Abstract
Sturgeon is known as a primitive fish with the ZZ/ZW sex determination system and is highly prized for its valuable caviar. Exploring the molecular mechanisms underlying gonadal differentiation would contribute to broadening our knowledge on the genetic regulation of sex differentiation of fish, enabling improved artificial breeding and management of sturgeons. However, the mechanisms are still poorly understood in sturgeons. This study aimed to profile expression patterns between female and male gonads at morphologically undifferentiated and early differentiated stages and identify vital genes involved in gonadal sex differentiation of sturgeons. The sexes of Yangtze sturgeon (Acipenser dabryanus) juveniles were identified via the sex-specific DNA marker and histological observation. Transcriptome analyses were carried out on female and male gonads at 30, 80 and 180 days post-hatching. The results showed that there was a total of 17 overlapped DEGs in the comparison groups of between female and male gonads at the three developmental stages, in which there were three DEGs related to ovarian steroidogenesis, including hsd17b1, foxl2 and cyp19a1. The three DEGs were highly expressed in the female gonads, of which the expression levels were gradually increased with the number of days after hatching. No well-known testis-related genes were found in the overlapped DEGs. Additionally, the expression levels of hsd17b1 and cyp19a1 mRNA were decreased with the knockdown of foxl2 mRNA via siRNA. The results further suggested that foxl2 should play a crucial role in the ovarian differentiation of sturgeons. In conclusion, this study showed that more genes involved in ovarian development than testis development emerged with sexually dimorphic expression during early gonadal sex differentiation, and it provided a preliminary understanding of the molecular regulation on gonadal differentiation of sturgeons.
1. Introduction
The order Acipenseriformes (consisting of sturgeons and paddlefishes) is considered to be a primitive group of actinopterygian fish that plays vital roles in fish phylogeny. Sex determination of sturgeons and paddlefishes belongs to the female heterogametic type of genetic determination [1,2]. As yet, little is known of the sex-determining molecular mechanisms in the order Acipenseriformes. Elucidating the molecular process during gonadal differentiation of sturgeons would be beneficial by broadening our understanding of their sex differentiation, further uncovering their sex-determining mechanisms.
In contrast to mammals and birds with obvious heterotypic chromosomes, most fish species have no highly distinct and differentiated sex chromosomes. Moreover, different sex determination systems and master sex-determining genes exist in fishes. Medaka fishes of the genus Oryzias possess different sex determination systems. For instance, Oryzias latipes, Oryzias curvinotus, Oryzias dancena, Oryzias minutillus and Oryzias luzonensis have an XX/XY system, whereas Oryzias hubbsi and Oryzias javanicus have a ZZ/ZW system [3,4]. They also have different master sex-determining genes; for instance, Dmy (DM-domain gene on the Y chromosome) has been found to be a master sex-determining gene in O. latipes [5] and O. curvinotus [6], while the master sex-determining gene in O. luzonensis was GsdfY (gonadal-soma-derived growth factor on the Y chromosome), which has replaced Dmy in the evolutionary process [7]. Therefore, the mechanism of sex determination in fish is highly diverse and plastic, which also increases the difficulty of exploring the molecular process of sex differentiation.
To investigate key genes involved in sex determination and differentiation in sturgeons, expression patterns of sex-related genes such as dmrt1, sox9, foxl2 and cyp19a1 have been analyzed by other researchers. These genes exhibited sexually dimorphic expression in the ovary and testis at certain developmental stages in their study [8,9,10,11,12,13]. With the rapid development of genomics, transcriptomics and other omics technologies, omics has become one of the effective methods for mining master sex-determining genes and uncovering molecular mechanisms of sex determination and differentiation [14,15,16]. Gonadal transcriptome sequencing was employed to discover more putative sex-related genes in Adriatic sturgeon (Acipenser naccarii) [17], Chinese sturgeon (Acipenser sinensis) [18,19,20], Russian sturgeon (Acipenser gueldenstaedtii) [21,22,23], Yangtze sturgeon (Acipenser dabryanus) [24], Siberian sturgeon (Acipenser baeri) [25,26], lake sturgeon (Acipenser fulvescens) [27,28] and Amur sturgeon (Acipenser schrenckii) [29,30,31,32,33]. Whole genomes have been sequenced from the sterlet (Acipenser ruthenus) [34,35] and American paddlefish [36]. And, to explore the mechanism of sex determination of sturgeons, whole-genome inter-sex variation was comprehensively analyzed using whole-genome sequencing on DNA from five female and five male adult Russian sturgeon [37]. Nevertheless, the molecular mechanism of sexual determination and differentiation remain unclear in sturgeons.
Nearly non-existent obvious secondary sexual characteristics, long lifespan and late maturation [38,39,40,41] might make it difficult to discover master genes or molecular mechanisms underlying the sex differentiation of sturgeons. At present, sex-specific DNA sequences and markers of sturgeons were obtained by comparative genomics with high-throughput sequencing [2,42], which could contribute to identifying the genetic gender of sturgeons early in development. And, the sex-specific DNA markers have been used to identify the sexes of lake sturgeon (Acipenser fulvescens) [43] and Yangtze sturgeon [44]. Morphological sex differentiation of gonads could be observed with histological section, for instance, in Yangtze sturgeon by 78 days post-hatching (dph) [45]. However, vital sex-differentiation-related genes might express differentially prior to morphological sex differentiation of gonads. The previous gonadal transcriptome analyses mainly focused on differentiated gonads, which did not reflect genes involved in the onset of sex differentiation. With the help of sex-specific DNA markers, profiling changes in gene expression during gonadal differentiation would be more feasible to carry out using transcriptome analysis.
In this study, undifferentiated and differentiated gonads of Yangtze sturgeon were collected and examined using sex-specific DNA marker and histological sections. Gene expression profiles among these gonads were investigated using mRNA-Seq. By integrated analyses of these transcriptome profiles, differentially expressed genes (DEGs) during sex differentiation were screened out. This study aimed to provide basic information on the transcriptome level during gonadal differentiation to assist future studies to elucidate the molecular mechanisms of sex determination and differentiation of sturgeons.
2. Materials and Methods
2.1. Fish and Sampling
Yangtze sturgeon juveniles were reared in 1600-L tanks from Taihu station, Yangtze River Fisheries Research Institute, Chinese Academy of Fisheries Science. A total of 60 juveniles from 30 dph, 70 dph, 80 dph, 90 dph and 180 dph were randomly selected, twelve fishes from each developmental stage. They were anesthetized with 0.001% ethyl 3-aminobenzoic acid ethyl ester methanesulfonate-222 (Sigma, Burlington, MA, USA) and were sacrificed by quick decapitation. The tail fin tissue was collected and kept in ethanol for genomic DNA extraction. Additionally, a piece of gonadal tissue was fixed in Bouin’s solution for histological identification, and the remnant was preserved in RNA Save (Biological Industries, Kibbutz Beit Haemek, Israel) for RNA extraction. These experimental procedures complied with the guiding principles of the Animal Care and Use Committee of the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences.
2.2. Sex Identification Using Sex-Specific DNA Marker
Genomic DNA was extracted from the tail fin tissue of the juveniles using the TIANamp Genomic DNA kit (Tiangen, Beijing, China) according to the manufacturer’s instructions and assessed using a 1.5% agarose gel and a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). A pair of sex-specific primers were synthesized according to the published sequences (5′-TAATCAATTGTAAGTCGCCAAG-3′ and 5′-ATTTTATTACGGTGAGTATACGAA-3′) [2]. A traditional PCR was conducted with the following steps: 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 52 °C for 45 s and 72 °C 45 s; and 72 °C for 7 min. Each amplification reaction contained 1 μL (approximately 100 ng) DNA template, 10.5 μL ddH2O, 0.5 μL each primer (10 μM) and 12.5 μL 2 × Premix Taq (Takara, Shiga, Japan) in a total volume of 25 μL. The PCR products were detected using electrophoresis with 1.5% agarose gel.
2.3. Histological Analysis
Gonads fixed in Bouin’s solution overnight were transferred to 70% ethanol at 4 °C until paraffin sectioning. Samples were cut into 4 μm thick slices in a standard paraffin embedding method and stained with hematoxylin-eosin (HE). Images of sections were obtained using a light microscope (BX-51, Olympus, Tokyo, Japan) and a digital camera (DP-73, Olympus).
2.4. Transcriptome Sequencing and Analyses
Total RNA was extracted from gonads with RNeasy Plus Mini Kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s instructions. RNA degradation and concentration were preliminarily assessed by 1.5% agarose gel electrophoresis and Nanodrop Lite spectrophotometer (Thermo Scientific, Waltham, MA, USA). The RNA samples were sent to Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China) for further assessment and library construction. The Illumina HiSeq 4000 platform was used to generate 150 bp paired-end reads. Clean data were obtained by removing low-quality raw reads that contained more than 10% poly-N or 50% bases with Qphred < 5. Due to no reference genome of Yangtze sturgeon, de novo assembly of a Yangtze sturgeon reference transcriptome was carried out using Trinity [46] with min_kmer_cov set to 2 by default and all other parameters set to default. To refine the final transcriptome dataset, a further hierarchical clustering step was performed using Corset [47]. The longest transcript of multiple isoform Corset clusters was selected as representative of the cluster, called ‘unigene’. Function annotation of the unigenes was conducted against the National Center for Biotechnology Information (NCBI) non-redundant protein sequences database (Nr), NCBI nucleotide sequences database (Nt), Swiss-Prot database, Protein families database (Pfam), clusters of orthologous groups for eukaryotic complete genomes database (KOG), Gene Ontology database (GO) and Kyoto Encyclopedia of Genes and Genomes Database (KEGG). Gene expression levels were estimated using RSEM [48] for each sample. Differential expression analyses between different groups were performed using DESeq2 [49], which used a negative binomial distribution to model the RNA-seq counts for determining differential expression in digital genes. The p-values from differential expression significance analysis were further adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted p-value < 0.05 and an absolute value of log2(fold change) > 1 were recognized as significant differential expression.
2.5. In Vivo Experiment and RNA Interference
The foxl2 siRNA was designed using DSIR (http://biodev.extra.cea.fr/DSIR/DSIR.html, accessed on 1 November 2023). The double-strand foxl2 siRNA (sense 5′-CAUGUGAAGACAUGUUUGAGA-3′, antisense 5′-UCAAACAUGUCUUCACAUGCG-3′) and negative control siRNA (sense 5′-UUCAAUUACAUUCAGGCUUAG-3′, antisense 5′-AAGCCUGAAUGUAAUUGAAUG-3′) were synthesized by AuGCT Biotech Co., Ltd., Beijing, China, respectively. Transfection of siRNA was performed using the reagent of EntransterTM in vivo for RNA (Engreen Biosystem, Auckland, New Zealand) according to the manufacturer’s protocol. The foxl2 siRNA and negative control siRNA were diluted to 1 μg/μL in endotoxin-free pure water. The transfection reagent was diluted to 50% using normal saline. For in vivo transfection, twenty 3-month-old juvenile Yangtze sturgeon (body weight: 21.7 ± 4.7 g) including fifteen females and five males were selected according to the results of sex identification using sex-specific DNA marker. Equal volumes of siRNA (1 μg/μL) and diluted transfection reagent were mixed together. The mixture with 16.7 μg siRNA was injected into the abdominal cavity of female sturgeon through the ventral side of the body. Each of the experimental groups (normal males, normal females, females treated with negative control siRNA, females treated with foxl2 siRNA) consisted of five individuals. At 48 h post-injection, the gonads were collected to quantify the relative expression levels of foxl2, cyp19a1a and hsd17b1 using qRT-PCR.
2.6. Real-Time qPCR
Primers of foxl2, cyp19a1, hsd17b1 and β-actin for qRT-PCR were designed using Oligo software (version 7), of which the sequences were shown as follows: foxl2 (5′-CTTCCTTTCCCCACCGCCTT-3′ and 5′-ACTCTGTCCGGCATCTACCAGT-3′), cyp19a1 (5′-TGCACAACACCCCGAGGTTGA-3′ and 5′-TTCCCTTTCTCACAGTGTAGCCTT-3′), hsd17b1 (5′-AGTTCCATCTTCCACGGTCAGCTT-3′ and 5′-CCAGTCTGCAGCACGTCGACCC-3′) and β-actin (5′-TGACAATGCCGTGCTCGATT-3′ and 5′-CATGGAAGACGAAATTGCCGCACT-3′). The cDNA templates were reverse-transcribed with total RNA from the gonads using the PrimerScript™ RT reagent kit with gDNA Eraser (TaKaRa, Shiga, Japan) following the manufacturer’s instructions. qRT-PCR was performed on a QuantStudio6 Flex Real time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using the TB Green® Premix Ex Taq™ II (Tli RNaseH Plus; TaKaRa, Shiga, Japan). Each sample was amplified in triplicate with the following steps: 94 °C for 5 min; 40 cycles of 94 °C for 30 s, 58 °C for 15 s and 72 °C for 15 s. The relative expression levels were calculated using the arithmetic formula 2−ΔΔCt method based on the expression level of β-actin. One-way analysis of variance (ANOVA) was performed to compare the relative expression levels between two experimental groups. p-values < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS software version 20.0.
3. Results
3.1. Sex Identification and Histological Observation
Twelve juveniles from each developmental stage—30 dph, 70 dph, 80 dph, 90 dph and 180 dph—were randomly selected for sex identification and histological observation of the gonads. Based on the results of sex identification using traditional PCR (Figure 1A), five females and five males were chosen from each developmental stage, of which the gonads were histologically examined. The gonads of Yangtze sturgeon at 30 dph, 70 dph and 80 dph did not show evident morphological differentiation between females and males (Figure S1). Morphological gonadal differentiation of female and male Yangtze sturgeon appeared at 90 dph and 180 dph. Female gonads were characterized by a slightly invaginated gonadal epithelium, while male gonads still showed smooth gonadal epithelium (Figure 1 and Figure S1).
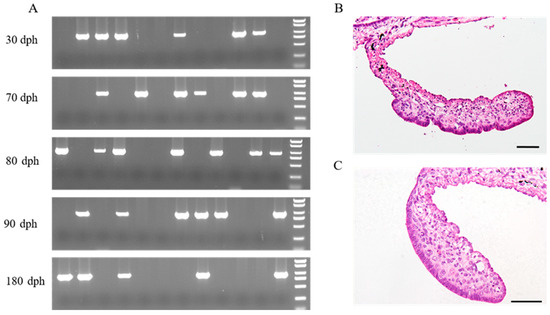
Figure 1.
Sex identification of Yangtze sturgeon juveniles and morphological sex differentiation. (A) Genetic sex identification of Yangtze sturgeon juveniles using female-specific DNA marker; (B) Transverse section of female gonad at 90 days post-hatching (dph); (C) Transverse section of male gonad at 90 dph. The rightmost lanes in five agarose gel electrophoresis maps were DL2000 marker DNA (top to bottom: 2000, 1500, 1000, 750, 500, 250 and 100 bp). The sample with a bright band (approximately 900 bp) was identified to be female.
3.2. Transcriptome Sequencing and Analyses
To explore the gonadal gene expression profiles and vital genes of Yangtze sturgeon in the process of gonadal differentiation, three female and three male gonads during each period (at 30 dph, 80 dph and 180 dph) were selected for transcriptome sequencing. More than 8 Gb (gigabase) of clean data for each sample were obtained for subsequent analyses (Table S1), which were submitted to the NCBI Sequence Read Archive under the accession number PRJNA1022591. In the present study, all the clean data were assembled with the software Trinity, thus generating 698487 transcripts (N50: 930 bp) with a mean length of 627 bp (Table S2). Furthermore, Corset was used to eliminate redundant sequences. The clustered non-redundant transcripts acquired were designated as ‘unigenes’, which included 436558 with an average length of 828 bp and N50 of 1186 bp (Table S2). To obtain comprehensive information on each gene function, unigenes were aligned against seven databases. In total, 217,367 unigenes (49.79%) were annotated in at least one database (Table S3).
The DEGs were identified with the criteria of |log2(fold change)| > 1 and adjusted p-value < 0.05 for comparing differential expression patterns between female and male gonads at 30 dph, 80 dph and 180 dph. A total of 215 DEGs were determined from a comparison of female and male gonads at 30 dph. With the increase in the number of days after hatching, the numbers of DEGs in the comparison groups of female vs. male gonads at 80 dph and 180 dph were increased to 314 and 729, respectively. The clustering patterns of DEGs (1258) from each comparison group were analyzed on the basis of their FPKM values (Figure S2). The result indicated that different expression patterns of these DEGs existed among the different comparison groups, which was consistent with the result of the DEGs Venn diagram in the three comparison groups. The Venn plot showed that only one DEG (hsd17b1) was shared by the three comparison groups, with a total of 17 overlapped DEGs in the three comparison groups (Figure 2A). In addition, there were eight of the same DEGs between female vs. male gonads at 80 dph and 180 dph, of which two, including foxl2 and cyp19a1, were related to estrogen synthesis on the basis of their functional annotation. According to the FPKM values of hsd17b1, foxl2 and cyp19a1, the three DEGs upregulated in female rather than male gonads, the expression level gradually increased in female gonads within the development period (Table S4). In addition, the three DEGs were chosen to verify the reliability of transcriptome data using qRT-PCR. The results of qRT-PCR showed that the expression patterns of the three DEGs were consistent with those obtained from RNA-seq (Figure 2B, Table S5), which showed that the expression analyses based on transcriptome sequencing were reliable.
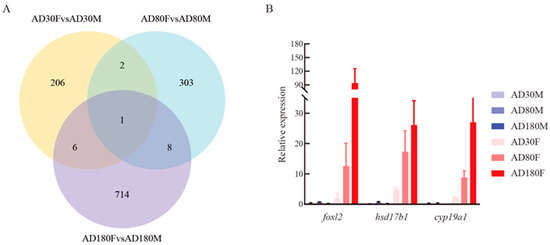
Figure 2.
DEGs Venn diagram and validation of vital DEGs using qRT-PCR. (A) Venn diagram of DEGs among comparisons of female vs. male gonads at 30 dph, female vs. male gonads at 80 dph and female vs. male gonads at 180 dph. (B) The relative expression levels of three overlapped DEGs (foxl2, hsd17b1 and cyp19a1) in the three comparison groups were validated using qRT-PCR. AD30F, AD80F and AD180F represent female gonads at 30 dph, 80 dph and 180 dph, respectively; AD30M, AD80M and AD180M represent male gonads at 30 dph, 80 dph and 180 dph, respectively.
Because genes known to be involved in testis development were not detected in the overlapped DEGs in the three comparison groups, we chose eleven genes reported as active in male sexual development to search for unigenes annotated in this study and DEGs in different comparison groups. The ten genes considered were: dmrt1, star, amh, sox9, gsdf, igf1, wt1, cyp17a1, ar and lh [8,11]. Only gsdf was not found in the set of unigenes; the other genes were matched in the unigenes. Unfortunately, they did not differentially express between female and male gonads at three sampling points (Table S4).
In order to further investigate the DEGs involved in the putative pathways, KEGG pathway enrichment analyses were conducted. No enriched KEGG pathway with a corrected p-value less than 0.05 was found among the three comparison groups (Tables S6–S8). In the comparison group of female vs. male gonads at 30 dph, eight pathways—DNA replication, nucleotide excision repair, mismatch repair, fatty acid elongation, TGF-beta signaling pathway, apoptosis, glycerophospholipid metabolism and NF-kappa B signaling pathway (except the class of disease pathways)—were significantly enriched with no corrected p-value < 0.05 (Figure 3A). Seven pathways—ovarian steroidogenesis, steroid hormone biosynthesis, RIG-I-like receptor signaling pathway, vitamin digestion and absorption, glycerophospholipid metabolism, protein processing in endoplasmic reticulum and allograft rejection (except the class of disease pathways)—were significantly enriched with no corrected p-value < 0.05 in the comparison group of female vs. male gonads at 80 dph (Figure 3B). In the comparison group of female vs. male gonads at 180 dph, we found that ten pathways—steroid hormone biosynthesis, ovarian steroidogenesis, arginine and proline metabolism, neuroactive ligand-receptor interaction, TGF-beta signaling pathway, alanine, aspartate and glutamate metabolism, lysine degradation, tryptophan metabolism, cell adhesion molecules (CAMs) and TNF signaling pathway (except the class of disease pathways)—were significantly enriched with no corrected p-value < 0.05 (Figure 3C). Two significantly enriched KEGG pathways—ovarian steroidogenesis and steroid hormone biosynthesis—involved in the process of estrogen biosynthesis were found in two comparison groups, female vs. male gonads at 80 dph and 180 dph.
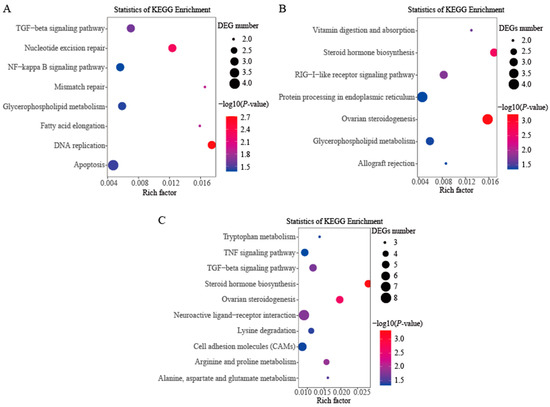
Figure 3.
KEGG significantly enriched pathways under no corrected p-value among the three comparison groups. (A) The comparison group of female vs. male gonads at 30 dph; (B) The comparison group of female vs. male gonads at 80 dph; (C) The comparison group of female vs. male gonads at 180 dph.
3.3. foxl2 Knockdown In Vivo
foxl2 knockdown by siRNA was performed in 3-month-old female Yangtze sturgeon juveniles. The result of qRT-PCR demonstrated that foxl2 siRNA could significantly reduce the expression of foxl2 in female gonads (Figure 4). In the female gonads treated with foxl2 siRNA, the expression levels of hsd17b1 and cyp19a1 were checked. Their expression also presented a significant decrease in comparison with control females, while the expression of the three genes showed no difference between normal and negative females (Figure 4, Table S9).
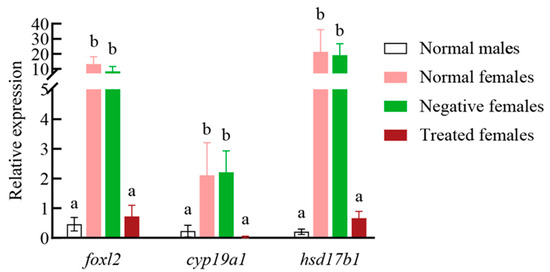
Figure 4.
Relative expression levels of foxl2, cyp19a1 and hsd17b1 in Yangtze sturgeon female gonads treated with foxl2 siRNA. Normal males: males with no treatment. Normal females: females with no treatment. Negative females: females treated with negative control siRNA. Treated females: Females treated with foxl2 siRNA. Different lower-case letters indicate significantly different means: p < 0.05.
4. Discussion
At present, the molecular mechanism of the ZZ/ZW sex determination system remains unclear in vertebrates [50,51,52], especially in fishes. Sturgeon is a primitive fish with the ZZ/ZW sex determination system, with a long lifespan and late puberty (5–30 years of age) [41]. Their preovulatory oocytes are prized as valuable caviar. Investigating expression profiles of sturgeons’ gonads during molecular sex differentiation would be of great benefit for artificial breeding and management and would contribute to our understanding of the mechanism of sex differentiation in fish with the ZZ/ZW sex determination system. In this study, the Yangtze sturgeon was used as a model species of sturgeons to investigate gene expression patterns during gonadal differentiation at the transcriptome level and to discover vital genes involved in gonadal sex differentiation.
To our knowledge, this was the first characterization of the gene expression profiles of undifferentiated and differentiated gonads that occurred in the process of morphological sex differentiation in Yangtze sturgeon juvenile fish with known genetic sex. Morphological sex differentiation of germinal epithelium was observed at 90 dph in this study. Previous studies reported that two distinguishable types of gonads were detected for Yangtze surgeon at 78 dph [45], Russian sturgeon at 3 months post-hatching (mph) [53], Amur sturgeon at 9 mph [10] and shortnose sturgeon (Acipenser brevirostrum) at 6 mph [53]. These results showed that there was variation at the beginning of morphological sex differentiation both among different species of sturgeons and within populations of the same sturgeon species.
Generally, molecular changes precede the morphological sex differentiation of gonads in vertebrates [21,54,55]. Since there was no evident external morphological variation between female and male sturgeon, it was difficult to determine the sex of the fish before the onset of morphological gender differentiation by observing external features or examining the sections of gonads. To solve the problem, the genetic gender of Yangtze sturgeon was identified using our previously developed sex-specific DNA marker before the period of morphological gonadal differentiation. Thus, the gene expression profiles of the gonads from female and male Yangtze sturgeon could be determined during the process of gonadal differentiation. In this study, two stages before morphological sex differentiation and one stage after morphological sex differentiation were selected to explore DEGs between female and male gonads. KEGG pathway enrichment analysis was conducted for the DEGs in each comparative group. No KEGG pathway relating to steroidogenesis was significantly enriched in the comparison group of female vs. male gonads at 30 dph. There were two significantly enriched KEGG pathways relative to steroidogenesis, including ovarian steroidogenesis and steroid hormone biosynthesis, in the comparison groups at 80 dph and 180 dph. These results indicated that pathways involved in steroidogenesis had taken part in gonadal development before morphological sex differentiation, which is consistent with the known function of steroidogenesis playing a crucial role in the process of gonadal development including gonadal differentiation, growth and maturation [56].
We found that DEGs in the ovarian steroidogenesis and steroid hormone biosynthesis KEGG pathways were upregulated in female gonads, except one DEG, cpla2 (Tables S4, S6 and S7). The Venn diagram also showed that three overlapped DEGs (hsd17b1, foxl2 and cyp19a) in the three comparison groups were upregulated in female gonads, which were related to female gonadal development. Similarly, the three genes were sexually dimorphic expressions in morphologically differentiated gonads of Russian sturgeon at 9 mph [21] and in morphologically undifferentiated gonads of Siberian sturgeon at 3 mph [57]. To examine the expression level of foxl2 in gonads of Amur sturgeon during morphological sex differentiation, the results also showed that sexual dimorphic expression of foxl2 was observed in morphologically differentiated gonads [10]. Similar to most other fish, foxl2 exhibits a significant sex dimorphic and ovary-dominant expression pattern [55,58,59,60,61,62,63] and is one of the earliest known markers of ovarian differentiation [59]. In a previous work on beluga (Huso huso), the results showed that foxl2 and cyp19a1 mRNA were sexually dimorphically expressed in female gonads during gonadal sex differentiation and development [12]. The sex-dimorphic expressions in foxl2 and cyp19a1 were also validated in 4-year-old Russian sturgeon [23]. cyp19a1, which encodes the aromatase-converting androgens to estrogens, plays a critical role in ovarian differentiation, development and growth of teleosts [56]. Additionally, hsd17b1 is responsible for interconversion between oestrone and oestradiol and between androstenedione and testosterone [64,65]. Since sexually dimorphic expression of the three genes (hsd17b1, foxl2 and cyp19a) involved in ovarian steroidogenesis were observed before morphological sex differentiation, the expression levels of which gradually increased with the number of days after hatching, these results suggested that estrogens were indispensable for the ovarian differentiation and development of Yangtze sturgeon.
Though our data showed that hsd17b1 was the earliest DEG with sex dimorphic expression among the three overlapped DEGs (hsd17b1, foxl2 and cyp19a), knockdown of foxl2 using siRNAs could lead to a decrease in both hsd17b1 and cyp19a1 mRNA. foxl2 regulating cyp19a1 expression is common to all teleost species [56,66]. It is rarely reported that foxl2 could affect the expression level of hsd17b1 in fish. The result further suggested that foxl2 is critical for ovarian differentiation of Yangtze sturgeon as it could affect hsd17b1 and cyp19a1 expression. Meanwhile, foxl2, except for maintaining cyp19a1 expression, might play a role during ovarian differentiation by regulating hsd17b1 expression in sturgeons. Unfortunately, the three genes do not locate on the sex-variable region of Russian sturgeon [37].
Unlike in female gonads of Yangtze sturgeon, it was surprising that no testis-related DEGs were found at the early development stage of Yangtze sturgeon with the ZZ/ZW sex determination system in this study. It is generally believed that males do not rely on testosterone for testicular determination, yet they ask sex-determining or testis-related genes for the onset of testicular differentiation [56]. However, we did not find DEGs related to testicular determination, for instance, dmrt1 and amh. In previous studies on sturgeons, sexual dimorphic expression of dmrt1 (dmrt1a and dmrt1b) in gonads was not observed in Amur sturgeon [10] or Russian sturgeon [21] during morphological sex differentiation, but dmrt1 presenting testis-dominant expression was found in 2-year-old sterlet [11], 3-year-old Siberian sturgeon [9], 3- and 4-year-old Chinese sturgeon [67] and adult Amur sturgeon [10]. It seems that dmrt1 is not required for early gonadal differentiation of sturgeons, yet it is critical for testis development and growth.
This study provided a snapshot of gene expression profiles during gonadal differentiation at the transcriptome level in Yangtze sturgeon. The results showed that foxl2, cyp19a1 and hsd17b1 appeared to be involved in the onset of ovarian differentiation, as their sex-dimorphic expression was clearly observed in undifferentiated and differentiated morphological gonads. In future, identifying how foxl2 affects expression levels of cyp19a1 and hsd17b1 would lead to a greater understanding of the onset of ovarian steroidogenesis during sexual development in sturgeons, a primitive fish species. Additionally, research is necessary to investigate expression profiles of the onset of testicular differentiation and development, which would enhance our understanding of the molecular mechanisms of sex differentiation in sturgeons.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14112058/s1, Figure S1: Transverse section of female and male gonads at four developmental stages including 30 dph, 70 dph, 80 dph, 90 dph and 180 dph; Figure S2: Cluster analysis of DEGs in the three comparison groups. AD30F represents the group of female gonads at 30 days post hatching (dph); AD30M represents the group of male gonads at 30 dph; AD80F represents the group of female gonads at 80 dph; AD80M represents the group of male gonads at 80 dph; AD180F represents the group of female gonads at 180 dph; AD180M represents the group of male gonads at 180 dph; Table S1: Information of high-throughput sequencing data from gonads at the three developmental stages; Table S2: Statistics of the de novo assembly of all clean data; Table S3: Summary of unigenes annotated in seven public databases; Table S4: Differentially expressed genes between female and male gonads at the three developmental stages; Table S5. Raw qRT-PCR data of foxl2, cyp19a1 and hsd17b1 in the three comparison groups including female vs. male gonads at 30 dph, female vs. male gonads at 80 dph and female vs. male gonads at 180 dph; Table S6: KEGG pathway enrichment analysis of DEGs between female and male gonads at 30 dph; Table S7: KEGG pathway enrichment analysis of DEGs between female and male gonads at 80 dph; Table S8: KEGG pathway enrichment analysis of DEGs between female and male gonads at 180 dph; Table S9. Raw qRT-PCR data of foxl2, cyp19a1 and hsd17b1 in Yangtze sturgeon female gonads treated with foxl2 siRNA.
Author Contributions
Conceptualization, R.R. and C.L.; methodology, R.R.; software, R.R.; validation, Y.L., H.Y. (Huamei Yue) and H.Y. (Huan Ye); formal analysis, R.R.; resources, J.W. and H.D.; data curation, J.J.; writing—original draft preparation, R.R.; writing—review and editing, H.D. and C.L.; supervision, H.D.; project administration, C.L.; funding acquisition, R.R. and H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31702395), the National Key R&D Program of China (2018YFD0900200) and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD23, 2023TD08).
Institutional Review Board Statement
This study was conducted according to the Guidelines for the Care and Use of Laboratory Animals developed by the Ministry of Science and Technology (Beijing, China), and approved by the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (the ethical approval code: 2022YFI-RR-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The clean data from gonadal transcriptome sequencing of Yangtze sturgeon juveniles are available from the NCBI Sequence Read Archive under accession number PRJNA1022591.
Acknowledgments
We thank Xinmei Qiao, Wei Xiong and Jiang Luo for their help in sample and data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shelton, W.L.; Mims, S.D. Evidence for female heterogametic sex determination in paddlefish Polyodon spathula based on gynogenesis. Aquaculture 2012, 356–357, 116–118. [Google Scholar]
- Ruan, R.; Feng, T.; Li, Y.; Yue, H.; Ye, H.; Du, H.; Liu, Q.; Ruan, J.; Li, C.; Wei, Q. Screening and identification of female-specific DNA sequences in octaploid sturgeon using comparative genomics with high-throughput sequencing. Genomics 2021, 113, 4237–4244. [Google Scholar]
- Takehana, Y.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Evolution of ZZ/ZW and XX/XY sex-determination systems in the closely related medaka species, Oryzias hubbsi and O. dancena. Chromosoma 2007, 116, 463–470. [Google Scholar]
- Takehana, Y.; Hamaguchi, S.; Sakaizumi, M. Different origins of ZZ/ZW sex chromosomes in closely related medaka fishes, Oryzias javanicus and O. hubbsi. Chromosome Res. 2008, 16, 801–811. [Google Scholar]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar]
- Matsuda, M.; Sato, T.; Toyazaki, Y.; Nagahama, Y.; Hamaguchi, S.; Sakaizumi, M. Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zoolog. Sci. 2003, 20, 159–161. [Google Scholar]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar]
- Berbejillo, J.; Martinez-Bengochea, A.; Bedo, G.; Brunet, F.; Volff, J.N.; Vizziano-Cantonnet, D. Expression and phylogeny of candidate genes for sex differentiation in a primitive fish species, the Siberian sturgeon, Acipenser baerii. Mol. Reprod. Dev. 2012, 79, 504–516. [Google Scholar]
- Berbejillo, J.; Martinez-Bengochea, A.; Bedó, G.; Vizziano-Cantonnet, D. Expression of dmrt1 and sox9 during gonadal development in the Siberian sturgeon (Acipenser baerii). Fish. Physiol. Biochem. 2013, 39, 91–94. [Google Scholar] [CrossRef]
- Okada, H.; Hagihara, S.; Yamashita, K.; Ijiri, S.; Adachi, S. Expression pattern of foxl2 and dmrt1 in gonad of Amur sturgeon Acipenser schrenckii in relation to sex differentiation. Aquaculture 2017, 479, 712–720. [Google Scholar]
- Wang, W.; Zhu, H.; Dong, Y.; Tian, Z.; Dong, T.; Hu, H.; Niu, C. Dimorphic expression of sex-related genes in different gonadal development stages of sterlet, Acipenser ruthenus, a primitive fish species. Fish. Physiol. Biochem. 2017, 43, 1557–1569. [Google Scholar] [CrossRef]
- Yarmohammadi, M.; Pourkazemi, M.; Kazemi, R. Differential expression of foxl2 and cyp19a1a mRNA during gonad developmental stages in great sturgeon Huso huso. J. Fish. Biol. 2017, 90, 1104–1111. [Google Scholar] [CrossRef]
- Burcea, A.; Popa, G.O.; Florescu Gune, I.E.; Maereanu, M.; Dudu, A.; Georgescu, S.E.; Costache, M. Expression characterization of six genes possibly involved in gonad development for stellate sturgeon individuals (Acipenser stellatus, Pallas 1771). Int. J. Genom. 2018, 2018, 7835637. [Google Scholar]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar]
- Qu, M.; Liu, Y.; Zhang, Y.; Wan, S.; Ravi, V.; Qin, G.; Jiang, H.; Wang, X.; Zhang, H.; Zhang, B.; et al. Seadragon genome analysis provides insights into its phenotype and sex determination locus. Sci. Adv. 2021, 7, eabg5196. [Google Scholar] [CrossRef]
- Zheng, S.; Tao, W.; Yang, H.; Kocher, T.D.; Wang, Z.; Peng, Z.; Jin, L.; Pu, D.; Zhang, Y.; Wang, D. Identification of sex chromosome and sex-determining gene of southern catfish (Silurus meridionalis) based on XX, XY and YY genome sequencing. Proc. Biol. Sci. 2022, 289, 20212645. [Google Scholar] [CrossRef]
- Vidotto, M.; Grapputo, A.; Boscari, E.; Barbisan, F.; Coppe, A.; Grandi, G.; Kumar, A.; Congiu, L. Transcriptome sequencing and de novo annotation of the critically endangered Adriatic sturgeon. BMC Genom. 2013, 14, 407. [Google Scholar] [CrossRef]
- Yue, H.; Li, C.; Du, H.; Zhang, S.; Wei, Q. Sequencing and de novo assembly of the gonadal transcriptome of the endangered Chinese Sturgeon (Acipenser sinensis). PLoS ONE 2015, 10, e0127332. [Google Scholar] [CrossRef]
- Du, H.; Jian, J.; Wang, B.; Liu, X.; Chen, J.; Xiao, K.; Xia, J.; Yang, J.; Gao, Y.; Chen, L. Hypothalamus-pituitary-gonad axis transcriptome profiling for sex differentiation in Acipenser sinensis. Sci. Data 2019, 6, 87. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, L.; Tian, H.; Yang, B.; Wang, E.; Zhu, B. Transcript annotation of Chinese sturgeon (Acipenser sinensis) using Iso-seq and RNA-seq data. Sci. Data 2023, 10, 105. [Google Scholar] [CrossRef]
- Hagihara, S.; Yamashita, R.; Yamamoto, S.; Ishihara, M.; Abe, T.; Ijiri, S.; Adachi, S. Identification of genes involved in gonadal sex differentiation and the dimorphic expression pattern in undifferentiated gonads of Russian sturgeon Acipenser gueldenstaedtii Brandt & Ratzeburg, 1833. J. Appl. Ichthyol. 2014, 30, 1557–1564. [Google Scholar]
- Chen, Y.; Xia, Y.; Shao, C.; Han, L.; Chen, X.; Yu, M.; Sha, Z. Discovery and identification of candidate sex-related genes based on transcriptome sequencing of Russian sturgeon (Acipenser gueldenstaedtii) gonads. Physiol. Genom. 2016, 48, 464–476. [Google Scholar] [CrossRef][Green Version]
- Degani, G.; Hurvitz, A.; Eliraz, Y.; Meerson, A. Sex-related gonadal gene expression differences in the Russian sturgeon (Acipenser gueldenstaedtii) grown in stable aquaculture conditions. Anim. Reprod. Sci. 2019, 200, 75–85. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Gong, Q.; Lai, J.; Song, M.; Du, J.; Deng, X. Gonadal transcriptome sequencing of the critically endangered Acipenser dabryanus to discover candidate sex-related genes. PeerJ 2018, 6, e5389. [Google Scholar] [CrossRef]
- Song, W.; Jiang, K.; Zhang, F.; Lin, Y.; Ma, L. Transcriptome sequencing, de novo assembly and differential gene expression analysis of the early development of Acipenser baeri. PLoS ONE 2015, 10, e0137450. [Google Scholar] [CrossRef]
- Klopp, C.; Lasalle, A.; Di Landro, S.; Vizziano-Cantonnet, D. RNA-Seq transcriptome data of undifferentiated and differentiated gonads of Siberian sturgeon. Data Brief. 2020, 31, 105741. [Google Scholar] [CrossRef]
- Hale, M.C.; McCormick, C.R.; Jackson, J.R.; Dewoody, J.A. Next-generation pyrosequencing of gonad transcriptomes in the polyploid lake sturgeon (Acipenser fulvescens): The relative merits of normalization and rarefaction in gene discovery. BMC Genom. 2009, 10, 203. [Google Scholar] [CrossRef]
- Hale, M.C.; Jackson, J.R.; Dewoody, J.A. Discovery and evaluation of candidate sex-determining genes and xenobiotics in the gonads of lake sturgeon (Acipenser fulvescens). Genetica 2010, 138, 745–756. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, X.; Li, L.; Jiang, H.; Chen, J. High-throughput sequencing of microRNA transcriptome and expression assay in the sturgeon, Acipenser schrenckii. PLoS ONE 2014, 9, e115251. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Li, L.; Jiang, H.; Chen, J. Conservation, sex-biased expression and functional annotation of microRNAs in the gonad of Amur sturgeon (Acipenser schrenckii). Comp. Biochem. Physiol. D Genom. Proteom. 2016, 18, 54–61. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Sun, Y.; Zhu, Y.; Zhang, Z.; Wang, Y. Transcriptome analysis provides insights into differentially expressed genes and long noncoding RNAs involved in sex-related differences in Amur sturgeon (Acipenser schrenckii). Mol. Reprod. Dev. 2019, 86, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, J.; Li, L.; Huang, W.; Ahmad, H.I.; Li, H.; Jiang, H.; Chen, J. Full-length transcriptome sequencing and comparative transcriptomic analysis to uncover genes involved in early gametogenesis in the gonads of Amur sturgeon (Acipenser schrenckii). Front. Zool. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Zhang, Y.; Dong, X.L.; Xi, Q.K.; Song, D.; Fu, H.T.; Sun, D.J. Comparative transcriptome analysis of testes and ovaries for the discovery of novel genes from Amur sturgeon (Acipenser schrenckii). Genet. Mol. Res. 2015, 14, 18913–18927. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Huang, Y.; Du, H.; Li, C.; Lv, Y.; Ruan, R.; Ye, H.; Bian, C.; You, X.; Xu, J.; et al. Draft genome and complete Hox-cluster characterization of the Sterlet (Acipenser ruthenus). Front. Genet. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Stöck, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.; Kichigin, I.; et al. The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Huang, Y.; Lv, Y.; Du, H.; Ruan, Z.; Li, C.; Ye, H.; Zhang, H.; Wu, J.; Wang, C.; et al. The American paddlefish genome provides novel insights into chromosomal evolution and bone mineralization in early vertebrates. Mol. Biol. Evol. 2021, 38, 1595–1607. [Google Scholar] [CrossRef]
- Degani, G.; Nevo Sarel, M.; Hajouj, A.; Hurvitz, A.; Veksler-Lublinsky, I.; Meerson, A. Whole-genome inter-sex variation in Russian sturgeon (Acipenser gueldenstaedtii). Int. J. Mol. Sci. 2022, 23, 9469. [Google Scholar] [CrossRef]
- Wildhaber, M.L.; Papoulias, D.M.; DeLonay, A.J.; Tillitt, D.E.; Bryan, J.L.; Annis, M.L.; Allert, J.A. Gender identification of shovelnose sturgeon using ultrasonic andendoscopic imagery and the application of the method to the pallidsturgeon. J. Fish. Biol. 2010, 67, 114–132. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Leng, X.; Zhang, S.; Luo, J.; Liu, Z.; Qiao, X.; Kynard, B.; Wei, Q. Gender and gonadal maturity stage identification of captive Chinese sturgeon, Acipenser sinensis, using ultrasound imagery and sex steroids. Gen. Comp. Endocrinol. 2016, 245, 36–43. [Google Scholar] [CrossRef]
- Keyvanshokooh, S.; Gharaei, A. A review of sex determination and searches for sex-specific markers in sturgeon. Aquac. Res. 2010, 41, e1–e7. [Google Scholar] [CrossRef]
- Billard, R.; Lecointre, G. Biology and conservation of sturgeon and paddlefish. Rev. Fish. Biol. Fisher 2001, 10, 355–392. [Google Scholar] [CrossRef]
- Kuhl, H.; Guiguen, Y.; Höhne, C.; Kreuz, E.; Du, K.; Klopp, C.; Lopez-Roques, C.; Yebra-Pimentel, E.S.; Ciorpac, M.; Gessner, J.; et al. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200089. [Google Scholar] [CrossRef] [PubMed]
- Scribner, K.T.; Kanefsky, J. Molecular sexing of lake sturgeon. J. Great Lahes Res. 2021, 47, 934–936. [Google Scholar] [CrossRef]
- Ye, H.; Takeuchi, Y.; Du, H.; Yue, H.; Ruan, R.; Li, C.; Wei, Q. Spermatogonia from cryopreserved testes of critically endangered chinese sturgeon efficiently colonized and preferentially proliferated in the recipient gonads of Yangtze sturgeon. Mar. Biotechnol. 2022, 24, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, C.J.; Yue, H.M.; Du, H.; Yang, X.G.; Yoshino, T.; Hayashida, T.; Takeuchi, Y.; Wei, Q.W. Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis. Theriogenology 2017, 94, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Davidson, N.M.; Oshlack, A. Corset: Enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biol. 2014, 15, 410. [Google Scholar]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Okada, E.; Umemoto, H.; Tamura, K.; Uno, Y.; Nishida-Umehara, C.; Matsuda, Y.; Takamatsu, N.; Shiba, T.; Ito, M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2008, 105, 2469–2474. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Hu, W. Progress in research on fish sex determining genes. Water Biol. Secur. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Li, X.Y.; Mei, J.; Ge, C.T.; Liu, X.L.; Gui, J.F. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci. China Life Sci. 2022, 65, 1091–1122. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.R.; Benfey, T.J. Sex differentiation and aspects of gametogenesis in shortnose sturgeon Acipenser brevirostrum Lesueur. J. Fish. Biol. 2007, 70, 1027–1044. [Google Scholar] [CrossRef]
- Hayman, E.S.; Fairgrieve, W.T.; Luckenbach, J.A. Molecular and morphological sex differentiation in sablefish (Anoplopoma fimbria), a marine teleost with XX/XY sex determination. Gene 2021, 764, 145093. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.Y.; Gong, Y.; Guo, X.F.; Liu, M.; Zhou, Y.L.; Li, Z.; Zhou, L.; Wang, Z.W.; Gui, J.F. Gonadal transcriptomes reveal sex-biased expression genes associated with sex determination and differentiation in red-tail catfish (Hemibagrus wyckioides). BMC Genom. 2023, 24, 183. [Google Scholar] [CrossRef]
- Tenugu, S.; Pranoty, A.; Mamta, S.K.; Senthilkumaran, B. Development and organisation of gonadal steroidogenesis in bony fishes—A review. Aquac. Fish. 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Lasalle, A.; Norbis, W.; Vizziano-Cantonnet, D. Sex identification of morphologically-undifferentiated Siberian sturgeon with statistical analysis of gene expression patterns. J. Appl. Ichthyol. 2021, 37, 835–846. [Google Scholar] [CrossRef]
- Wang, D.; Kobayashi, T.; Zhou, L.; Nagahama, Y. Molecular cloning and gene expression of Foxl2 in the Nile tilapia, Oreochromis niloticus. Biochem. Biophys. Res. Commun. 2004, 320, 83–89. [Google Scholar] [CrossRef]
- Nakamoto, M.; Matsuda, M.; Wang, D.S.; Nagahama, Y.; Shibata, N. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2006, 344, 353–361. [Google Scholar] [CrossRef]
- Vizziano, D.; Randuineau, G.; Baron, D.; Cauty, C.; Guiguen, Y. Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Dev. Dyn. 2007, 236, 2198–2206. [Google Scholar] [CrossRef]
- Ijiri, S.; Kaneko, H.; Kobayashi, T.; Wang, D.S.; Sakai, F.; Paul-Prasanth, B.; Nakamura, M.; Nagahama, Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biol. Reprod. 2008, 78, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Zhang, G.R.; Wei, K.J.; Ji, W.; Gardner, J.P.; Yang, R.B.; Chen, K.C. Molecular identification and expression of the Foxl2 gene during gonadal sex differentiation in northern snakehead Channa argus. Fish. Physiol. Biochem. 2015, 41, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zou, Y.; Liang, D.; Tan, X.; Jiao, S.; Wu, Z.; Li, J.; Zhang, P.; You, F. Roles of forkhead box protein L2 (foxl2) during gonad differentiation and maintenance in a fish, the olive flounder (Paralichthys olivaceus). Reprod. Fertil. Dev. 2019, 31, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Desai, R.; Ståhle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Gauri, M.; Li, T.; Wang, R.; Lin, S.X. Current knowledge of the multifunctional 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1). Gene 2016, 588, 54–61. [Google Scholar] [CrossRef]
- Wang, D.S.; Kobayashi, T.; Zhou, L.Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.; Nagahama, Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef]
- Leng, X.Q.; Du, H.J.; Li, C.J.; Cao, H. Molecular characterization and expression pattern of dmrt1 in the immature Chinese sturgeon Acipenser sinensis. J. Fish. Biol. 2016, 88, 567–579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).