Uncoupling Protein 3 Promotes the Myogenic Differentiation of Type IIb Myotubes in C2C12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. C2C12 Cells Culture and Myogenic Differentiation
2.2. RNA Extraction and cDNA Synthesis
2.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. Lentiviral-Mediated Transduction
2.5. Immunofluorescence Staining
2.6. Western Blot
2.7. Statistical Analysis
3. Results
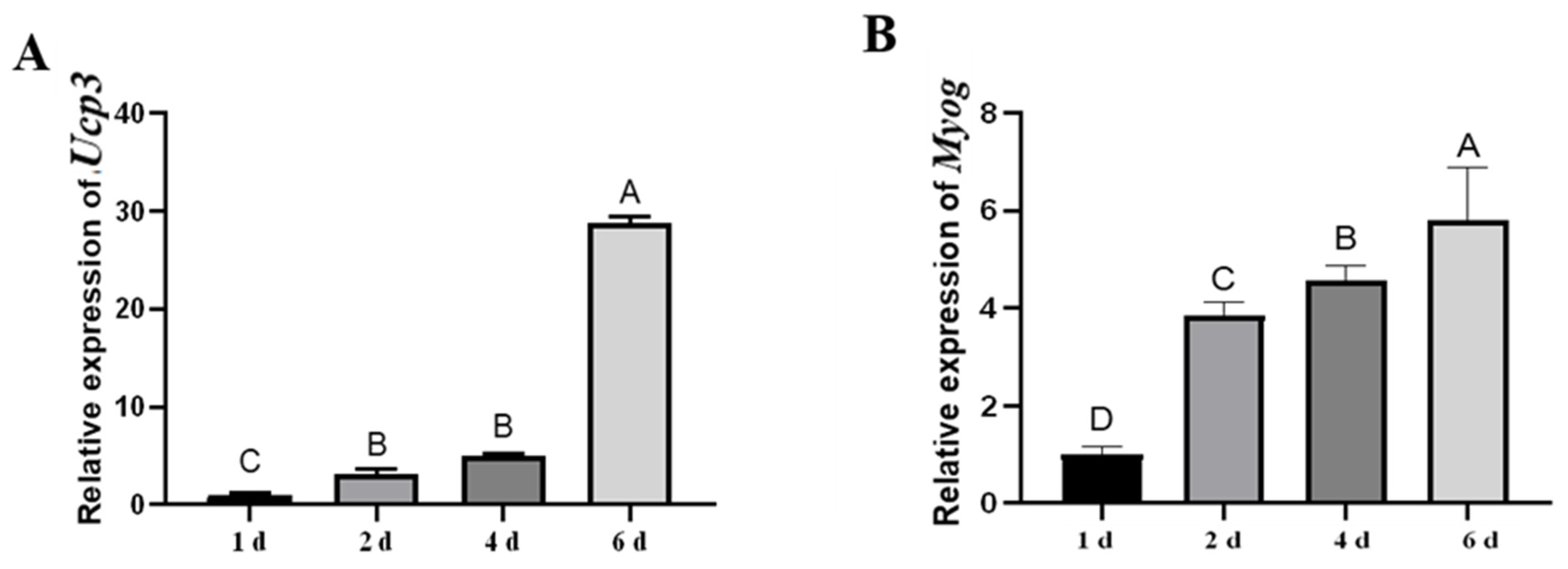
3.1. The Expression Patterns of Ucp3
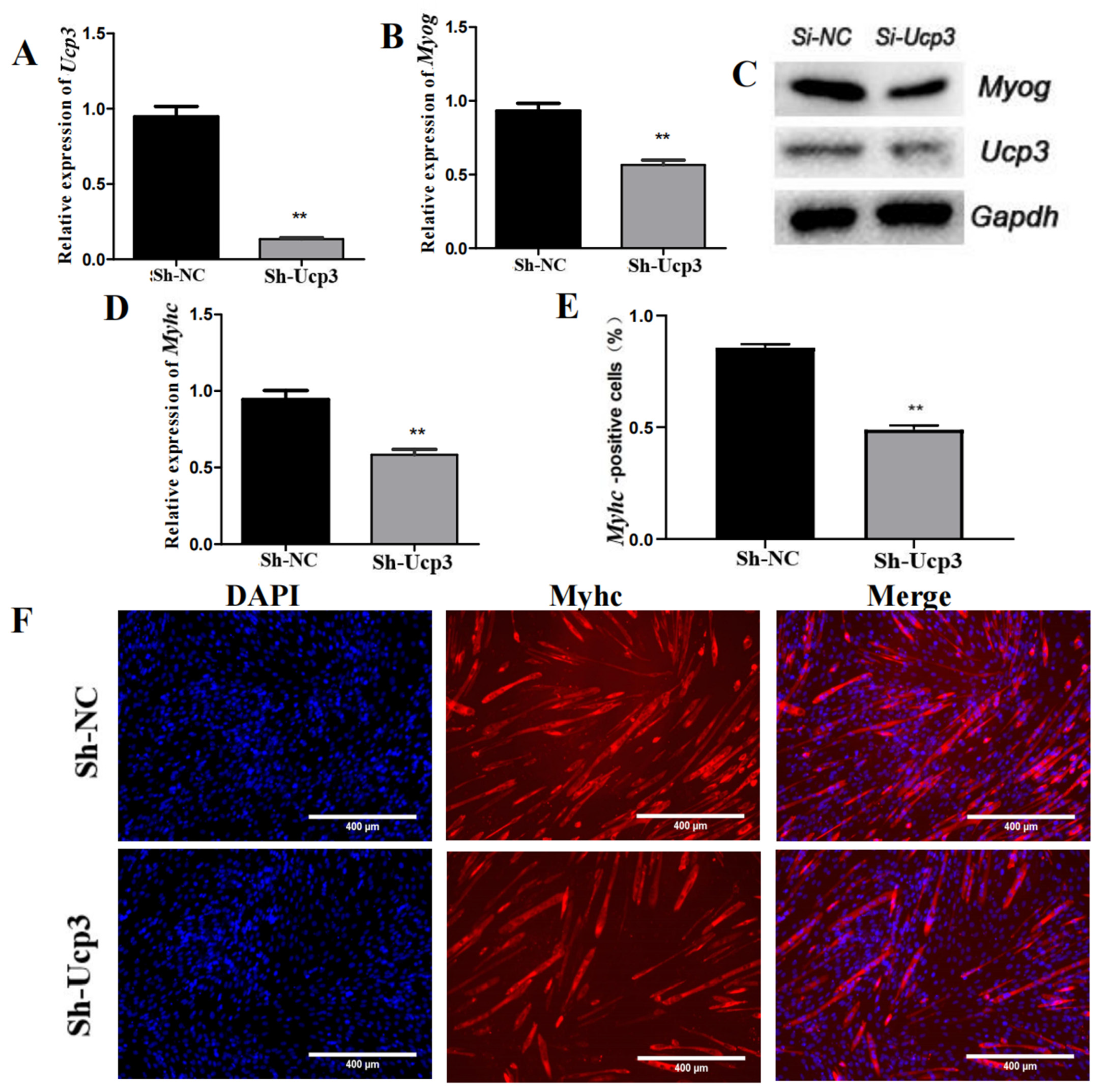
3.2. Ucp3 Promoted Myogenic Differentiation of C2C12 Cells
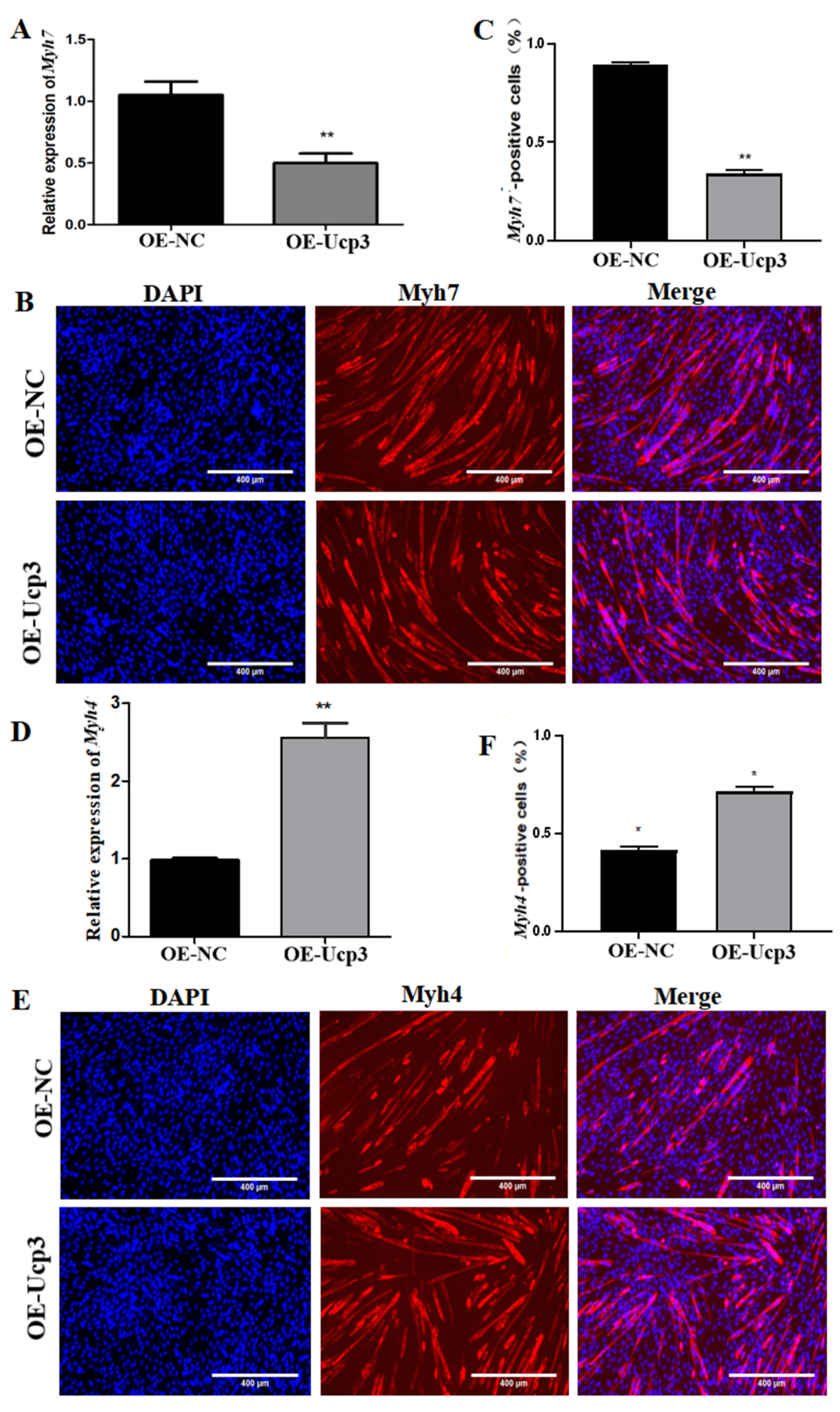
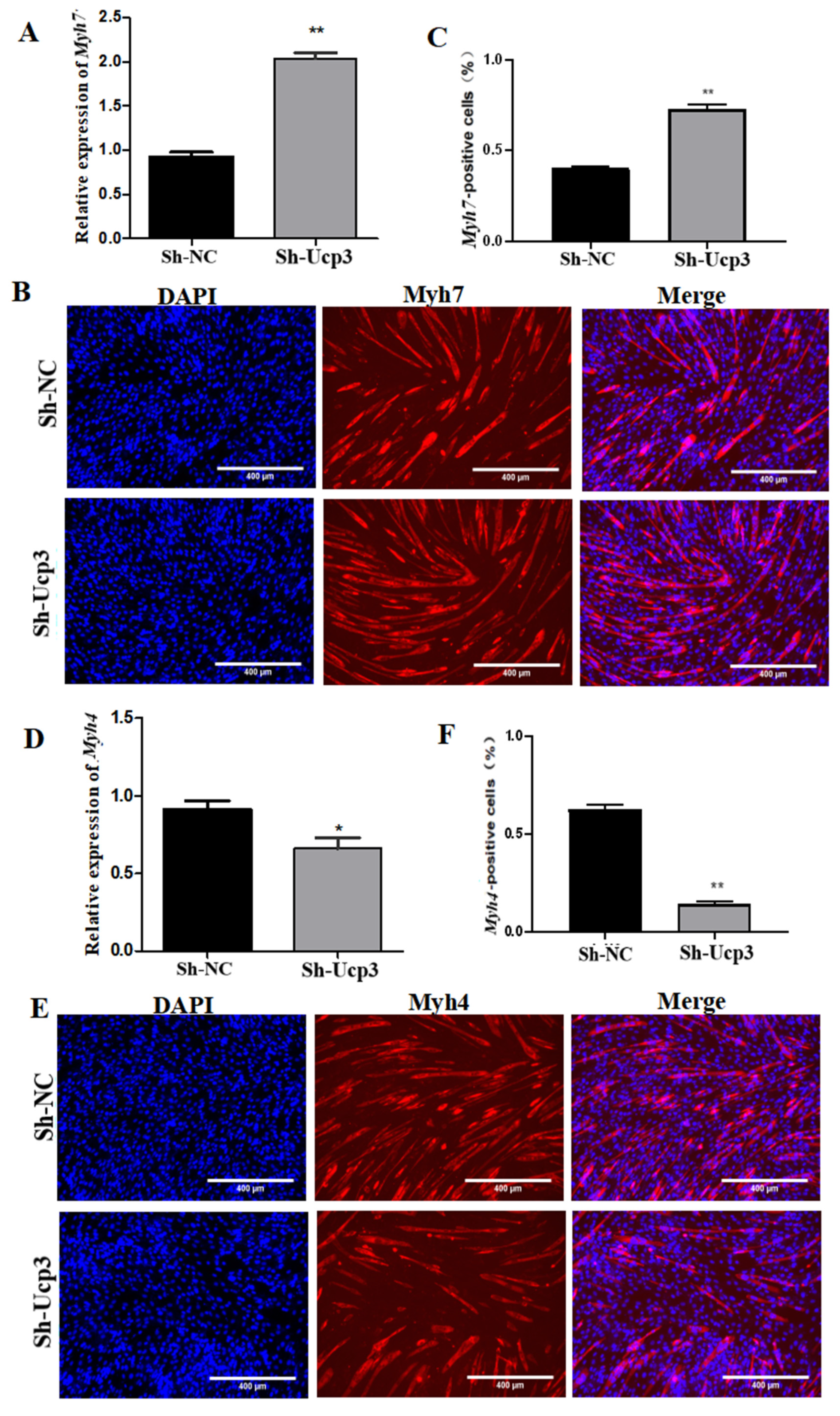
3.3. Effects of Ucp3 on Myofiber Conversion
3.4. Regulation Effects of Ucp3 on Energy Metabolism in C2C12 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qaisar, R.; Renaud, G.; Hedstrom, Y.; Pöllänen, E.; Ronkainen, P.; Kaprio, J.; Alen, M.; Sipilä, S.; Artemenko, K.; Bergquist, J.; et al. Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J. Physiol. 2013, 591, 2333–2344. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Lefaucheur, L. A second look into fibre typing--relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Bakhsh, A.; Lee, J.G.; Joo, S.T. Differences in Muscle Fiber Characteristics and Meat Quality by Muscle Type and Age of Korean Native Black Goat. Food Sci. Anim. Resour. 2019, 39, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Damon, M.; Vincent, A.; Lombardi, A.; Herpin, P. First evidence of uncoupling protein-2 (UCP-2) and -3 (UCP-3) gene expression in piglet skeletal muscle and adipose tissue. Gene 2000, 246, 133–141. [Google Scholar] [CrossRef]
- Millet, L.; Vidal, H.; Andreelli, F.; Larrouy, D.; Riou, J.P.; Ricquier, D.; Laville, M.; Langin, D. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J. Clin. Investig. 1997, 100, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cao, C.; Tao, C.; Ye, R.; Dong, M.; Zheng, Q.; Wang, C.; Jiang, X.; Qin, G.; Yan, C.; et al. Cold adaptation in pigs depends on UCP3 in beige adipocytes. J. Mol. Cell Biol. 2017, 9, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Qian, L.; Jiang, S.; Sun, Y.; Wang, Q.; Ma, D.; Xiao, G.; Li, B.; Xie, S.; Gao, T.; et al. Loss-of-function myostatin mutation increases insulin sensitivity and browning of white fat in Meishan pigs. Oncotarget 2017, 8, 34911–34922. [Google Scholar] [CrossRef] [PubMed]
- Bezaire, V.; Spriet, L.L.; Campbell, S.; Sabet, N.; Gerrits, M.; Bonen, A.; Harper, M.E. Constitutive UCP3 overexpression at physiological levels increases mouse skeletal muscle capacity for fatty acid transport and oxidation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 977–979. [Google Scholar] [CrossRef]
- MacLellan, J.D.; Gerrits, M.F.; Gowing, A.; Smith, P.J.; Wheeler, M.B.; Harper, M.E. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes 2005, 54, 2343–2350. [Google Scholar] [CrossRef]
- Costford, S.R.; Chaudhry, S.N.; Crawford, S.A.; Salkhordeh, M.; Harper, M.E. Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1018–E1024. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, M.K.; Keizer, H.A.; Borghouts, L.B.; Schaart, G.; Kornips, C.F.; Slieker, L.J.; Sloop, K.W.; Saris, W.H.; Schrauwen, P. Protein expression of UCP3 differs between human type 1, type 2a, and type 2b fibers. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1071–1073. [Google Scholar]
- Zheng, Q.; Lin, J.; Huang, J.; Zhang, H.; Zhang, R.; Zhang, X.; Cao, C.; Hambly, C.; Qin, G.; Yao, J.; et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E9474–E9482. [Google Scholar] [CrossRef] [PubMed]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lee, K.A.; Willemsen, P.H.; Van Der Vusse, G.J.; Van Bilsen, M. Effects of fatty acids on uncoupling protein-2 expression in the rat heart. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 495–502. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.J.; Seong, J.K. UCP2 KO mice exhibit ameliorated obesity and inflammation induced by high-fat diet feeding. BMB Rep. 2022, 55, 500–505. [Google Scholar] [CrossRef]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.P. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef]
- Vidal-Puig, A.; Solanes, G.; Grujic, D.; Flier, J.S.; Lowell, B.B. UCP3: An uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem. Biophys. Res. Commun. 1997, 235, 79–82. [Google Scholar] [CrossRef]
- Razeghi, P.; Young, M.E.; Ying, J.; Depre, C.; Uray, I.P.; Kolesar, J.; Shipley, G.L.; Moravec, C.S.; Davies, P.J.; Frazier, O.H.; et al. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology 2002, 97, 203–209. [Google Scholar] [CrossRef]
- Hayashi, M.; Futawaka, K.; Matsushita, M.; Koyama, R.; Fun, Y.; Fukuda, Y.; Nushida, A.; Nezu, S.; Tagami, T.; Moriyama, K. GH directly stimulates UCP3 expression. Growth Horm. IGF Res. 2018, 40, 44–54. [Google Scholar] [CrossRef]
- Nau, K.; Fromme, T.; Meyer, C.W.; von Praun, C.; Heldmaier, G.; Klingenspor, M. Brown adipose tissue specific lack of uncoupling protein 3 is associated with impaired cold tolerance and reduced transcript levels of metabolic genes. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2008, 178, 269–277. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Lanni, A.; Beneduce, L.; Lombardi, A.; Moreno, M.; Boss, O.; Muzzin, P.; Giacobino, J.P.; Goglia, F. Expression of uncoupling protein-3 and mitochondrial activity in the transition from hypothyroid to hyperthyroid state in rat skeletal muscle. FEBS Lett. 1999, 444, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Costford, S.R.; Seifert, E.L.; Bézaire, V.; Gerrits, M.F.; Bevilacqua, L.; Gowing, A.; Harper, M.E. The energetic implications of uncoupling protein-3 in skeletal muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 884–894. [Google Scholar] [CrossRef]
- Son, J.S.; Chae, S.A.; Zhao, L.; Wang, H.; de Avila, J.M.; Zhu, M.J.; Jiang, Z.; Du, M. Maternal exercise intergenerationally drives muscle-based thermogenesis via activation of apelin-AMPK signaling. EBioMedicine 2022, 76, 103842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, X.; Zhang, M.; Tong, F.; Chen, H.; Wang, X.; Xiu, N.; Liu, Z.; Wang, Y. microRNA-181a Promotes Mitochondrial Dysfunction and Inflammatory Reaction in a Rat Model of Intensive Care Unit-Acquired Weakness by Inhibiting IGFBP5 Expression. J. Neuropathol. Exp. Neurol. 2022, 81, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jung, J.I.; Jeon, Y.E.; Kim, S.M.; Oh, T.K.; Lee, J.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract and Gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes. Nutr. Res. Pract. 2022, 16, 14–32. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Li, F.; Wang, W.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Yin, Y. Effect of branched-chain amino acid ratio on the proliferation, differentiation, and expression levels of key regulators involved in protein metabolism of myocytes. Nutrition 2017, 36, 8–16. [Google Scholar] [CrossRef]
- Watamoto, Y.; Futawaka, K.; Hayashi, M.; Matsushita, M.; Mitsutani, M.; Murakami, K.; Song, Z.; Koyama, R.; Fukuda, Y.; Nushida, A.; et al. Insulin-like growth factor-1 directly mediates expression of mitochondrial uncoupling protein 3 via forkhead box O4. Growth Horm. IGF Res. 2019, 46–47, 24–35. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hesselink, M. Uncoupling protein 3 and physical activity: The role of uncoupling protein 3 in energy metabolism revisited. Proc. Nutr. Soc. 2003, 62, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Xiang, Y.; Ai, Z.; You, Z.; Jin, X.; Wan, Y.; Yang, Y. UCP3 Ablation Exacerbates High-Salt Induced Cardiac Hypertrophy and Cardiac Dysfunction. Cell. Physiol. Biochem. 2018, 46, 1683–1692. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Jiang, L.; Hou, J.; Yang, Z. Curcumin Improves Cardiopulmonary Resuscitation Outcomes by Modulating Mitochondrial Metabolism and Apoptosis in a Rat Model of Cardiac Arrest. Front. Cardiovasc. Med. 2022, 9, 908755. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Begani, G.; Guida, L.; Magnone, M.; Galante, D.; D’Arrigo, C.; Scotti, C.; Iamele, L.; De Jonge, H.; Zocchi, E.; et al. LANCL1 binds abscisic acid and stimulates glucose transport and mitochondrial respiration in muscle cells via the AMPK/PGC-1α/Sirt1 pathway. Mol. Metab. 2021, 53, 101263. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.; Xiong, Y.; Yang, J. Skeletal Muscle-Specific Overexpression of PGC-1α Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Lantier, L.; Fentz, J.; Mounier, R.; Leclerc, J.; Treebak, J.T.; Pehmøller, C.; Sanz, N.; Sakakibara, I.; Saint-Amand, E.; Rimbaud, S.; et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 3211–3224. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hoeks, J.; Hesselink, M.K. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: Involvement in fatty acid metabolism? Prog. Lipid Res. 2006, 45, 17–41. [Google Scholar] [CrossRef]
- Aguer, C.; Fiehn, O.; Seifert, E.L.; Bézaire, V.; Meissen, J.K.; Daniels, A.; Scott, K.; Renaud, J.M.; Padilla, M.; Bickel, D.R.; et al. Muscle uncoupling protein 3 overexpression mimics endurance training and reduces circulating biomarkers of incomplete β-oxidation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 4213–4225. [Google Scholar] [CrossRef]
- Nagai, S.; Ikeda, K.; Horie-Inoue, K.; Shiba, S.; Nagasawa, S.; Takeda, S.; Inoue, S. Estrogen modulates exercise endurance along with mitochondrial uncoupling protein 3 downregulation in skeletal muscle of female mice. Biochem. Biophys. Res. Commun. 2016, 480, 758–764. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Zhou, P.; Zhang, Z.; Liu, J.; Zhang, H. MicroRNA-152 Promotes Slow-Twitch Myofiber Formation via Targeting Uncoupling Protein-3 Gene. Anim. Open Access J. MDPI 2019, 9, 669. [Google Scholar] [CrossRef]
- Lima, T.I.; Guimarães, D.; Sponton, C.H.; Bajgelman, M.C.; Palameta, S.; Toscaro, J.M.; Reis, O.; Silveira, L.R. Essential role of the PGC-1α/PPARβ axis in Ucp3 gene induction. J. Physiol. 2019, 597, 4277–4291. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequences (5′–3′) |
|---|---|
| Ucp3 | F: GCCGGCACTGCGGCCTGTTTT R: TGTGCGCACCATAGTCAGGAT |
| Myog | F: TCCCAACCCAGGAGATCATT R: AGTTGGGCATGGTTTCGTCT |
| Myhc | F: ACTTGTGGTGTCGGTCACTC R: CTGAAAATCAGCCGCACGTC |
| Myh4 | F: CTCACCTACCAGACCGAGGA R: CTCCTGTCACCTCTCAACAGA |
| Myh7 | F: GATTCCTCTAGGACAGCAGCG R: TTCCTTTCTCTGAGCCACCTTG |
| Pgc1-α | F: TGTGTGCTGTGTGTCAGAGT R: ACCAGAGCAGCACACTCTAT |
| Atp5a1 | F: TTGTTGGTGCAAGAAATCTCCA R: TACCATCACCAATGCTTAACACA |
| Ldha | F: AACTTGGCGCTCTACTTGCT R: GGACTTTGAATCTTTTGAGACCTTG |
| Ldhb | F: AAAGGCTACACCAACTGGGC R: GCCGTACATTCCCTTCACCA |
| Uqcrc2 | F: CCGGGTCCTTCTCGAGATTTT R: TGCTTCAATCCCACGGGTTA |
| Ndufa9 | F: TTCCAATGTCACGTCCTGCC R: CTTGTGACCCCATTCGTCCA |
| 18S rRNA | F: ATAAACGATGCCGACTGGCGAT R: CAATCTGTCAATCCTGTCCGTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Z.; Wang, J.; Li, F.; Hei, W.; Li, M.; Guo, X.; Gao, P.; Cao, G.; Cai, C.; Li, B. Uncoupling Protein 3 Promotes the Myogenic Differentiation of Type IIb Myotubes in C2C12 Cells. Genes 2023, 14, 2049. https://doi.org/10.3390/genes14112049
You Z, Wang J, Li F, Hei W, Li M, Guo X, Gao P, Cao G, Cai C, Li B. Uncoupling Protein 3 Promotes the Myogenic Differentiation of Type IIb Myotubes in C2C12 Cells. Genes. 2023; 14(11):2049. https://doi.org/10.3390/genes14112049
Chicago/Turabian StyleYou, Ziwei, Jieyu Wang, Faliang Li, Wei Hei, Meng Li, Xiaohong Guo, Pengfei Gao, Guoqing Cao, Chunbo Cai, and Bugao Li. 2023. "Uncoupling Protein 3 Promotes the Myogenic Differentiation of Type IIb Myotubes in C2C12 Cells" Genes 14, no. 11: 2049. https://doi.org/10.3390/genes14112049
APA StyleYou, Z., Wang, J., Li, F., Hei, W., Li, M., Guo, X., Gao, P., Cao, G., Cai, C., & Li, B. (2023). Uncoupling Protein 3 Promotes the Myogenic Differentiation of Type IIb Myotubes in C2C12 Cells. Genes, 14(11), 2049. https://doi.org/10.3390/genes14112049





