miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Culture
2.2. Cumulus Cell Treatment
2.3. Cell Transfection
2.4. RNA Extraction and RT–qPCR
2.5. γH2AX Detection
2.6. Cell Proliferation Assays
2.7. Cell Cycle Analysis
2.8. Apoptosis Analysis
2.9. Analysis of Steroid Hormone Secretion
2.10. DualLuciferase Reporter Gene Analysis
2.11. Statistical Analysis
3. Results
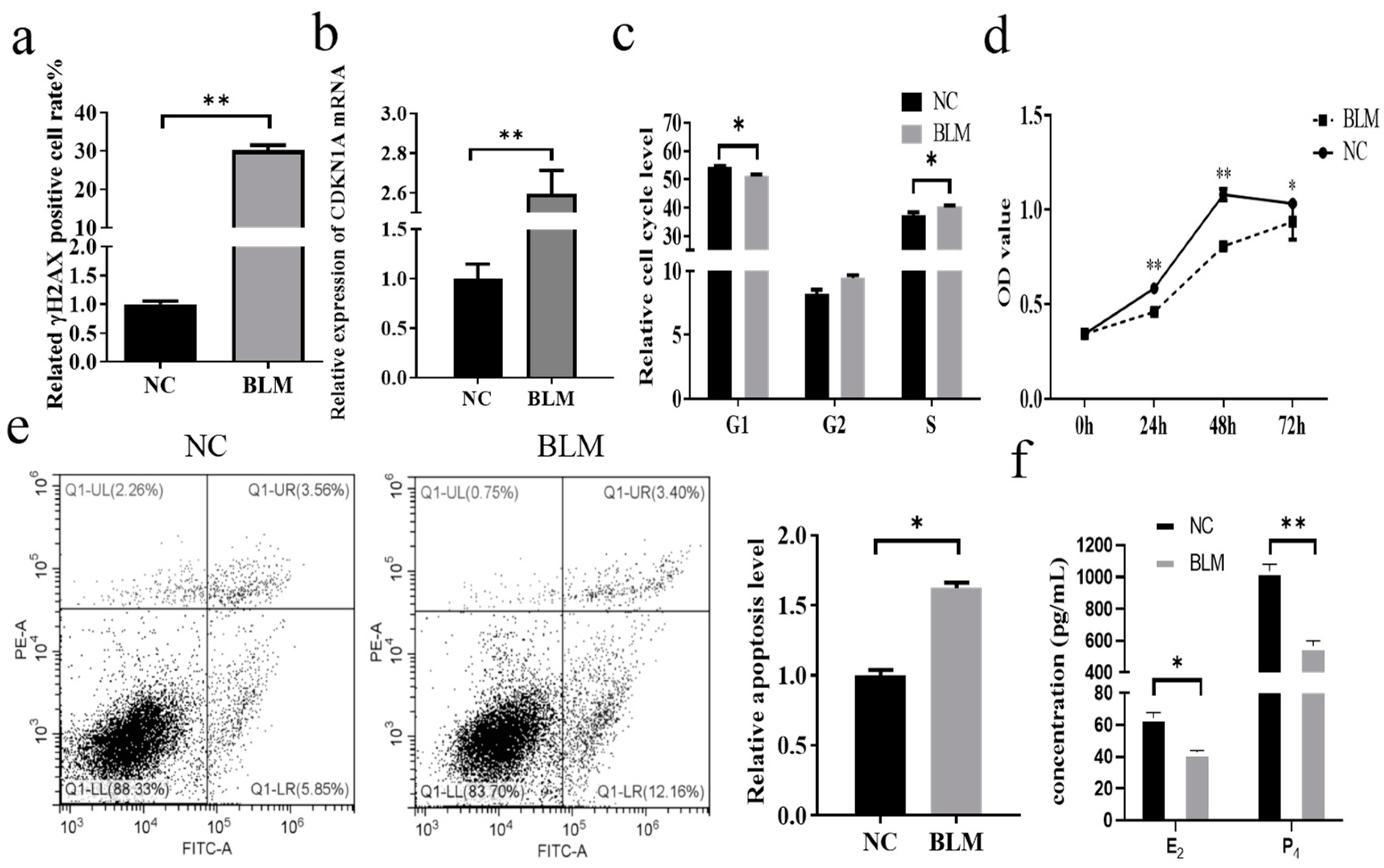
3.1. Effect of DNA Damage on Cumulus Cells in Bovines
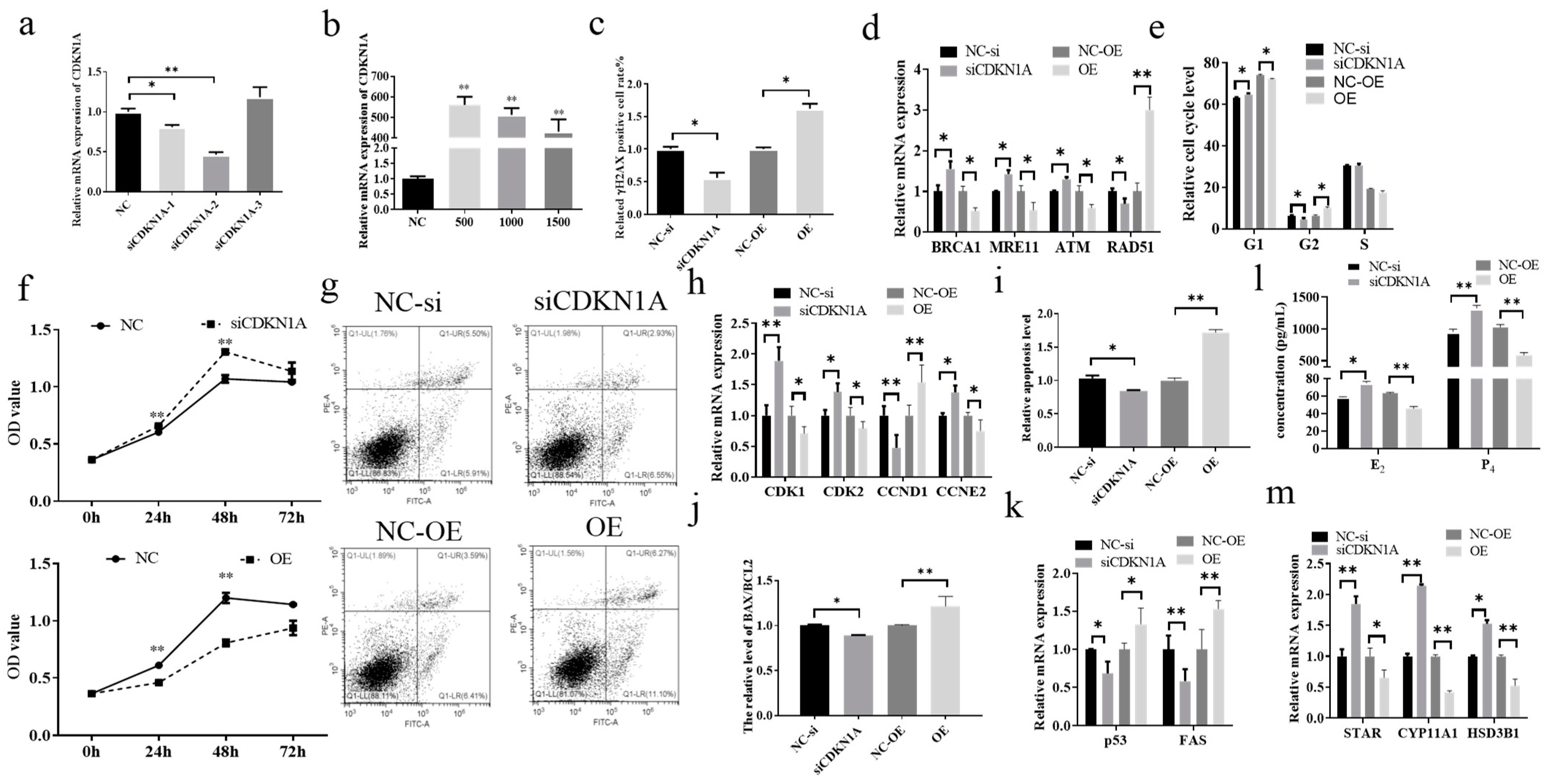
3.2. Effect of CDKN1A on DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells
3.3. miR-302d Targeted Binding to the CDKN1A 3′UTR and Inhibited Its Expression
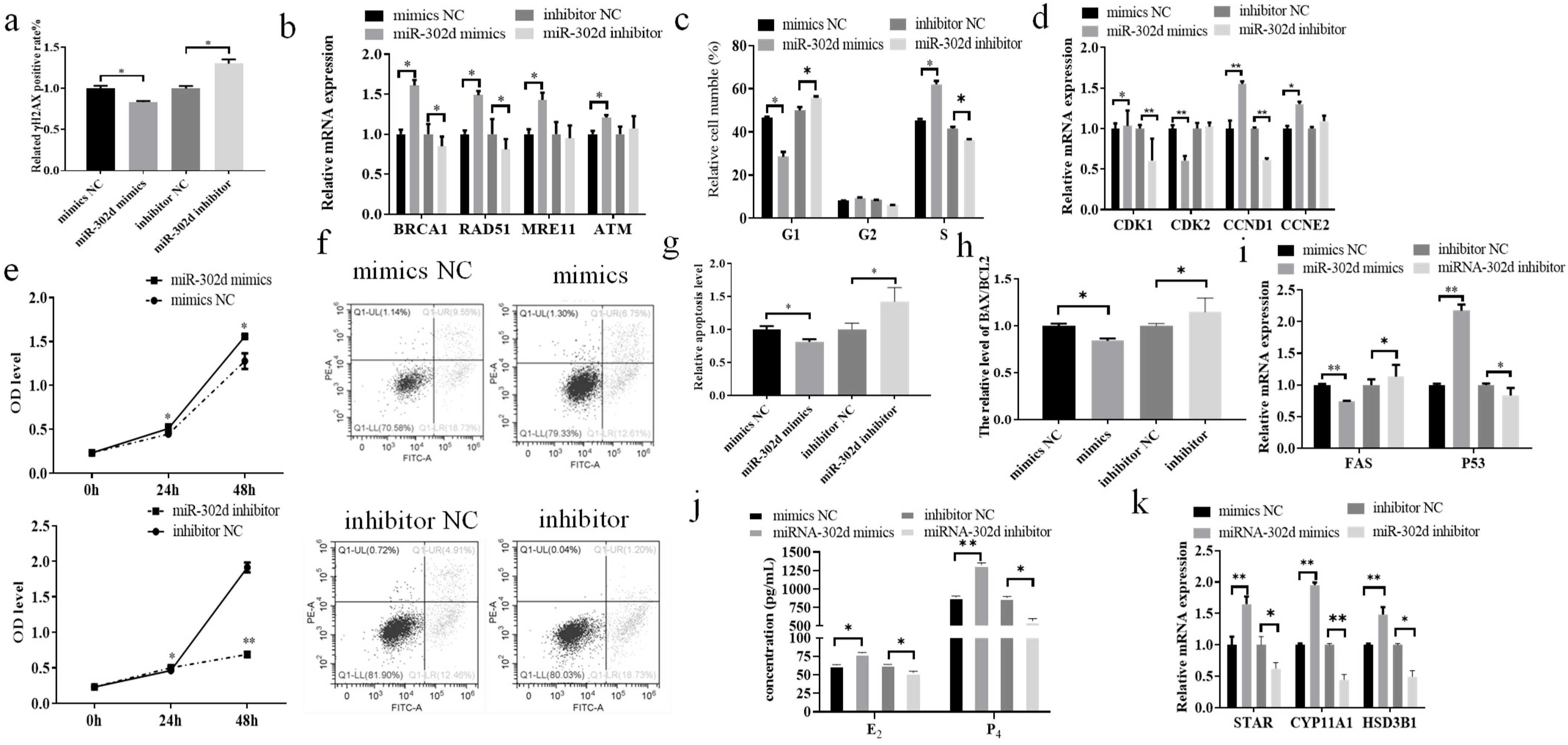
3.4. miR-302d Regulated DNA Damage and Steroid Hormone Secretion in Bovine CCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.Q.; Sugiura, K.; Eppig, J.J. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 2009, 27, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Ding, Z.; Meng, F.; Zhang, X.; Wang, Y.; Chen, F.; Duan, Z.; Wu, D.; Zhang, S.; Miao, Y.; et al. The toxic effects of Fluorene-9-bisphenol on porcine oocyte in vitro maturation. Environ. Toxicol. 2020, 35, 152–158. [Google Scholar] [CrossRef] [PubMed]
- De Bont, R.; van Larebeke, N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Chen, M.; Zhao, X.; Liu, Y.; Ren, X.; Zhang, L. Versican expression level in cumulus cells is associated with human oocyte developmental competence. Syst. Biol. Reprod. Med. 2020, 66, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.R.; Lan, D.L.; Li, J.; Yin, S.; Xiong, Y.; Zi, X.D. Identification of differential abundances of mRNA transcript in cumulus cells and CCND1 associated with yak oocyte developmental competence. Anim. Reprod. Sci. 2019, 208, 106135. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.S.; Chan, P.J.; Corselli, J.U.; Patton, W.C.; Jacobson, J.D.; Chan, S.R.; King, A. Comet assay of cumulus cell DNA status and the relationship to oocyte fertilization via intracytoplasmic sperm injection. Hum. Reprod. 2001, 16, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Tuppi, M.; Kehrloesser, S.; Coutandin, D.W.; Rossi, V.; Luh, L.M.; Strubel, A.; Hotte, K.; Hoffmeister, M.; Schafer, B.; De Oliveira, T.; et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat. Struct. Mol. Biol. 2018, 25, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Marangos, P.; Carroll, J. Oocytes progress beyond prophase in the presence of DNA damage. Curr. Biol. 2012, 22, 989–994. [Google Scholar] [CrossRef]
- Pandita, T.K.; Richardson, C. Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res. 2009, 37, 1363–1377. [Google Scholar] [CrossRef]
- van Gent, D.C.; Hoeijmakers, J.H.; Kanaar, R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2001, 2, 196–206. [Google Scholar] [CrossRef]
- Bohgaki, T.; Bohgaki, M.; Hakem, R. DNA double-strand break signaling and human disorders. Genome Integr. 2010, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Teng, M.; Zhao, G.; Lei, A. Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes. Int. J. Mol. Sci. 2020, 21, 8892. [Google Scholar] [CrossRef] [PubMed]
- Maneschi, F.; Benedetti-Panici, P.; Scambia, G.; Salerno, M.G.; D’Agostino, G.; Mancuso, S. Menstrual and hormone patterns in women treated with high-dose cisplatin and bleomycin. Gynecol. Oncol. 1994, 54, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Harries, L. MicroRNAs as Mediators of the Ageing Process. Genes 2014, 5, 656–670. [Google Scholar] [CrossRef]
- Wei, S.; Xue, J.; Sun, B.; Zou, Z.; Chen, C.; Liu, Q.; Zhang, A. miR-145 via targeting ERCC2 is involved in arsenite-induced DNA damage in human hepatic cells. Toxicol. Lett. 2018, 295, 220–228. [Google Scholar] [CrossRef]
- Pan, B.; Toms, D.; Shen, W.; Li, J. MicroRNA-378 regulates oocyte maturation via the suppression of aromatase in porcine cumulus cells. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E525–E534. [Google Scholar] [CrossRef]
- Andreas, E.; Pandey, H.O.; Hoelker, M.; Salilew-Wondim, D.; Gebremedhn, S.; Schellander, K.; Tesfaye, D. The regulatory role of miR-20a in bovine cumulus cells and its contribution to oocyte maturation. Zygote 2021, 29, 435–444. [Google Scholar] [CrossRef]
- Li, Y.; Huo, J.; Pan, X.; Wang, C.; Ma, X. MicroRNA 302b-3p/302c-3p/302d-3p inhibits epithelial-mesenchymal transition and promotes apoptosis in human endometrial carcinoma cells. Onco Targets Ther. 2018, 11, 1275–1284. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Z.; Liu, F.; Bai, Y.; Wu, F. FGD5-AS1 Inhibits Osteoarthritis Development by Modulating miR-302d-3p/TGFBR2 Axis. Cartilage 2021, 13, 1412S–1420S. [Google Scholar] [CrossRef]
- Gartel, A.L. Is p21 an oncogene? Mol. Cancer Ther. 2006, 5, 1385–1386. [Google Scholar] [CrossRef]
- Tom, S.; Ranalli, T.A.; Podust, V.N.; Bambara, R.A. Regulatory roles of p21 and apurinic/apyrimidinic endonuclease 1 in base excision repair. J. Biol. Chem. 2001, 276, 48781–48789. [Google Scholar] [CrossRef]
- Wang, Y.; Fisher, J.C.; Mathew, R.; Ou, L.; Otieno, S.; Sublet, J.; Xiao, L.; Chen, J.; Roussel, M.F.; Kriwacki, R.W. Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nat. Chem. Biol. 2011, 7, 214–221. [Google Scholar] [CrossRef]
- Chan, T.A.; Hwang, P.M.; Hermeking, H.; Kinzler, K.W.; Vogelstein, B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000, 14, 1584–1588. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, J.B.; Yan, X.M.; Xie, P.G.; Fu, Y.; Fu, X.H.; Sun, X.L.; Han, D.X.; Li, S.P.; Zheng, Y.; et al. DNA Double-Strand Break-Related Competitive Endogenous RNA Network of Noncoding RNA in Bovine Cumulus Cells. Genes 2023, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Todorova, T.; Miteva, D.; Chankova, S. DNA susceptibility of Saccharomyces cerevisiae to Zeocin depends on the growth phase. Int. Microbiol. 2019, 22, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.Z.; Li, B.; Huang, B.; Wang, Y.; Liu, X.D.; Guan, H.; Zhang, S.M.; Tang, Y.; Rang, W.Q.; Zhou, P.K. gammaH2AX foci formation in the absence of DNA damage: Mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS Lett. 2013, 587, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Cappelen, K.; Aase, K.; Storm, M.; Hetland, J.; Harris, A. Psychometric properties of the Nursing Home Survey on Patient Safety Culture in Norwegian nursing homes. BMC Health Serv. Res. 2016, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, G.L.; Ma, J.Y.; Qi, S.T.; Wang, Z.B.; Wang, Z.W.; Luo, Y.B.; Jiang, Z.Z.; Schatten, H.; Sun, Q.Y. Effects of DNA damage and short-term spindle disruption on oocyte meiotic maturation. Histochem. Cell Biol. 2014, 142, 185–194. [Google Scholar] [CrossRef]
- Stucki, M.; Jackson, S.P. gammaH2AX and MDC1: Anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair 2006, 5, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwata, H.; Goto, H.; Shiratuki, S.; Tanaka, H.; Monji, Y.; Kuwayama, T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol. Reprod. Dev. 2010, 77, 595–604. [Google Scholar] [CrossRef]
- Sun, M.H.; Yang, M.; Xie, F.Y.; Wang, W.; Zhang, L.; Shen, W.; Yin, S.; Ma, J.Y. DNA Double-Strand Breaks Induce the Nuclear Actin Filaments Formation in Cumulus-Enclosed Oocytes but Not in Denuded Oocytes. PLoS ONE 2017, 12, e0170308. [Google Scholar] [CrossRef] [PubMed]
- Best, B.P. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 2009, 12, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Jiang, H.; Han, D.X.; Fu, Y.; Liu, J.B.; Gao, Y.; Hu, S.M.; Yuan, B.; Zhang, J.B. The activated DNA double-strand break repair pathway in cumulus cells from aging patients may be used as a convincing predictor of poor outcomes after in vitro fertilization-embryo transfer treatment. PLoS ONE 2018, 13, e0204524. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.H.; Nystrom, S.; Nygren, J.; Hammarsten, O. Activation of ataxia telangiectasia mutated by DNA strand break-inducing agents correlates closely with the number of DNA double strand breaks. J. Biol. Chem. 2005, 280, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Rzepka-Gorska, I.; Tarnowski, B.; Chudecka-Glaz, A.; Gorski, B.; Zielinska, D.; Toloczko-Grabarek, A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res. Treat. 2006, 100, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tarsounas, M.; Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Turan, V.; Titus, S.; Stobezki, R.; Liu, L. BRCA Mutations, DNA Repair Deficiency, and Ovarian Aging. Biol. Reprod. 2015, 93, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gong, Y.; Peng, B.; Shi, R.; Fan, D.; Zhao, H.; Zhu, M.; Zhang, H.; Lou, Z.; Zhou, J.; et al. MRE11 UFMylation promotes ATM activation. Nucleic Acids Res. 2019, 47, 4124–4135. [Google Scholar] [CrossRef]
- Govindaraj, V.; Keralapura Basavaraju, R.; Rao, A.J. Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod. Biomed. Online 2015, 30, 303–310. [Google Scholar] [CrossRef]
- Suwaki, N.; Klare, K.; Tarsounas, M. RAD51 paralogs: Roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin. Cell Dev. Biol. 2011, 22, 898–905. [Google Scholar] [CrossRef]
- Bilotto, S.; Boni, R.; Russo, G.L.; Lioi, M.B. Meiosis progression and donor age affect expression profile of DNA repair genes in bovine oocytes. Zygote 2015, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.A.; Duong, M.T.; Carey, J.P.W.; Hunt, K.K.; Keyomarsi, K. Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for Targeted Therapies. Cancer Res. 2018, 78, 5481–5491. [Google Scholar] [CrossRef]
- Niculescu, A.B., 3rd; Chen, X.; Smeets, M.; Hengst, L.; Prives, C.; Reed, S.I. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell Biol. 1998, 18, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- Huertas, P.; Cortes-Ledesma, F.; Sartori, A.A.; Aguilera, A.; Jackson, S.P. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 2008, 455, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F. History of the events leading to the formulation of the apoptosis concept. Toxicology 2002, 181–182, 471–474. [Google Scholar] [CrossRef]
- King, K.L.; Cidlowski, J.A. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Chauhan, P.; Sodhi, A.; Tarang, S. Cisplatin-treated murine peritoneal macrophages induce apoptosis in L929 cells: Role of Fas-Fas ligand and tumor necrosis factor-tumor necrosis factor receptor 1. Anti-Cancer Drugs 2007, 18, 187–196. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Ehmcke, J.; Hou, M.; Schlatt, S. Testicular function and fertility preservation in male cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kawashima, I.; Gunji, Y.; Hishinuma, M.; Shimada, M. Progesterone is essential for maintenance of Tace/Adam17 mRNA expression, but not EGF-like factor, in cumulus cells, which enhances the EGF receptor signaling pathway during in vitro maturation of porcine COCs. J. Reprod. Dev. 2010, 56, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Weihua, Z.; Makinen, S.; Makela, S.; Saji, S.; Warner, M.; Gustafsson, J.A.; Hovatta, O. A role for the androgen receptor in follicular atresia of estrogen receptor β knockout mouse ovary. Biol. Reprod. 2002, 66, 77–84. [Google Scholar] [CrossRef]
- Al-Bader, M.; Kilarkaje, N. Effects of bleomycin, etoposide and cisplatin treatment on Leydig cell structure and transcription of steroidogenic enzymes in rat testis. Eur. J. Pharmacol. 2015, 747, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.P.; Phillips, S.; Sar, M.; Foster, P.M.; Gaido, K.W. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol. Sci. 2004, 81, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Yeste, M.; Roca, J.; Llavanera, M.; Bucci, D.; Galeati, G.; Spinaci, M.; Barranco, I. Seminal extracellular vesicles subsets modulate gene expression in cumulus cells of porcine in vitro matured oocytes. Sci. Rep. 2022, 12, 19096. [Google Scholar] [CrossRef]
- Caillaud, M.; Gerard, N. In vivo and in vitro effects of interleukin-1beta on equine oocyte maturation and on steroidogenesis and prostaglandin synthesis in granulosa and cumulus cells. Reprod. Fertil. Dev. 2009, 21, 265–273. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Song, J.; Wang, L.; Guo, P.; Cao, J. MiRNA-181b-5p Modulates Cell Proliferation, Cell Cycle, and Apoptosis by Targeting SSX2IP in Acute Lymphoblastic Leukemia. Turk. J. Haematol. 2022, 39, 160–169. [Google Scholar] [CrossRef]
- Qiao, G.Y.; Dong, B.W.; Zhu, C.J.; Yan, C.Y.; Chen, B.L. Deregulation of WNT2/FZD3/β-catenin pathway compromises the estrogen synthesis in cumulus cells from patients with polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2017, 493, 847–854. [Google Scholar] [CrossRef]
- Maidarti, M.; Anderson, R.A.; Telfer, E.E. Crosstalk between PTEN/PI3K/Akt Signalling and DNA Damage in the Oocyte: Implications for Primordial Follicle Activation, Oocyte Quality and Ageing. Cells 2020, 9, 200. [Google Scholar] [CrossRef]
- Kwon, Y.; Rosner, H.; Zhao, W.; Selemenakis, P.; He, Z.; Kawale, A.S.; Katz, J.N.; Rogers, C.M.; Neal, F.E.; Badamchi Shabestari, A.; et al. DNA binding and RAD51 engagement by the BRCA2 C-terminus orchestrate DNA repair and replication fork preservation. Nat. Commun. 2023, 14, 432. [Google Scholar] [CrossRef]
- Bobyk, L.; Vianna, F.; Martinez, J.S.; Gruel, G.; Benderitter, M.; Baldeyron, C. Differential Recruitment of DNA Repair Proteins KU70/80 and RAD51 upon Microbeam Irradiation with α-Particles. Biology 2022, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. miRNA Biogenesis and Regulation of Diseases: An Updated Overview. Methods Mol. Biol. 2023, 2595, 1–12. [Google Scholar] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Lopez, A.; Betancourt, M.; Ducolomb, Y.; Rodriguez, J.J.; Casas, E.; Bonilla, E.; Bahena, I.; Retana-Marquez, S.; Juarez-Rojas, L.; Casillas, F. DNA damage in cumulus cells generated after the vitrification of in vitro matured porcine oocytes and its impact on fertilization and embryo development. Porc. Health Manag. 2021, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Barcena, P.; Lopez-Fernandez, C.; Garcia-Ochoa, C.; Obradors, A.; Vernaeve, V.; Gosalvez, J.; Vassena, R. Detection of DNA damage in cumulus cells using a chromatin dispersion assay. Syst. Biol. Reprod. Med. 2015, 61, 277–285. [Google Scholar] [PubMed]
- Haraguchi, H.; Hirota, Y.; Saito-Fujita, T.; Tanaka, T.; Shimizu-Hirota, R.; Harada, M.; Akaeda, S.; Hiraoka, T.; Matsuo, M.; Matsumoto, L.; et al. Mdm2-p53-SF1 pathway in ovarian granulosa cells directs ovulation and fertilization by conditioning oocyte quality. FASEB J. 2019, 33, 2610–2620. [Google Scholar] [CrossRef]
- Lin, Z.L.; Kim, N.H. Role of ataxia-telangiectasia mutated (ATM) in porcine oocyte in vitro maturation. Cell Biol. Int. 2015, 39, 710–720. [Google Scholar] [CrossRef]
- Sun, M.H.; Zheng, J.; Xie, F.Y.; Shen, W.; Yin, S.; Ma, J.Y. Cumulus Cells Block Oocyte Meiotic Resumption via Gap Junctions in Cumulus Oocyte Complexes Subjected to DNA Double-Strand Breaks. PLoS ONE 2015, 10, e0143223. [Google Scholar] [CrossRef]
- Sirini, M.A.; Anchordoquy, J.M.; Anchordoquy, J.P.; Pascua, A.M.; Nikoloff, N.; Carranza, A.; Relling, A.E.; Furnus, C.C. The presence of acylated ghrelin during in vitro maturation of bovine oocytes induces cumulus cell DNA damage and apoptosis, and impairs early embryo development. Zygote 2017, 25, 601–611. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, J.; Zheng, Y.; Zhao, G.; Jiang, H.; Yuan, B. miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells. Genes 2023, 14, 2195. https://doi.org/10.3390/genes14122195
Liu J, Zhang J, Zheng Y, Zhao G, Jiang H, Yuan B. miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells. Genes. 2023; 14(12):2195. https://doi.org/10.3390/genes14122195
Chicago/Turabian StyleLiu, Jianbo, Jiabao Zhang, Yi Zheng, Guokun Zhao, Hao Jiang, and Bao Yuan. 2023. "miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells" Genes 14, no. 12: 2195. https://doi.org/10.3390/genes14122195
APA StyleLiu, J., Zhang, J., Zheng, Y., Zhao, G., Jiang, H., & Yuan, B. (2023). miR-302d Targeting of CDKN1A Regulates DNA Damage and Steroid Hormone Secretion in Bovine Cumulus Cells. Genes, 14(12), 2195. https://doi.org/10.3390/genes14122195




