon, J.; Jego, L.; Lopez, E.; Deleuze, J.F.; Doummar, D.; Giles, R.H.; Johnson, C.A.; et al. Fifteen years of research on oral-facial-digital syndromes: From 1 to 16 causal genes. J. Med. Genet. 2017, 54, 371–380. [Google Scholar] [CrossRef]
- Miles, J.H. Autism spectrum disorders—A genetics review. Genet. Med. 2011, 13, 278–294. [Google Scholar] [CrossRef]
- Betancur, C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res. 2011, 1380, 42–77. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Abrahams, B.S.; Geschwind, D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008, 9, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, D.H. Genetics of autism spectrum disorders. Trends Cogn. Sci. 2011, 15, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Folstein, S.; Rutter, M. Infantile autism: A genetic study of 21 twin pairs. J. Child Psychol. Psychiatry 1977, 18, 297–321. [Google Scholar] [CrossRef]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef]
- Autism Genome Project Consortium; Szatmari, P.; Paterson, A.D.; Zwaigenbaum, L.; Roberts, W.; Brian, J.; Liu, X.Q.; Vincent, J.B.; Skaug, J.L.; Thompson, A.P.; et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007, 9, 319–328. [Google Scholar] [CrossRef]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Schaaf, C.P.; Betancur, C.; Yuen, R.K.C.; Parr, J.R.; Skuse, D.H.; Gallagher, L.; Bernier, R.A.; Buchanan, J.A.; Buxbaum, J.D.; Chen, C.A.; et al. A framework for an evidence-based gene list relevant to autism spectrum disorder. Nat. Rev. Genet. 2020, 21, 367–376. [Google Scholar] [CrossRef]
- Sebat, J.; Lakshmi, B.; Malhotra, D.; Troge, J.; Lese-Martin, C.; Walsh, T.; Yamrom, B.; Yoon, S.; Krasnitz, A.; Kendall, J.; et al. Strong association of de novo copy number mutations with autism. Science 2007, 316, 445–449. [Google Scholar] [CrossRef]
- Marshall, C.R.; Noor, A.; Vincent, J.B.; Lionel, A.C.; Feuk, L.; Skaug, J.; Shago, M.; Moessner, R.; Pinto, D.; Ren, Y.; et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008, 82, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e23. [Google Scholar] [CrossRef]
- Louvi, A.; Grove, E.A. Cilia in the CNS: The quiet organelle claims center stage. Neuron 2011, 69, 1046–1060. [Google Scholar] [CrossRef]
- Marley, A.; von Zastrow, M. A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PLoS ONE 2012, 7, e46647. [Google Scholar] [CrossRef]
- Karalis, V.; Donovan, K.E.; Sahin, M. Primary Cilia Dysfunction in Neurodevelopmental Disorders beyond Ciliopathies. J. Dev. Biol. 2022, 10, 54. [Google Scholar] [CrossRef]
- Migliavacca, E.; Golzio, C.; Männik, K.; Blumenthal, I.; Oh, E.C.; Harewood, L.; Kosmicki, J.A.; Loviglio, M.N.; Giannuzzi, G.; Hippolyte, L.; et al. A Potential Contributory Role for Ciliary Dysfunction in the 16p11.2 600 kb BP4-BP5 Pathology. Am. J. Hum. Genet. 2015, 96, 784–796. [Google Scholar] [CrossRef]
- Guemez-Gamboa, A.; Coufal, N.G.; Gleeson, J.G. Primary cilia in the developing and mature brain. Neuron 2014, 82, 511–521. [Google Scholar] [CrossRef]
- Patowary, A.; Won, S.Y.; Oh, S.J.; Nesbitt, R.R.; Archer, M.; Nickerson, D.; Raskind, W.H.; Bernier, R.; Lee, J.E.; Brkanac, Z. Family-based exome sequencing and case-control analysis implicate CEP41 as an ASD gene. Transl. Psychiatry 2019, 9, 4. [Google Scholar] [CrossRef]
- Pruski, M.; Lang, B. Primary cilia—An underexplored topic in major mental illness. Front. Psychiatry 2019, 10, 104. [Google Scholar] [CrossRef]
- Alhassen, W.; Chen, S.; Vawter, M.; Robbins, B.K.; Nguyen, H.; Myint, T.N.; Saito, Y.; Schulmann, A.; Nauli, S.M.; Civelli, O.; et al. Patterns of cilia gene dysregulations in major psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 11025. [Google Scholar] [CrossRef]
- Budisteanu, M.; Papuc, S.M.; Erbescu, A.; Iliescu, C.; Dobre, M.; Barca, D.; Tarta-Arsene, O.; Motoescu, C.; Dica, A.; Sandu, C.; et al. Clinical and genomic findings in brain heterotopia: Report of a pediatric patient cohort from Romania. Exp. Ther. Med. 2022, 23, 101. [Google Scholar] [CrossRef]
- Oda, H.; Sato, T.; Kunishima, S.; Nakagawa, K.; Izawa, K.; Hiejima, E.; Kawai, T.; Yasumi, T.; Doi, H.; Katamura, K.; et al. Exon skipping causes atypical phenotypes associated with a loss-of-function mutation in FLNA by restoring its protein function. Eur. J. Hum. Genet. 2016, 24, 408–414. [Google Scholar] [CrossRef]
- Papuc, S.M.; Budisteanu, M.; Erbescu, A.; Ionescu, V.; Iliescu, C.; Sandu, C.; Arghir, A. Novel DCX pathogenic variant in a girl with subcortical band heterotopia. Rev. Romana Med. Lab. 2022, 30, 345–351. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Thauvin-Robinet, C.; Cossée, M.; Cormier-Daire, V.; Van Maldergem, L.; Toutain, A.; Alembik, Y.; Bieth, E.; Layet, V.; Parent, P.; David, A.; et al. Clinical, molecular, and genotype-phenotype correlation studies from 25 cases of oral-facial-digital syndrome type 1: A French and Belgian collaborative study. J. Med. Genet. 2006, 43, 54–61. [Google Scholar] [CrossRef]
- Budny, B.; Chen, W.; Omran, H.; Fliegauf, M.; Tzschach, A.; Wisniewska, M.; Jensen, L.R.; Raynaud, M.; Shoichet, S.A.; Badura, M.; et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum. Genet. 2006, 120, 171–178. [Google Scholar] [CrossRef]
- Webb, T.R.; Parfitt, D.A.; Gardner, J.C.; Martinez, A.; Bevilacqua, D.; Davidson, A.E.; Zito, I.; Thiselton, D.L.; Ressa, J.H.; Apergi, M.; et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23). Hum. Mol. Genet. 2012, 21, 3647–3654. [Google Scholar] [CrossRef]
- Bouman, A.; Alders, M.; Oostra, R.J.; van Leeuwen, E.; Thuijs, N.; van der Kevie-Kersemaekers, A.M.; van Maarle, M. Oral-facial-digital syndrome type 1 in males: Congenital heart defects are included in its phenotypic spectrum. Am. J. Med. Genet. A 2017, 173, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, I.M.; Johnston, J.J.; Patton, J.H.; Graham, J.M.; Sapp, J.C.; Biesecker, L.G. Exome sequencing identifies a mutation in OFD1 in a male with Joubert syndrome, orofaciodigital spectrum anomalies and complex polydactyly. Hum. Genome Var. 2016, 3, 15069. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, N.; Morisada, N.; Nozu, K.; Nagatani, K.; Ohta, T.; Shimizu, J.; Wada, T.; Shima, Y.; Yamamura, T.; Minamikawa, S.; et al. Clinical spectrum of male patients with OFD1 mutations. J. Hum. Genet. 2019, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Morleo, M.; Franco, B. OFD Type I syndrome: Lessons learned from a rare ciliopathy. Biochem. Soc. Trans. 2020, 48, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- de Conciliis, L.; Marchitiello, A.; Wapenaar, M.C.; Borsani, G.; Giglio, S.; Mariani, M.; Consalez, G.G.; Zuffardi, O.; Franco, B.; Ballabio, A.; et al. Characterization of Cxorf5 (71-7A), a novel human cDNA mapping to Xp22 and encoding a protein containing coiled-coil alpha-helical domains. Genomics 1998, 51, 243–250. [Google Scholar] [CrossRef]
- D’Angelo, A.; De Angelis, A.; Avallone, B.; Piscopo, I.; Tammaro, R.; Studer, M.; Franco, B. Ofd1 controls dorso-ventral patterning and axoneme elongation during embryonic brain development. PLoS ONE 2012, 7, e52937. [Google Scholar] [CrossRef]
- Romio, L.; Fry, A.M.; Winyard, P.J.; Malcolm, S.; Woolf, A.S.; Feather, S.A. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J. Am. Soc. Nephrol. 2004, 15, 2556–2568. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, M.G.; Stowe, T.R.; Chen, S.; Zhu, M.; Stearns, T.; Franco, B.; Zhong, Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 2013, 502, 254–257. [Google Scholar] [CrossRef]
- Morleo, M.; Brillante, S.; Formisano, U.; Ferrante, L.; Carbone, F.; Iaconis, D.; Palma, A.; Buonomo, V.; Maione, A.S.; Grumati, P.; et al. Regulation of autophagosome biogenesis by OFD1-mediated selective autophagy. EMBO J. 2021, 40, e105120. [Google Scholar] [CrossRef]
- Emes, R.D.; Ponting, C.P. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet. 2001, 10, 2813–2820. [Google Scholar] [CrossRef]
- Alfieri, M.; Iaconis, D.; Tammaro, R.; Perone, L.; Calì, G.; Nitsch, L.; Dougherty, G.W.; Ragnini-Wilson, A.; Franco, B. The centrosomal/basal body protein OFD1 is required for microtubule organization and cell cycle progression. Tissue Cell. 2020, 64, 101369. [Google Scholar] [CrossRef]
- Franco, B.; Morleo, M. The role of OFD1 in selective autophagy. Mol. Cell. Oncol. 2021, 8, 1903291. [Google Scholar] [CrossRef]
- Morleo, M.; Franco, B. The OFD1 protein is a novel player in selective autophagy: Another tile to the cilia/autophagy puzzle. Cell Stress 2021, 5, 33–36. [Google Scholar] [CrossRef]
- Gangaram, B.; Devine, W.P.; Slavotinek, A. Expanding the phenotype of males with OFD1 pathogenic variants-a case report and literature review. Eur. J. Med. Genet. 2022, 65, 104496. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Morleo, M.; Franco, B. Dosage compensation of the mammalian X chromosome influences the phenotypic variability of X-linked dominant male-lethal disorders. J. Med. Genet. 2008, 45, 401–408. [Google Scholar] [CrossRef]
- Prattichizzo, C.; Macca, M.; Novelli, V.; Giorgio, G.; Barra, A.; Franco, B.; Oral-Facial-Digital Type I (OFDI) Collaborative Group. Mutational spectrum of the oral-facial-digital type I syndrome: A study on a large collection of patients. Hum. Mutat. 2008, 29, 1237–1246. [Google Scholar] [CrossRef]
- Towfighi, J.; Berlin, C.M.; Ladda, R.L., Jr.; Frauenhoffer, E.E.; Lehman, R.A. Neuropathology of oral-facial-digital syndromes. Arch. Pathol. Lab. Med. 1985, 109, 642–646. [Google Scholar]
- Krumm, N.; Turner, T.N.; Baker, C.; Vives, L.; Mohajeri, K.; Witherspoon, K.; Raja, A.; Coe, B.P.; Stessman, H.A.; He, Z.X.; et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 2015, 47, 582–588. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Guo, H.; Shi, L.; Zhang, K.; Tang, M.; Hu, S.; Dong, S.; Liu, Y.; Wang, T.; et al. Targeted sequencing and functional analysis reveal brain-size-related genes and their networks in autism spectrum disorders. Mol. Psychiatry 2017, 22, 1282–1290. [Google Scholar] [CrossRef]
- Tran, K.T.; Le, V.S.; Bui, H.T.P.; Do, D.H.; Ly, H.T.T.; Nguyen, H.T.; Dao, L.T.M.; Nguyen, T.H.; Vu, D.M.; Ha, L.T.; et al. Genetic landscape of autism spectrum disorder in Vietnamese children. Sci. Rep. 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Coene, K.L.; Roepman, R.; Doherty, D.; Afroze, B.; Kroes, H.Y.; Letteboer, S.J.; Ngu, L.H.; Budny, B.; van Wijk, E.; Gorden, N.T.; et al. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am. J. Hum. Genet. 2009, 85, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, C.; Liu, W.; Yang, H. Retinitis Pigmentosa and Bilateral Idiopathic Demyelinating Optic Neuritis in a 6-Year-Old Boy with OFD1 Gene Mutation. Case Rep. Ophthalmol. Med. 2017, 2017, 5310924. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sheng, X.; Liu, Y.; Li, Z.; Sun, X.; Jiang, C.; Qi, R.; Yuan, S.; Wang, X.; Zhou, G.; et al. Distinct mutations with different inheritance mode caused similar retinal dystrophies in one family: A demonstration of the importance of genetic annotations in complicated pedigrees. J. Transl. Med. 2018, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; DeBrosse, S.; Kinghorn, B.; Strausbaugh, S.; Aitken, M.L.; Rosenfeld, M.; Wolf, W.E.; Knowles, M.R.; Zariwala, M.A. The expanding phenotype of OFD1-related disorders: Hemizygous loss-of-function variants in three patients with primary ciliary dyskinesia. Mol. Genet. Genom. Med. 2019, 7, e911. [Google Scholar] [CrossRef]
- Bukowy-Bieryllo, Z.; Rabiasz, A.; Dabrowski, M.; Pogorzelski, A.; Wojda, A.; Dmenska, H.; Grzela, K.; Sroczynski, J.; Witt, M.; Zietkiewicz, E. Truncating mutations in exons 20 and 21 of OFD1 can cause primary ciliary dyskinesia without associated syndromic symptoms. J. Med. Genet. 2019, 56, 769–777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papuc, S.M.; Erbescu, A.; Glangher, A.; Streata, I.; Riza, A.-L.; Budisteanu, M.; Arghir, A. Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome. Genes 2023, 14, 327. https://doi.org/10.3390/genes14020327
Papuc SM, Erbescu A, Glangher A, Streata I, Riza A-L, Budisteanu M, Arghir A. Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome. Genes. 2023; 14(2):327. https://doi.org/10.3390/genes14020327
Chicago/Turabian StylePapuc, Sorina Mihaela, Alina Erbescu, Adelina Glangher, Ioana Streata, Anca-Lelia Riza, Magdalena Budisteanu, and Aurora Arghir. 2023. "Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome" Genes 14, no. 2: 327. https://doi.org/10.3390/genes14020327
APA StylePapuc, S. M., Erbescu, A., Glangher, A., Streata, I., Riza, A.-L., Budisteanu, M., & Arghir, A. (2023). Autistic Behavior as Novel Clinical Finding in OFD1 Syndrome. Genes, 14(2), 327. https://doi.org/10.3390/genes14020327






