Transcriptomic Analysis Reveals CBF-Dependent and CBF-Independent Pathways under Low-Temperature Stress in Teak (Tectona grandis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Determination of Free Proline and Superoxide Dismutase
2.3. Library Construction and Sequencing
2.4. Data Processing and Differential Gene Expression (DEG) Analysis
2.5. Weighted Gene Correlation Network Analysis (WGCNA)
2.6. qRT-PCR Validation of DEGs
2.7. Transformation Verification of CBF Gene Function in Teak
3. Results
3.1. Teak Exhibits Signs of Damage under Temporary Low Temperatures
- The detrimental effect of cold stress on teak’s leaf morphology is evident from the observed damage at 4 °C (Figure 1). Firstly, the injured symptoms initially occurred at the top of the plant. After 3 h of 4 °C, the top leaves started to present brownish chlorosis spots sporadically, indicating freezing injury (Figure 1B). Subsequently, the top and bottom of the leaves were more injured, and the chlorosis spots increased and enlarged at 4 °C for 9 h (Figure 1C). These results indicate that teak is susceptible to temporary low-temperature stress, with young leaves being particularly vulnerable. These morphological alterations may be connected to secondary metabolites in the leaves.
- Teak showed injury symptoms at low temperatures, as described above, implying physiological and biochemical changes in teak (Figure 2). In this study, it was discovered that the osmotic adjustment index, specifically the free proline content, as well as the oxidative stress index and superoxide dismutase (SOD) content, exhibited a significant increase during short-term low-temperature stress. As a result, during the initial treatment (4 °C for 3 h), the free proline concentration substantially increased from 8.15 μg/g to 16.14 μg/g, representing a significant difference. Moreover, the activity of SOD experienced a noteworthy enhancement from 309.7 U/g to 427.117 U/g. After that, the content of free proline and SOD continuously increased. The content of free proline reached 18.7 μ g/g, and the content of SOD was 461.5 U/g in the second treatment (4 °C for 9 h).
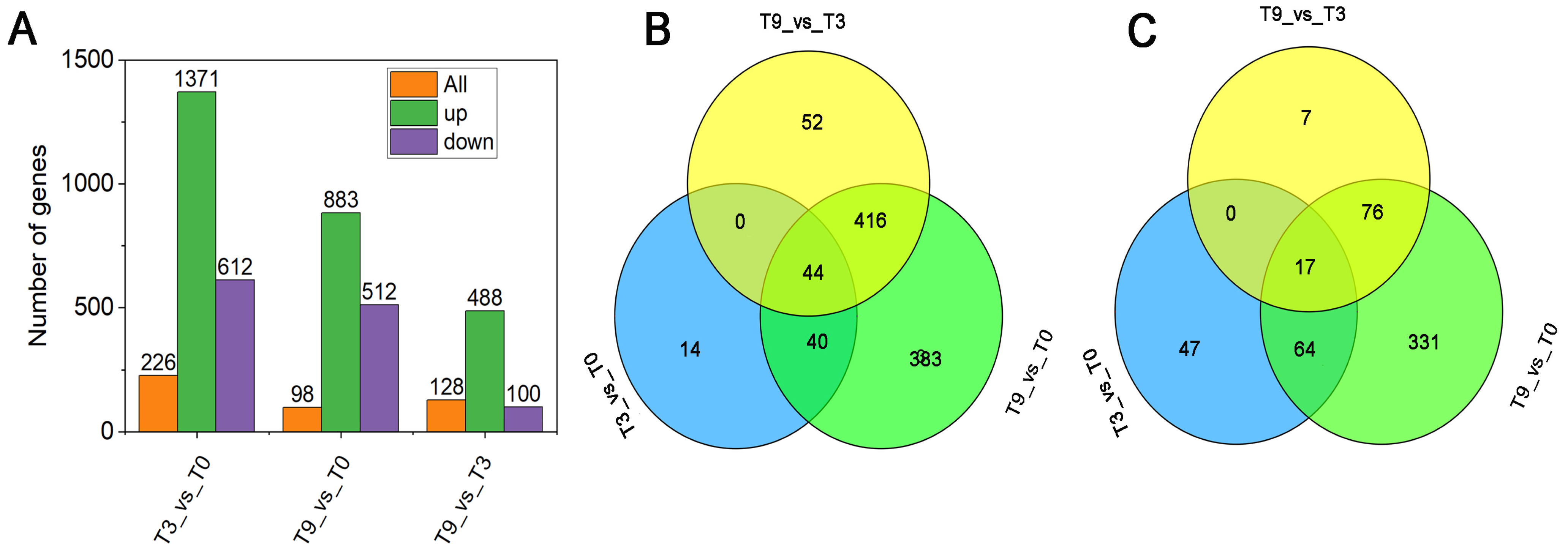
3.2. A Total of 1055 Novel Genes Were Identified via Transcriptome Analysis
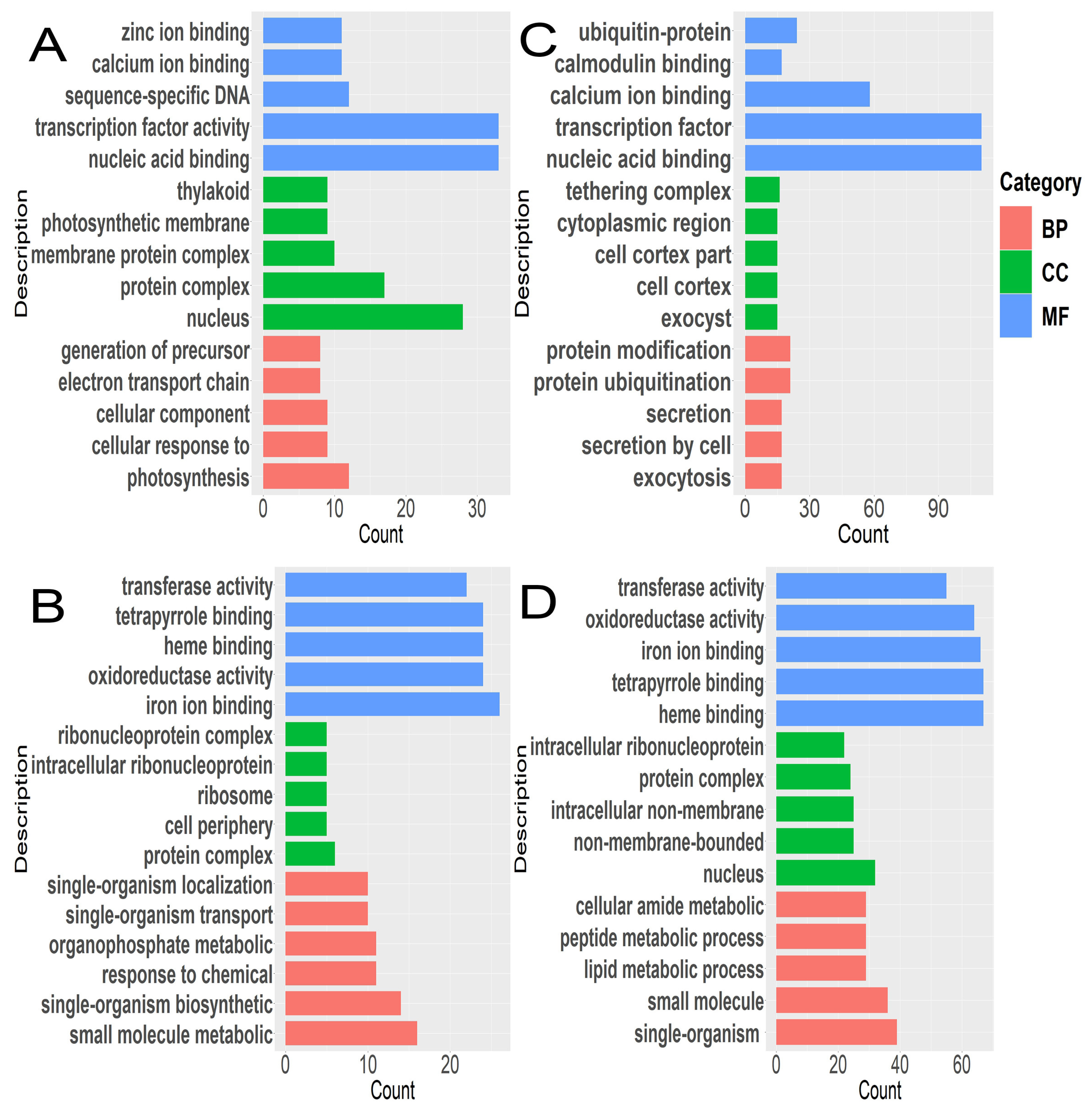
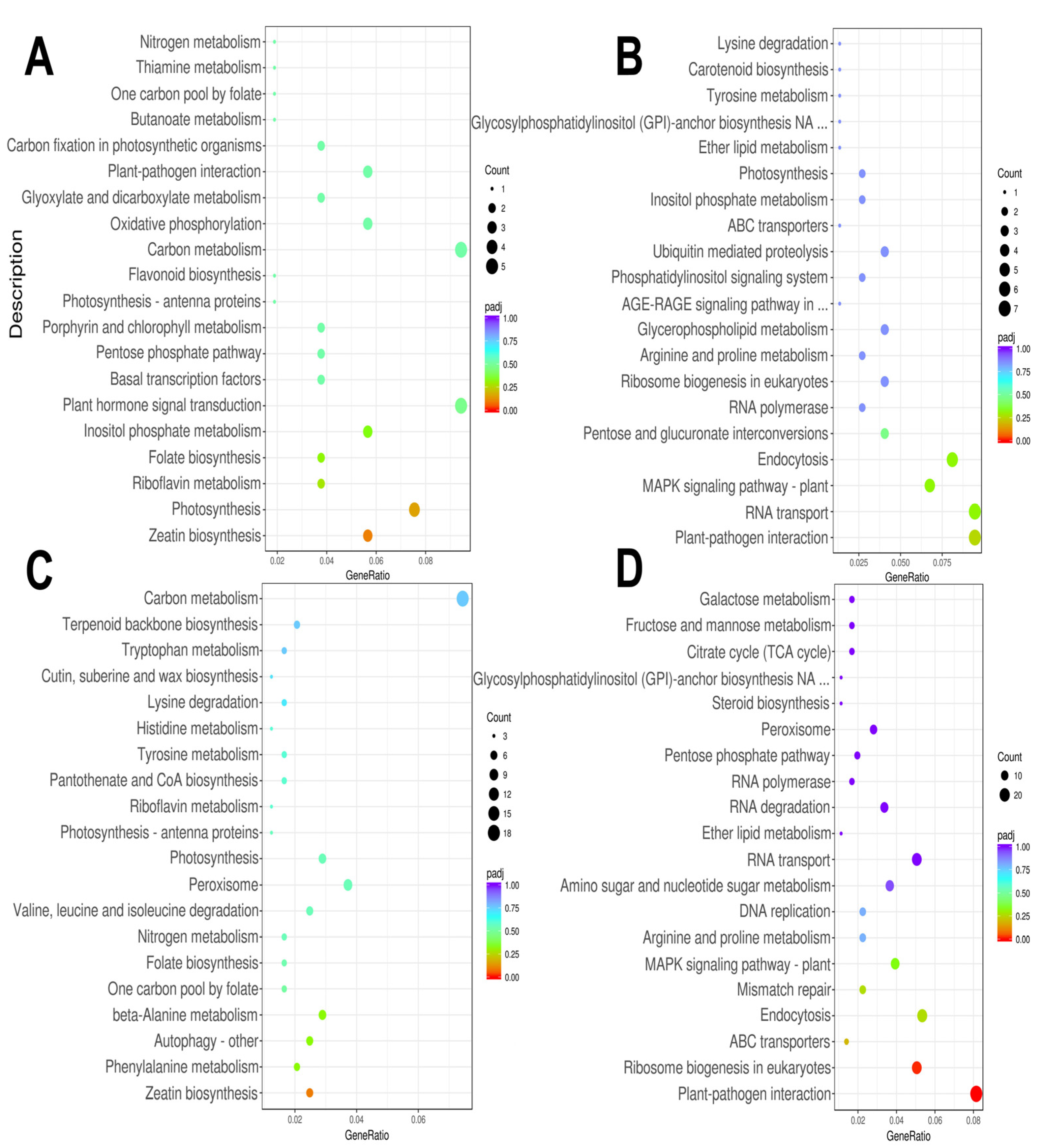
3.3. Enrichment Characteristics of KEGG and GO of DEGs
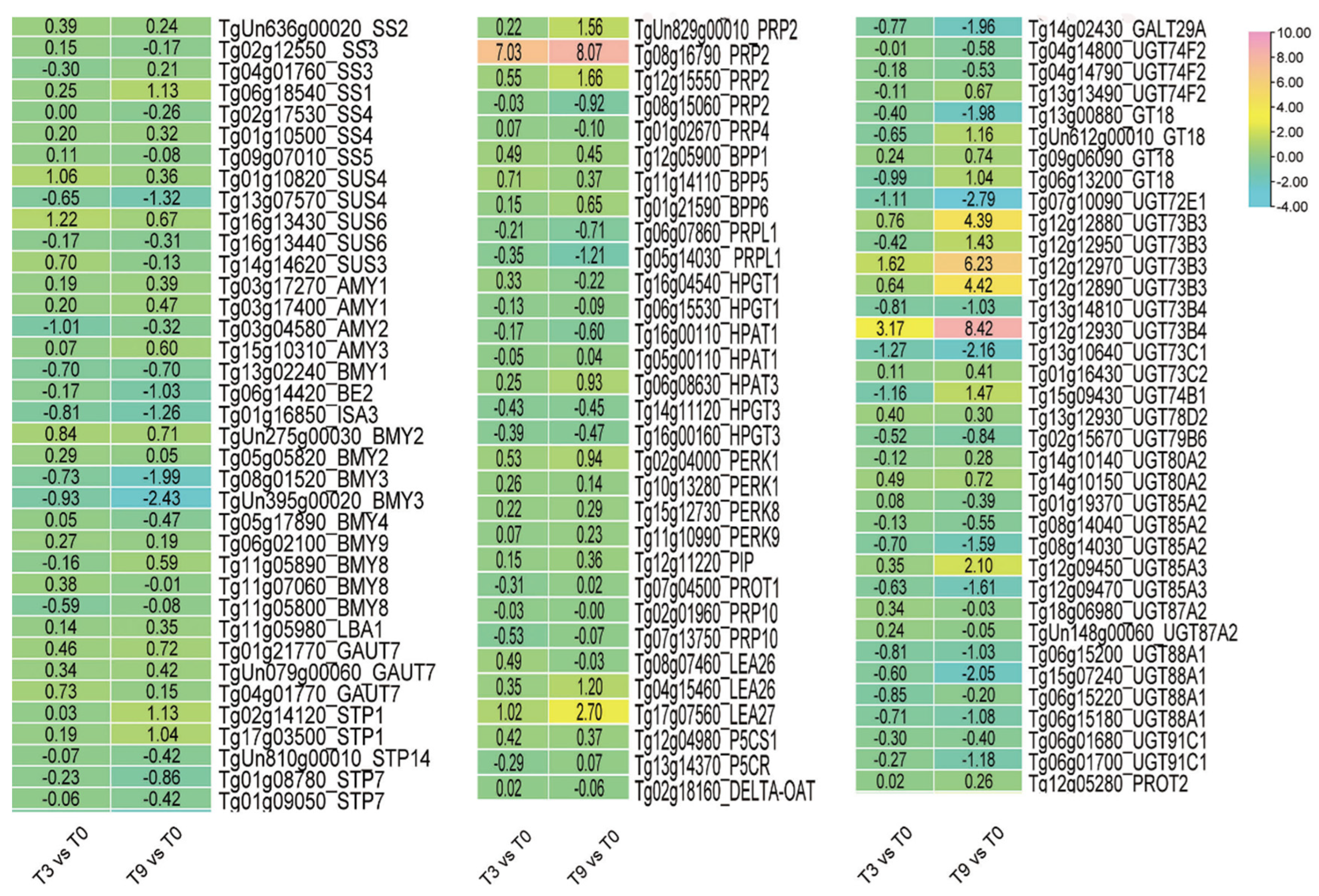
3.4. DEGs Involved in Osmoregulation Pathway
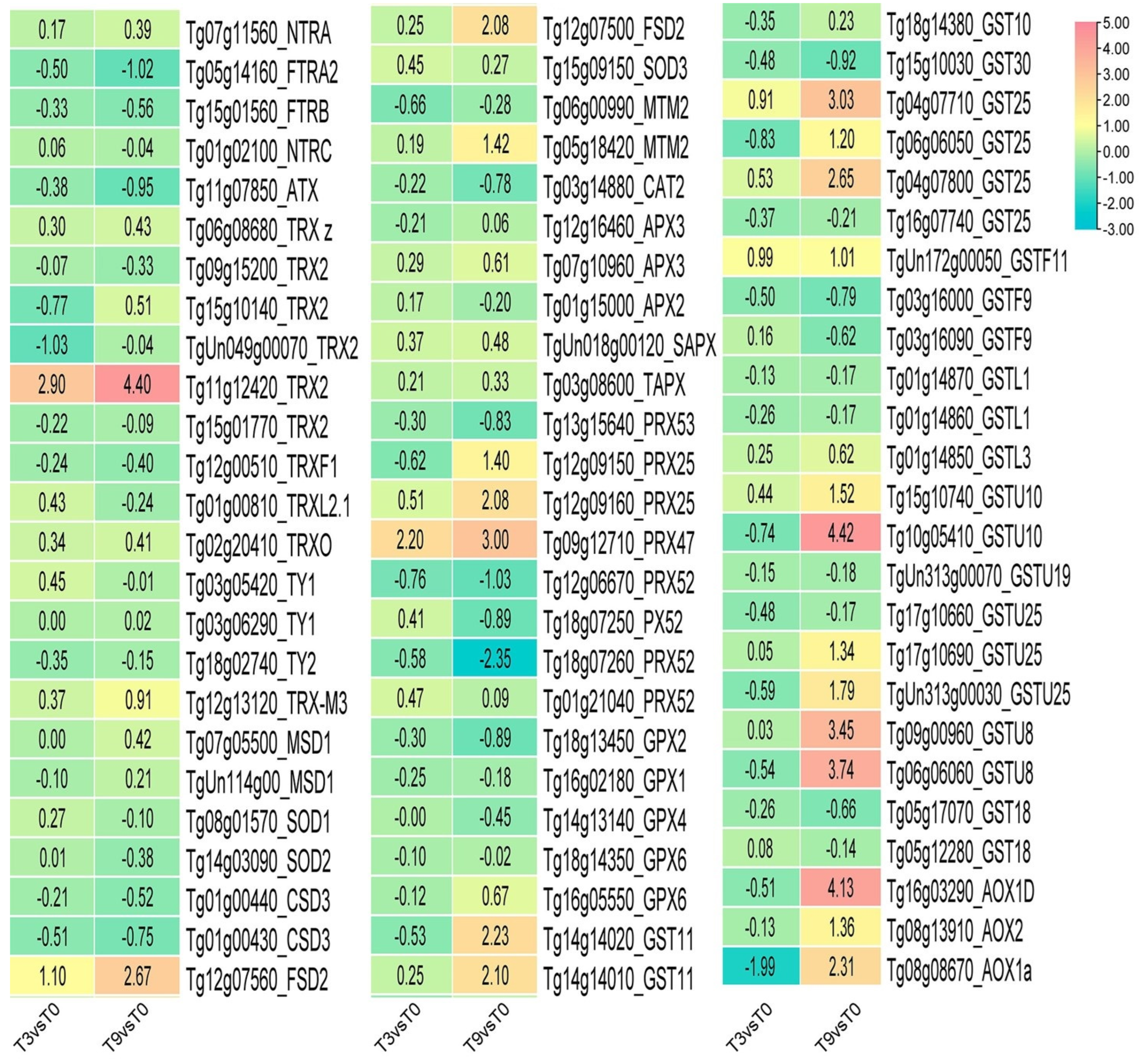
3.5. DEGs Involved in Antioxidant Enzyme Genes under Low-Temperature Stress
3.6. DEGs Involved in PSI and PSII under Low-Temperature Stress
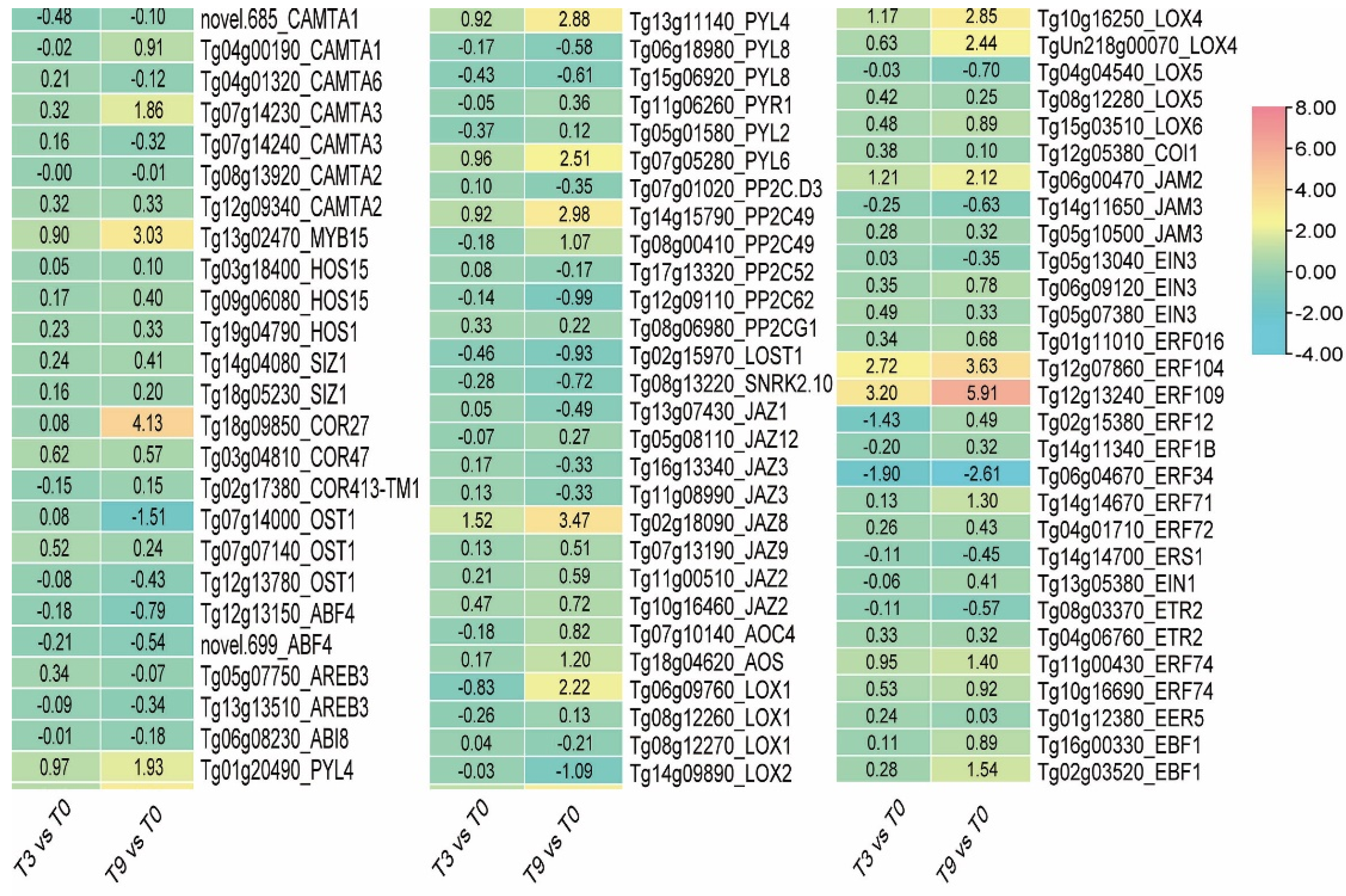
3.7. CBF Expression Pattern under Low-Temperature Stress
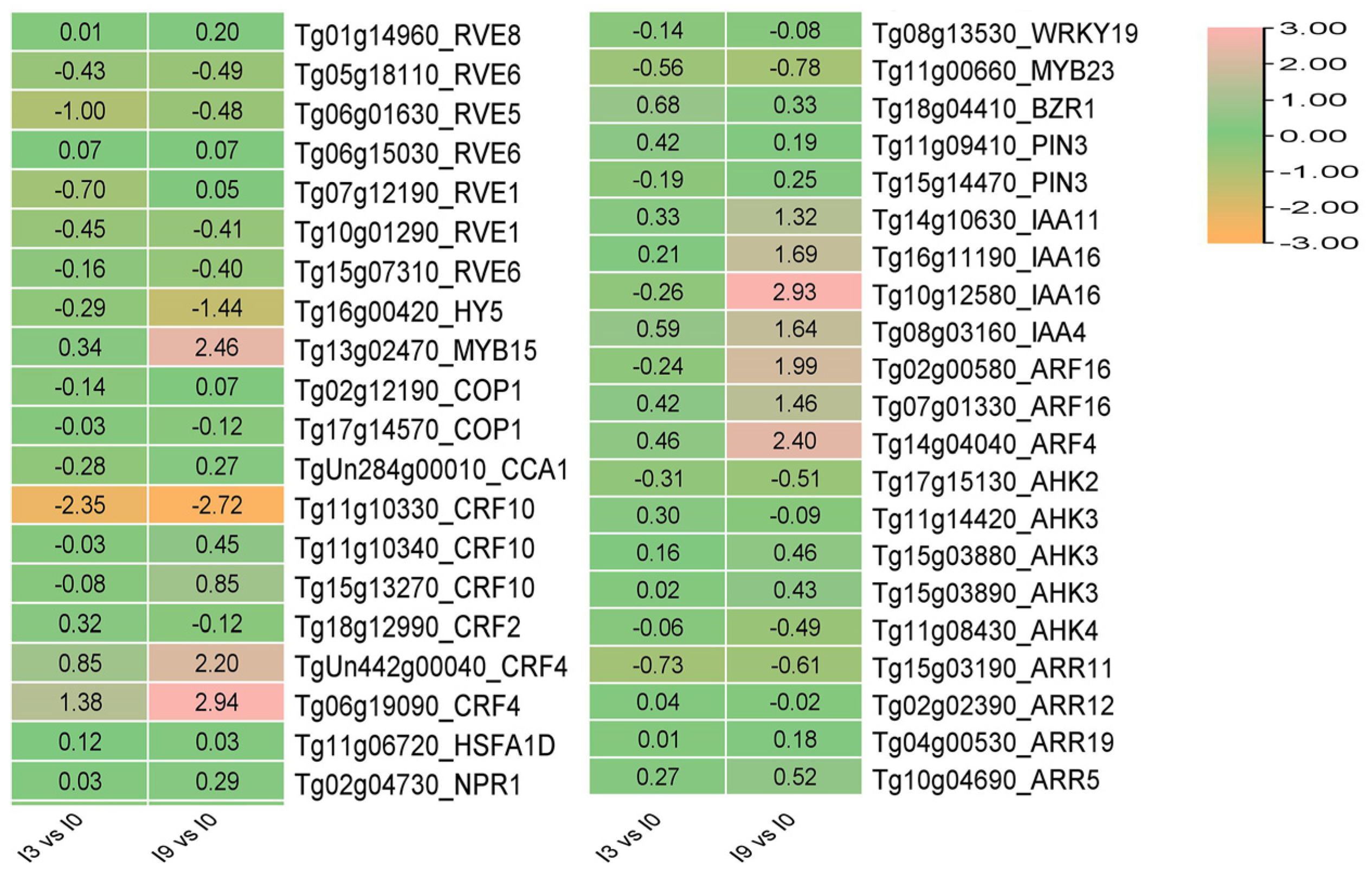
3.8. The Expression of CBF-Independent Genes HY5 and REV5 in Teak Changed Dramatically in Response to Cold Stress
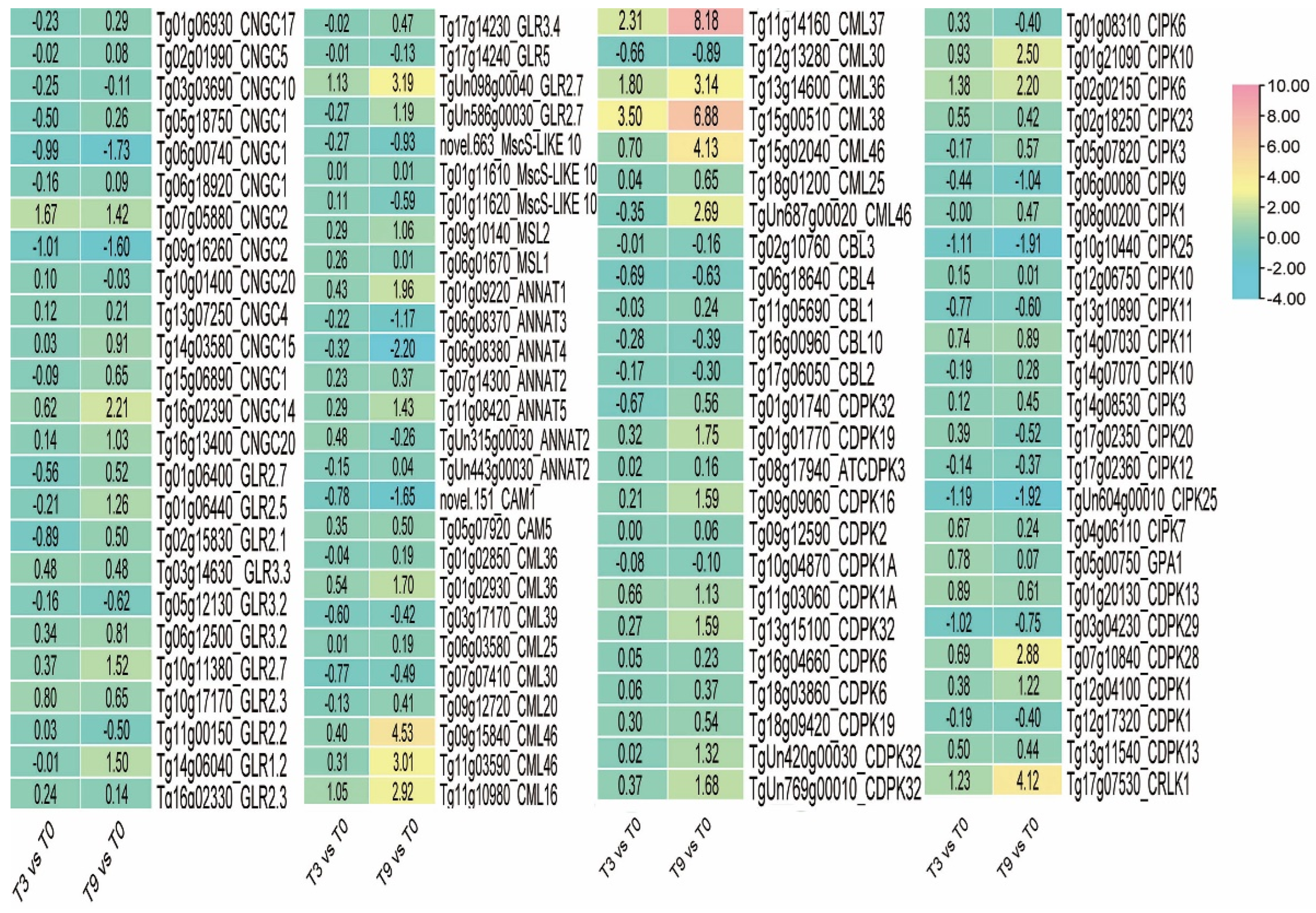
3.9. Calcium Signal Transduction in Teak Cells under Low-Temperature Stress
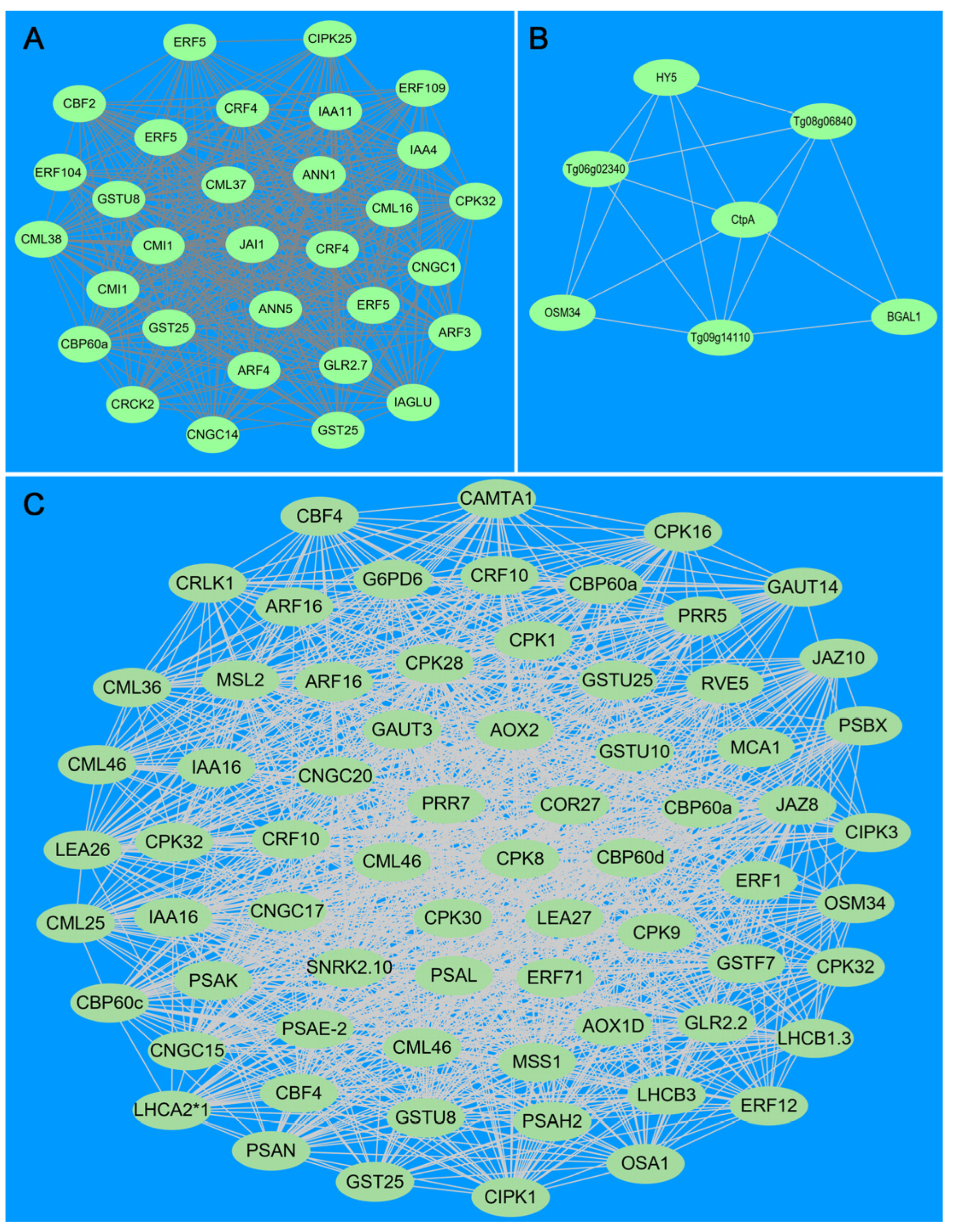
3.10. WGCNA and Hub Genes of DEGs Identified Three CBF-Dependent and CBF-Independent Regulatory Networks
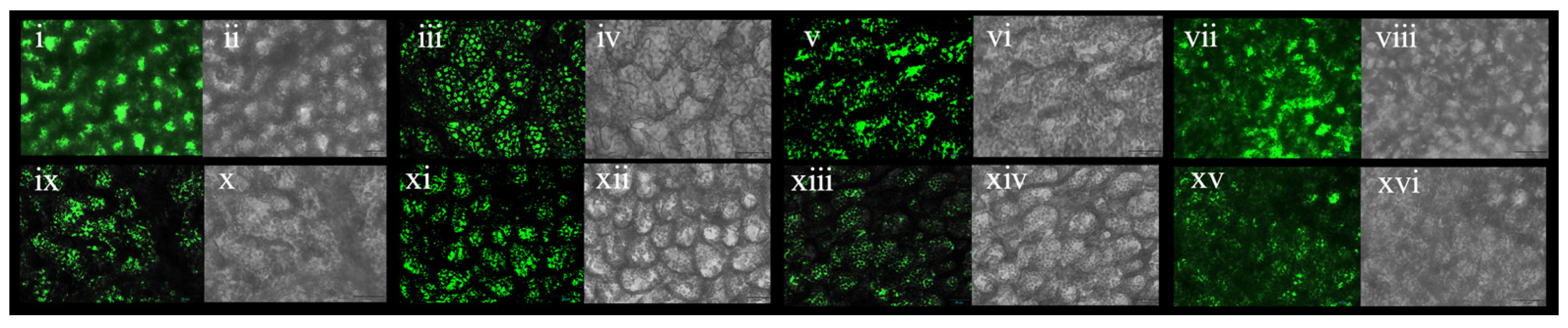
3.11. Morphologies of OE-TgCBF2 and OE-TgCBF4 under Low-Temperature Treatment
4. Discussion
4.1. Teak CBFs and Their Regulation Exhibit Conservation and Differentiation
4.2. The Signal Transduction of Cold Stress in Teak Also Exhibits Conservation and Diversity
4.3. The CBF-Independent Pathway Also Influences the Resistance of Teak against Cold Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Global Teak Trade in the Aftermath of Myanmar’s Log ExportBan. 2015. Available online: http://www.fao.org/3/a-i5023e.pdf (accessed on 15 January 2020).
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Gupta, K.; Lopato, S.; Agarwal, P. Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J. Exp. Bot. 2017, 68, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engi-neering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z.; Jin, Y.-N.; Ding, X.-H.; Wang, W.-J.; Zhai, S.-S.; Bai, L.-P.; Guo, Z.-F. Gene Regulation and Signal Transduction in the ICE-CBF-COR Signaling Pathway during Cold Stress in Plants. Biochemistry 2017, 82, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef]
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Kurbidaeva, A.; Ezhova, T.; Novokreshchenova, M. Arabidopsis thaliana ICE 2 gene: Phylogeny, structural evolution and functional diversification from ICE1. Plant Sci. 2014, 229, 10–22. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef]
- Kidokoro, S.; Yoneda, K.; Takasaki, H.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Different Cold-Signaling Pathways Function in the Responses to Rapid and Gradual Decreases in Temperature. Plant Cell 2017, 29, 760–774. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.; Xu, C.; Wang, C.; Liu, M.; Zhang, Z.; Wu, H.; Yuan, Z.; Zhou, J. Interferon-γ decreases ATP-binding cassette subfamily G member 1-mediated cholesterol efflux through small ubiquitin-like modifier/ubiquitin-dependent liver X receptor-α degradation in macrophages. Biotechnol. Appl. Biochem. 2020, 68, 1412–1420. [Google Scholar] [CrossRef]
- Miura, K.; Jin, J.B.; Hasegawa, P.M. Sumoylation, a post-translational regulatory process in plants. Curr. Opin. Plant Biol. 2007, 10, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin response in Arabidopsis under cold stress: Underlying molecular mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rahman, A. Cellular Protein Trafficking: A New Player in Low-Temperature Response Pathway. Plants 2022, 11, 933. [Google Scholar] [CrossRef]
- Xing, H.; Wang, P.; Cui, X.; Zhang, C.; Wang, L.; Liu, X.; Yuan, L.; Li, Y.; Xie, Q.; Xu, X. LNK1 and LNK2 recruitment to the evening element require morning expressed circadian related MYB-like transcription factors. Plant Signal. Behav. 2015, 10, e1010888. [Google Scholar] [CrossRef][Green Version]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Duan, M.; Ma, N.-N.; Li, D.; Deng, Y.-S.; Kong, F.-Y.; Lv, W.; Meng, Q.-W. Antisense-mediated suppression of tomato thylakoidal ascorbate peroxidase influences anti-oxidant network during chilling stress. Plant Physiol. Biochem. 2012, 58, 37–45. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: II. Purification and Quantitative Relationship with Water-soluble Protein in Seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Rychlik, W. OLIGO 7 primer analysis software. Methods Mol. Biol. 2007, 402, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-X.; Wang, S.-B.; Xiao, H.-J.; Zhang, H.-X.; Zhang, Z.; Jing, H.; Zhang, Y.-L.; Chen, R.-G.; Gong, Z.-H. Overexpression of the CaTIP1-1 pepper gene in tobacco enhances resistance to osmotic stresses. Int. J. Mol. Sci. 2014, 15, 20101–20116. [Google Scholar] [CrossRef]
- Fu, J.; Huang, S.; Qian, J.; Qing, H.; Wan, Z.; Cheng, H.; Zhang, C. Genome-wide identification of Petunia HSF Genes and potential function of PhHSF19 in benzenoid/phenylpropanoid biosynthesis. Int. J. Mol. Sci. 2022, 23, 2974. [Google Scholar] [CrossRef]
- Zhao, D.; Hamilton, J.P.; Bhat, W.W.; Johnson, S.R.; Godden, G.T.; Kinser, T.J.; Boachon, B.; Dudareva, N.; Soltis, D.E.; Soltis, P.S. Data from: A chromosomal-scale genome assembly of Tectona grandis reveals the importance of tandem gene duplication and enables discovery of genes in natural product biosynthetic pathways. Gigascience 2019, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chan, C.-K.K. Analysis of RNA-Seq Data Using TopHat and Cufflinks. Methods Mol. Bioinform. 2016, 1374, 339–361. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, X.; Xu, Y.; Li, H.; Ma, L.; Yao, X.; Weng, Y.; Guo, Y.; Liu, C.; Chong, K. OsCIPK7 point-mutation leads to conformation and kinase-activity change for sensing cold response. J. Integr. Plant Biol. 2019, 61, 1194–1200. [Google Scholar] [CrossRef]
- Zhang, X.; Fowler, S.G.; Cheng, H.; Lou, Y.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, W.; Xia, X.; Wang, T.; Zhang, W.-H. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef]
- Shi, Y.T.; Tian, S.W.; Hou, L.Y.; Huang, X.Z.; Zhang, X.Y.; Guo, H.W.; Yang, S.H. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and Type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, Q.; Ahammed, G.J.; Lin, R.; Wang, L.; Shao, S.; Yu, J.; Zhou, Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef] [PubMed]






| Gene ID | Forword Primers | Reverse Primers | TAIR Description |
|---|---|---|---|
| Tg01g18270 | AATCTGACGATTCGCAACCC | CGAGCAGTACAATCCTCTCCC | NDR1/HIN1-like 2 |
| Tg07g05280 | AGAGCTGCCACGTCATCCTTG | CCACCACGCTGAAACTCGTC | RCAR9, regulatory components of ABA receptor 9 |
| Tg09g03150 | AGACTTCTAGATAAAGCTCGT | TTTCTCTATCCGCCACCGTA | SAUR30, small auxin upregulated rna 30 |
| Tg09g12620 | TCACTTTCCACAGAAGGCATC | GAACACGACATCGCTCCAC | AATP1, AAA-ATPase 1 |
| Tg16g05170 | TGCTGGTCTACCTATTGACAGT | CCAAATCGGAGAACTTCACCAC | BRH1, brassinosteroid-responsive RING-H2 |
| TgUn002g01760 | TTTGACTGATCCTGCCCCTG | TGTTCCTCACCAACGATCCGA | ATPB, ATP synthase subunit beta |
| TgUn195g00060 | TGGTATATCTCTTCCGGTGT | TCATCGCCATCAAAATCTCC | CBP60a, Calmodulin-binding protein 60a |
| Tg06g13830 | CGTCATCGGATCCTAAGGACA | CCTCCTCATCCATAAAGCACAC | CBF4 |
| Tg16g06080 | GAGCTACGTGAGCCAACCCAA | GCAACCGCCATACAGAGTCC | DDF1, Dwarf and delayed flowering 1 |
| TgUn466g00010 | AGGTCAGCAACAATTACACGG | ATTTCTCGGCAATTCCAGGTT | WRKY6 |
| Tg03g16060 | TCCAGGCTCAATATCCACCAC | GCCACCCATTTTCCCCAGT | WIND1, wound induced dedifferentiation 1 |
| Tg09g10240 | TCCGCCAGACTCTTTACTCCAC | CGAACCATTGCGACATCAGCAG | DIC2, dicarboxylate carrier 2 |
| Tg03g10220 | GCCTTTGGAGCTTCAGCAAC | CCCGAGAGAGCAAAACACGAT | SUT1, SUCROSE TRANSPORTER 1 |
| Tg13g10760 | TTCTGCCAAGACAAACACCAG | ATTCCGCCATCTATTTCACCAC | FER, FERONIA |
| Tg05g13790 | GTTGAACATGCTGCTACTCAC | TAGCTTTGCCCAAAATTCCAC | bZIP 23 |
| Tg06g00470 | TGTTCCCAAGATTTTCGGACA | TGTAGCGCCATGAAAACCA | JAM2, Jasmonate Associated MYC2 LIKE 2 |
| Tg01g19010 | TGAATTTAGCCTGCAGCCAA | TCAAATCCCCGTACATCCAC | LHW, LONESOME HIGHWAY |
| Tg18g02580 | TTGAGACCTTCAACGTGCC | ATAATCAGTGAGATCCCGACCA | Actin |
| Sample | Raw_Reads | Clean_Reads | Clean_Bases | Q30 (%) | Total_Map | Splice Reads(%) | GC (%) |
|---|---|---|---|---|---|---|---|
| T0_1 | 42,258,920 | 40,712,072 | 6.11 G | 93.4 | 36,128,928 (88.74%) | 12,002,426 (29.48%) | 44.82 |
| T0_2 | 45,101,940 | 44,351,094 | 6.65 G | 93.32 | 39,455,647 (88.96%) | 13,123,149 (29.59%) | 44.53 |
| T0_3 | 52,698,330 | 50,794,162 | 7.62 G | 93.16 | 45,374,885 (89.33%) | 15,642,780 (30.8%) | 44.90 |
| T3_1 | 61,704,656 | 59,371,024 | 8.91 G | 93.37 | 52,288,275 (88.07%) | 17,879,136 (30.11%) | 44.29 |
| T3_2 | 59,390,990 | 57,148,694 | 8.57 G | 93.22 | 50,258,279 (87.94%) | 15,592,721 (27.28%) | 43.93 |
| T3_3 | 47,792,662 | 45,960,592 | 6.89 G | 93.04 | 40,063,421 (87.17%) | 13,199,390 (28.72%) | 43.72 |
| T9_1 | 53,340,150 | 51,332,880 | 7.70 G | 93.24 | 44,434,695 (86.56%) | 13,681,417 (26.65%) | 43.77 |
| T9_2 | 55,819,212 | 53,679,450 | 8.05 G | 93.19 | 46,979,435 (87.52%) | 14,590,252 (27.18%) | 44.13 |
| T9_3 | 43,179,072 | 41,514,078 | 6.23 G | 93.41 | 34,961,459 (84.22%) | 10,024,613 (24.15%) | 44.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Yang, G.; Zhou, W.; Wang, X.; Han, Q.; Wang, J.; Huang, G. Transcriptomic Analysis Reveals CBF-Dependent and CBF-Independent Pathways under Low-Temperature Stress in Teak (Tectona grandis). Genes 2023, 14, 2098. https://doi.org/10.3390/genes14112098
Liu M, Yang G, Zhou W, Wang X, Han Q, Wang J, Huang G. Transcriptomic Analysis Reveals CBF-Dependent and CBF-Independent Pathways under Low-Temperature Stress in Teak (Tectona grandis). Genes. 2023; 14(11):2098. https://doi.org/10.3390/genes14112098
Chicago/Turabian StyleLiu, Miaomiao, Guang Yang, Wenlong Zhou, Xianbang Wang, Qiang Han, Jiange Wang, and Guihua Huang. 2023. "Transcriptomic Analysis Reveals CBF-Dependent and CBF-Independent Pathways under Low-Temperature Stress in Teak (Tectona grandis)" Genes 14, no. 11: 2098. https://doi.org/10.3390/genes14112098
APA StyleLiu, M., Yang, G., Zhou, W., Wang, X., Han, Q., Wang, J., & Huang, G. (2023). Transcriptomic Analysis Reveals CBF-Dependent and CBF-Independent Pathways under Low-Temperature Stress in Teak (Tectona grandis). Genes, 14(11), 2098. https://doi.org/10.3390/genes14112098





