Abstract
In this study, to explore the effect of growth hormone changes on the related genes and regulatory roles of the turtle, PCR amplification, real-time fluorescence quantitative analysis, and enzyme cutting technology were used to clone and sequence the somatostatin (SS) gene, growth hormone receptor (GHR), and insulin-like growth factor-1 (IGF-I) sequence of Chinemys reevesii. The effects of human growth hormone on the mRNA expression of growth-axis-related genes SS, GHR, and IGF-1 in different sexes were observed. The study of the SS gene in turtles using real-time fluorescence quantitative PCR showed that the SS gene was mainly expressed in the nervous system and the digestive system, with the highest expression found in the brain, while the GHR gene and the IGF-I gene were expressed in all tissues of Chinemys reevesii. The SS gene was expressed in the brain, pituitary, liver, stomach, and intestine, with the highest expression in the brain and the lowest expression in the liver. Within 4 weeks of the injection of exogenous growth hormone, the expression level of the SS gene in the brain of both sexes first increased and then decreased, showing a parabolic trend, and the expression level of the experimental group was lower than that of the control group. After the injection of growth hormone (GH), the expression of the GHR gene in the liver of both sexes showed a significant increase in the first week, decreasing to the control group level in the second week, and then gradually increasing. Finally, a significant level of difference in the expression of the GHR gene was reached at 3 and 4 weeks. In terms of the IGF-I gene, the changing trend of the expression level in the liver was the same as that of the GHR gene. After the injection of exogenous growth hormone, although the expression of the SS gene increased the inhibition of the secretion of the GHR gene by the Reeves’ turtle, exogenous growth hormone could replace the synthesis of GH and GHR, accelerating the growth of the turtle. The experiments showed that the injection of recombinant human growth hormone affects the expression of SS, GHR, and IGF-1 genes, and promotes the growth of the Reeves’ turtle.
1. Introduction
Turtles are long-living animals with complicated body growth, and there is both determinate and indeterminate body growth in this group [1,2,3,4]. The main difference is in the ability to continue growth throughout life in indeterminate growers, while the determinate ones cease their skeletal growth typically close to sexual maturation [5,6,7]. There are many kinds of turtles, roughly divided into three kinds: tortoise, terrapin, and semi-water turtle. Chinemys reevesii is a freshwater turtle belonging to Chordata, Reptilia, Testudoformes, and Geoemydidae that is mainly distributed in subtropical and temperate regions, such as China, Japan, and South Korea. Generally, turtles are long-living and slow-growing animals, making them suitable for use as animal models to study growth [8,9]. However, determining why turtles grow slowly remains an unsolved problem. Moreover, the causal relationship between growth retardation and gene expression patterns related to the growth axis is still unclear. Growth hormone and somatostatin (SS) are key factors that regulate the growth and development of an animal [10]. To date, the SS gene expression pattern of the Reeves’ turtle remains unknown. In previous studies on the hypophysis microstructure, adenohypophysis ultrastructure, and blood biochemical indexes [11,12], it was found that fewer granules are secreted by the GH cells of the hypophysis of turtles compared with other animals.
SS is a low molecular basic peptide secreted in the hypothalamus, and was first discovered by British scientists in 1968 [13]. After a series of experiments, such as separation, purification, and identification, Brazeau et al. extracted the substance successfully, determined its structure as a cyclic 14-peptide (SS-14), and officially named it as SS [14]. Pradayrol et al. later found that SS-28 and SS-25 of the SS-14 amino-terminal extension were both encoded by the same gene in mammals [15,16]. Since the discovery of SS, it has been detected in various vertebrates, mainly in the nervous system and the digestive system. It has many functions, such as inhibiting the activity of the nervous system and the spontaneous electrical activity of the brain [17]. An injection of SS into the animal brain and spinal cord can effectively relieve pain [18]. In the clinic, SS can inhibit the production of antibodies via B lymphocytes and can also inhibit the release of inflammatory factors [19,20,21,22] to resist the inflammatory reaction. In addition, it can inhibit the exocrine function of the gastrointestinal tract and pancreas, the release of gastrointestinal hormones and proteases, gastrointestinal peristalsis, and the repair of gastrointestinal mucosa [23]. In terms of regulating animal growth, growth hormone and insulin-like growth factor-1 (IGF-I) can promote animal growth, while SS can inhibit the release of various hormones, such as growth hormone (GH), thyroid-stimulating hormone (TSH), gonadotropin-releasing hormone (GnRH), etc. [24,25], which play a negative feedback role in the growth axis, and work with growth hormone and IGF-I to regulate the growth and metabolism of animals. Based on this function, SS can effectively inhibit the growth of tumors [26,27,28]. Mclean and Donaldson found that an exogenous injection of growth hormone extracted from mammals can promote the growth of amphibians and reptiles [29]. Therefore, in this study, we injected turtles with human growth hormone to observe the expression of SS mRNA, GHR mRNA, and IGF-I mRNA related to the growth axis of the Reeves’ turtle of different sexes, to explore the effect of growth hormone on related genes and its regulatory role, and to determine the main reason for the slow growth of turtles.
2. Materials and Methods
2.1. Experimental Animal
In this experiment, male and female turtles were identified and 100 healthy first-year young turtles were selected. There are two main criteria used for the discrimination of sex in C. reevesii. One is the turtle’s belly nail—the female’s is flat and the pattern is denser, while the male’s is concave and the pattern is sparser. The other is the turtle’s tail—the female tail is slender and close to the anus, while the male tail is shorter and far from the anus. The experimental animals were from Guangdong Zhangcheng Ecological Agriculture Investment Co., Ltd (Guangdong, China). with a weight of 80–100 g. The water temperature was kept at 24–26 °C. The change in the water temperature in the aquaculture pond was affected by the day/night cycle, but due to the thermal characteristics of the water itself, the change in water temperature in the aquaculture pond was much smaller than the air temperature. In addition, the water level was 1 cm higher than the back of the young turtle. The amount of feed was approximately 3% of the total body weight of the young turtle. The C. reevesii feed used in the experiment was purchased from Shanghai Derui Feed Co., Ltd (Shanghai, China). The main ingredients of the feed were crude protein 30%, total phosphorus 0.8%, lysine 1.1%, crude fiber 6.5%, 13% crude ash, 3% calcium, 3% crude fat, and 10% water. The Animal Care Committee of South China Agriculture University (Guangzhou, China) approved the current study under the trial registration number G027, and the research was accomplished according to the Experimental Animal Management Law of China.
2.2. Sample Preparation
The growth hormone used in the experiment was sourced from Shanghai Shushi Biotechnology Co., Ltd (Shanghai, China). The male and female turtles in the experimental group were injected with growth hormone from 10 μg/g via by intraperitoneal injection once a week, lasting 4 weeks. The Reeves’ turtles in the control group were injected with same volume of PBS, and the other breeding conditions were consistent with the experimental group. Every week, male and female turtles were randomly selected for dissection once a week, and seven organs (brain, hypophysis, liver, spleen, kidney, stomach, and intestine) were placed in liquid nitrogen, and then transferred to the refrigerator at −80 °C for preservation.
2.3. Tissue Collection and Isolation of RNA
In order to determine the expression pattern of the tissue-specific gene, seven organs and tissues were removed from the turtles: liver, spleen, kidney, brain, hypophysis gland, stomach, and intestine. The total RNA was extracted with the Eastep Super RNA Extraction Kit (Promega, Beijing, China), and the cDNA was then synthesized via reverse transcription from 1 μg RNA (OD260/OD280) with the GoScriptTM RT Mix Kit (Promega, Beijing, China).
2.4. Cloning and DNA Sequencing of the SS Gene of the Reeves’ Turtle
The NCBI website was used to query the cDNA sequence of the SS gene of the species close to the Reeves’ turtle in GenBank, and DNAMAN software (LynnonBiosoft, American) was used to determine its conservative region. Primer Premier 6.0 software was employed to design specific nucleotide sequence primers for the SS gene of the turtles. Accordingly, the forward and reverse primers were 5′-GGCAAACAGGAACTGGC-3′ and 5′-ACAGTCTTCGGCTCGTGTCGTG-3′, respectively. PCR was performed by using the DNA polymerase of 2 × TransStart® KD Plus PCR SuperMix (TransGen Biotech, Beijing, China) under the following conditions: an initial denaturing cycle at 94 °C for 5 min, followed by 30 cycles of amplification consisting of denaturation at 94 °C for 30 s, annealing at 52 °C for 1 min, and extension at 68 °C for 1 min. A final extension at 68 °C for 10 min was added after the last cycle. The PCR product was run using 1% (w/v) agarose gel electrophoresis with 30 mM Tris-acetate buffer (pH 8.0) containing 1 mM EDTA and visualized using ethidium bromide (0.5 µg/mL). The PCR product was linked with a pMD18-T simple vector (Takara Bio, Beijing, China) to form a recombinant plasmid. The recombinant plasmid DNA was introduced into DH5α-sensitive Escherichia coli for culture and amplification. Sequencing was then carried out by Qingke Biotechnology Co., Ltd. (Beijing, China). The cloned SS PCR product was expected to be 294 base pairs (bp).
2.5. RT-PCR
The nucleotide sequence of the SS gene of turtles was obtained via sequencing, and RT-PCR primers were designed. The forward and reverse primers were 5′-GGCAAACAGGAACTGGC-3′ and 5′-TTAGCCGATCGCTCCAACTC-3′, respectively. The gene expression of each turtle’s organ was detected using the newly acquired SS sequence, GHR gene, and IGF-I gene obtained from previous experiments in the laboratory. This was carried out under the following conditions: an initial denaturing cycle at 95 °C for 2 min, followed by 40 cycles of amplification at 95 °C for 15 s, the annealing temperature was then adjusted according to primer temperature for 1 min, the extension was at 72 °C for 1 min, the fluorescence signal was collected at 72 °C, and dissolution curve analysis was added to the reaction conditions at the end of the cycle. In this study, β-actin was used as the internal reference gene of RT-PCR.
2.6. Statistical Analysis
Considering the influence of the size, age, and kinship of the experimental animals, first-year turtles of the same size were selected for the experiment. The turtles selected in this experiment were full-sibs. The data in this study were expressed as the mean ± SEM (standard error of mean; the standard error of the sample mean was used to measure the gap between the sample mean and the population mean). One-way ANOVA was used to test the differences between the groups. The difference was significant (p < 0.05). All the statistics were calculated using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and the data were logarithmically transformed.
3. Results
3.1. Tissue Distribution of SS Gene Expression in the Reeves’ Turtle
To determine the expression of the SS gene in the liver, spleen, kidney, brain, hypophysis, stomach, and intestinal tissue of the Reeves’ turtle, we cloned part of the SS gene for subsequent research. The qRT-PCR results showed that the expression level of SS mRNA in the brain was the highest, whether in male or female Reeves’ turtles. The expression level of SS in the hypophysis was nearly half that of the brain. The expression of SS in these two tissues was higher than in other tissues. Compared with the SS mRNA expression in the brain, the SS gene expression levels in the stomach, intestine, and liver tissues were approximately 7.9%, 1%, and 0.2%, respectively. Interestingly, the expression levels of the SS gene in the male brain and the hypophysis were significantly higher than those in the female (p < 0.05). However, the expression of SS in other tissues shared no sex difference. The transcription of SS could not be detected either in the male/female spleen or kidney (Figure 1).
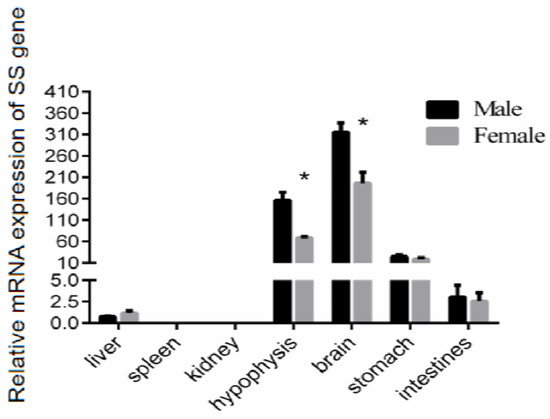
Figure 1.
Relative gene expression of SS in male and female Reeves’ turtles. Total RNA from different tissues of three Reeves’ turtles was used to perform the real-time PCR. Each sample was performed in duplicate. The expression levels of the SS gene in the Reeves’ turtles were normalized to the expression of the β-actin gene. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and were subjected to variance analysis (ANOVA) using the Open GL Mathematics (GLM) process. Different superscripts signify significant differences (* p < 0.05). Data are expressed as mean ± standard error of the mean (n = 3).
3.2. Modulation of SS Gene Expression in the Brain under Periodic Injection of Growth Hormone
The growth hormone/insulin-like growth factor (GH/IGF) axis is an essential endocrine system regulating vertebrate growth [30]. However, the effect of growth hormone on the expression of SS in Reeves’ turtles was still unclear. Given that the highest mRNA expression level of SS was found in the brain, we analyzed the modulation of SS expression in the brain after periodic injections of growth hormone. The qRT-PCR results showed that the SS mRNA expression in the brain tissue did not change significantly in the first week after injection (p > 0.05, effect size of Cohen d = 0.2). After two weeks of continuous injection, the expression of SS in the brain tissue of female turtles increased significantly (p < 0.01, effect size of Cohen d = 0.8) (Figure 2a). After two or three weeks of continuous injection, the expression of SS mRNA in the male turtle brain increased significantly (p < 0.01, effect size of Cohen d = 0.8) (Figure 2b). Interestingly, in the fourth week of continuous injection, the SS gene expression in the brain tissue of male and female turtles shared no significant difference compared with the control group (p > 0.05, effect size of Cohen d = 0.2). Collectively, the SS gene expression in the brain of male and female turtles presented a trend of increasing first, and then decreasing. Further data analysis revealed that, in the third week of continuous GH injection, expression of the SS gene in the brain tissue of female turtles was significantly higher than that of males (p < 0.05) (Figure 2c).
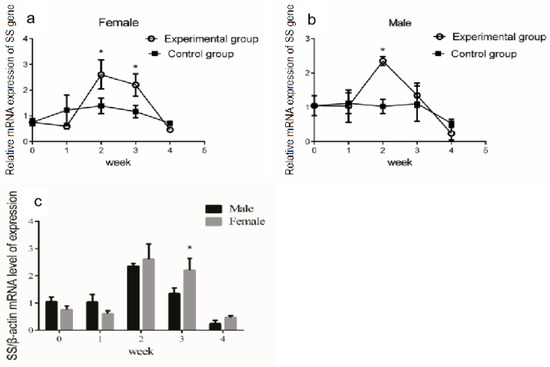
Figure 2.
Modulation of SS expression in the brain after injection of GH. The turtles were divided into two groups, the experimental group and the control group, which contained the same number of male and female turtles. We first measured the body weight of the turtles. Male or female Reeves’ turtles in the experimental group were injected with a certain amount of growth hormone weekly. The turtles to be injected were weighed and were injected according to the standard 10 μg/g. Meanwhile, male or female Reeves’ turtles in the control group were injected with same volume of PBS. (a) Relative SS gene mRNA expression in the brain tissue of male Reeves’ turtle. (b) Relative SS gene mRNA expression in the brain tissue of female Reeves’ turtle. (c) Sex differences regarding SS gene expression in the brain after four weeks of GH injection. β-actin was used as the internal gene of qRT-PCR. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and were subjected to variance analysis (ANOVA) using the GLM process. Different superscripts signify significant differences (* p < 0.05). Data are expressed as mean ± standard error of the mean (n = 3).
3.3. Effect of Exogenous Growth Hormone on SS Gene Expression in Tissues of the Reeves’ Turtle
Given the modulation of SS gene expression in the brain through GH injection, we further analyzed whether a similar effect existed in other organs of the Reeves’ turtle. The turtles were divided into two groups: the experimental group and the control group. The male and female Reeves’ turtles in the experimental group were injected weekly with a certain amount of growth hormone on Mondays for four weeks. The Reeves’ turtles in the control group were injected with the same volume of PBS. Four weeks after the injection of GH, the mRNA expression of the SS gene in different tissues of male and female Reeves’ turtles was analyzed via qRT-PCR. Compared with the control group, the expression of the SS gene mRNA in the hypophysis decreased significantly (p < 0.05, effect size of Cohen d = 0.5) (Figure 3a,b). The expression of the SS gene in the stomach and intestinal tissues did not change significantly under the stimulation of GH (p > 0.05, effect size of Cohen d = 0.2). In both male and female turtles, the expression of SS in the liver, spleen, and kidney were so low that they were almost undetectable. Interestingly, when facing the GH stimulation, the mRNA expression profile of SS had a similar response trend in different tissues both in male and female turtles.
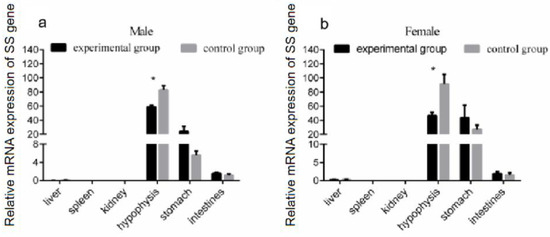
Figure 3.
Expression of the SS gene in each organ after 4 weeks of growth hormone injection. The turtles were divided into two groups, the experimental group and the control group, containing the same number of male and female turtles. We first measured the body weight of the turtles. Male or female Reeves’ turtles in the experimental group were injected with a certain amount of growth hormone weekly. The turtles to be injected were weighed and were injected according to the standard 10 μg/g. Meanwhile, male or female Reeves’ turtles in the control group were injected with same volume of PBS. (a) The mRNA expression of the SS gene in the male Reeves’ turtle after four weeks of GH stimulation. (b) The mRNA expression of the SS gene in the female Reeves’ turtle after four weeks of GH stimulation. β-actin was used as the internal gene of qRT-PCR. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and were subjected to variance analysis (ANOVA) using the GLM process. Different superscripts signify significant differences (* p < 0.05). Data are expressed as mean ± SEM (n = 3).
3.4. Modulation of GHR Gene Expression in the Liver under Periodic Injection of Growth Hormone
GHR is an essential endocrine regulator of the growth axis of GH/IGF-I. The biological role of growth hormone is mediated by GHR [8]. In the liver of fish and other vertebrates, the expression level of GHR was found to be high [8,31,32]. We measured the mRNA expression of the GHR gene in the liver, spleen, kidney, brain, hypophysis, stomach, and intestine of turtles via the qRT-PCR method. The GHR gene was highly expressed in the liver, brain, stomach, and hypophysis of the Reeves’ turtle, but the abundance of GHR mRNA in the spleen, kidney, and intestines was low (Figure 4a).
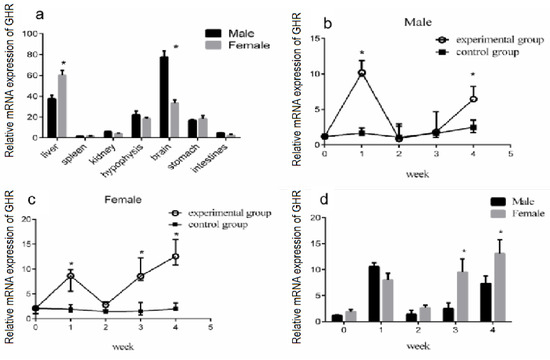
Figure 4.
Effect of GH stimulation on GHR gene expression in the liver. The mRNA expression of GHR in different organs of the Reeves’ turtle and the effect of GH stimulation on GHR expression in the liver. (a) The mRNA expression of GHR in different organs of the Reeves’ turtle. The turtles were divided into two groups, the experimental group and the control group, containing the same number of male and female turtles. We first measured the body weight of the turtles. Male or female Reeves’ turtles in the experimental group were injected with a certain amount of growth hormone weekly for four weeks. The turtles to be injected were weighed and injected according to the standard 10 μg/g. Meanwhile, male or female Reeves’ turtles in the control group were injected with same volume of PBS. (b) Relative GHR gene mRNA expression of male Reeves’ turtle liver under GH stimulation. (c) Relative GHR gene mRNA expression of female Reeves’ turtle liver under GH stimulation. (d) Sex differences in GHR gene expression in the liver after four weeks of GH injection. β-actin was used as the internal gene of qRT-PCR. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and were subjected to variance analysis (ANOVA) using the GLM process. Different superscripts signify significant differences (* p < 0.05). Data are expressed as mean ± SEM (n = 3).
To further clarify the effect of GH stimulation on GHR gene expression in turtle liver, the turtles were divided into two groups, the experimental group and the control group, containing the same number of male and female turtles. We first measured the body weight of the turtles. Male or female Reeves’ turtles in the experimental group were injected with a certain amount of growth hormone weekly for four weeks. The turtles to be injected were weighed and were injected according to the standard 10 μg/g. Meanwhile, male or female Reeves’ turtles in the control group were injected with the same volume of PBS. In the first week of the GH injection experiment, the mRNA expression of GHR was significantly increased in both the male and female Reeves’ turtles in the experimental group (p < 0.05). However, during the second and third week of the GH injection experiment, the GHR expression in the male turtle liver in the experimental group was decreased, and there was no significant difference between the control group and the experimental group (p > 0.05, effect size of Cohen d = 0.2) (Figure 4b). Meanwhile, in the second week of the GH injection experiment, the GHR expression in the female turtle liver in the experimental group was decreased, and there was no significant difference with the control group (p > 0.05, effect size of Cohen d = 0.2) (Figure 4c). Interestingly, in the fourth week of the GH injection experiment, the GHR gene expression in the male turtle liver was increased significantly again (p < 0.01, effect size of Cohen d = 0.8) (Figure 4b). In the third and fourth weeks of GH injection, the GHR gene expression of the female turtle liver was significantly higher than that of the control group (p < 0.01, effect size of Cohen d = 0.8) (Figure 4c). The GHR gene expression in the liver of male and female turtles presented a change trend of increasing first, and then decreasing, but eventually increasing again. Further data analysis revealed that in the third and fourth week of GH injection, the expression of the GHR gene in the female turtle liver was significantly higher than that of the male turtle (p < 0.05) (Figure 4d).
3.5. Modulation of GHR mRNA Expression in Different Tissues of Reeves’ Turtles after Four Weeks of GH Injection
In order to clarify the effect of GH injection on GHR mRNA expression in different organs, the modulation of GHR expression in the Reeves’ turtle was detected after four weeks of GH injection. The GHR mRNA expression in different organs of male and female Reeves’ turtles did not change significantly after four weeks of GH stimulation (p > 0.05) (Figure 5a,b). The data analyzed with regard to sex differences showed that the GHR mRNA expression in the male turtle brain was significantly higher than that in the female turtle brain (p < 0.05) (Figure 5c).
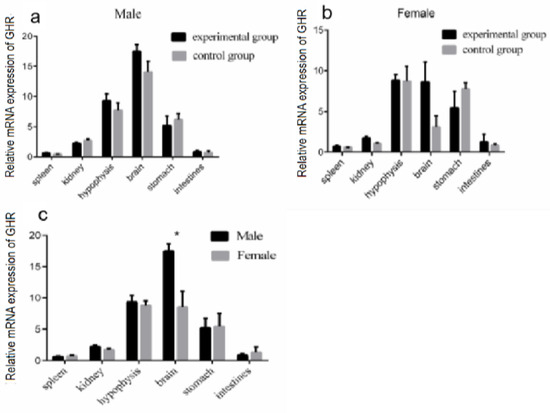
Figure 5.
Effect of four weeks of GH stimulation on GHR expression. Expression of GHR in different tissues of the Reeves’ turtle after four weeks of GH injection. (a) The mRNA expression of the GHR gene in the male Reeves’ turtle after four weeks of GH stimulation. (b) The mRNA expression of the GHR gene in the female Reeves’ turtle after four weeks of GH stimulation. (c) The mRNA expression of the GHR gene in each tissue of female and male Reeves’ turtles. β-actin was used as the internal gene of qRT-PCR. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and were subjected to variance analysis (ANOVA) using the GLM process. Different superscripts signify significant differences (* p < 0.05). Data are expressed as mean ± SEM (n = 3).
3.6. Effect of Periodic Exogenous GH Injection on IGF-I Gene Expression in the Liver
IGF-I is a hormone that is fundamental to reproduction and lifespan, as well as to cell growth, differentiation, migration, and survival [33]. In vertebrates, it is produced both centrally by the liver and peripherally by diverse tissues [34]. To verify the effect of GH stimulation on IGF-I gene expression in the liver of male and female Reeves’ turtles, we first measured the expression of the IGF-I gene in the liver, spleen, kidney, brain, hypophysis, stomach, and intestine. The qRT-PCR data showed that IGF-I mRNA was expressed in different organs of the Reeves’ turtle. There are differences in the expression of IGF-I mRNA between the male and female. The IGF-I mRNA expression levels in the liver, kidney, and spleen of the female turtle were significantly higher than those of the male turtle (p < 0.05) (Figure 6a). However, the highest mRNA expression of IGF-I was found in both the male and female liver.
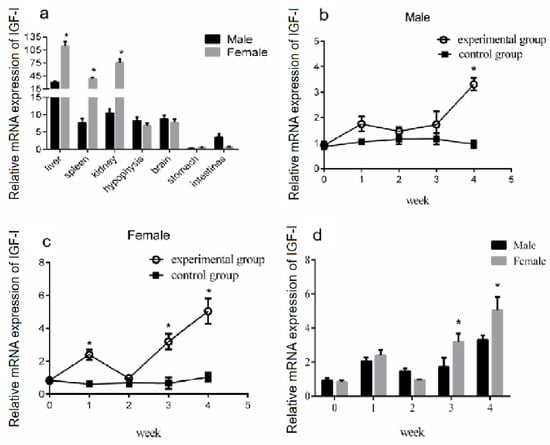
Figure 6.
Effect of GH stimulation on IGF-I gene expression in the liver. (a) The mRNA expression of the IGF-1 gene in each tissue of female and male Reeves’ turtles. (b) The mRNA expression of the IGF-1 gene in the liver of the male Reeves’ turtle after four weeks of GH stimulation. (c) The mRNA expression of the IGF-1 gene in the liver of the female Reeves’ turtle after four weeks of GH stimulation. (d) The mRNA expression of the IGF-1 gene in the liver of the female and male Reeves’ turtles. The expression of the IGF-I gene in different tissues of the Reeves’ turtle. The average expression in the male liver is the highest at 30.72, while the expression in the stomach is the lowest at 0.28. The average expression in the female liver is the highest at 113.60, while the expression in the stomach is the lowest at 0.47. Different superscripts signify significant differences (* p < 0.05). Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and expressed as the mean ± SEM. Variance analysis was operated using GLM procedures, based on at least three replicates for each treatment.
3.7. Changes in IGF-I Gene mRNA Expression in the Liver after Periodic Injection of Growth Hormone
To study the effect of GH injection on the expression of liver IGF-I, Reeves’ turtles in the experimental group were injected with a certain amount of growth hormone weekly for four weeks. Meanwhile, the turtles in the control group were injected with the same volume of PBS. After the injection of growth hormone, the expression of IGF-I mRNA in the liver of males and females in the control group remained basically stable, and the changing trend of IGF-I mRNA expression levels in the male and female liver were similar: the expression increased in the first week of the experiment, decreased in the second week, and continuously increased in the third and fourth weeks of the experiment. The expression of mRNA in the experiment group of the male turtle was significantly higher than that in the control group at the fourth week (p < 0.01, effect size of Cohen d = 0.8), while the expression in the female group was significantly higher than that in the control group at the first, second, and fourth weeks (p < 0.05, effect size of Cohen d = 0.5). The expression of IGF-I mRNA in the liver of male turtles in the experimental group was only slightly higher than that of female turtles in the second week, while that of female turtles was higher than that of male turtles in the first, third, and fourth weeks, with the difference significant in the third and fourth weeks (p < 0.05). The results are shown in Figure 6. Four weeks after the injection of growth hormone, the expression of the IGF-I gene mRNA in the liver and kidney of the male turtles in the experimental group was significantly higher than that in the control group (p < 0.05, effect size of Cohen d = 0.8), while the expression in the spleen was significantly lower than that in the control group (p < 0.05, effect size of Cohen d = 0.8), and the expression levels in the hypophysis, brain, and stomach were slightly lower than those in the control group (p > 0.05, effect size of Cohen d = 0.2). The expression of the IGF-I gene mRNA in the liver of the female turtles was significantly higher than that in the control group (p < 0.05, effect size of Cohen d = 0.8), and expression in the spleen was slightly lower than that in the control group (p > 0.05, effect size of Cohen d = 0.2). The results are shown in Figure 7.
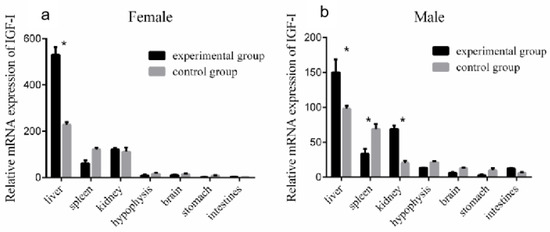
Figure 7.
Expression of the IGF-I gene mRNA in each organ after 4 weeks of injection of growth hormone. (a) Compared with the control group, the expression of the IGF-I gene mRNA in the liver and kidney of the male experimental group increased significantly, while the expression in the spleen decreased significantly. (b) Compared with the control group, the expression of the IGF-I gene mRNA in the female experimental group increased significantly. Data were processed using Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA), and expressed as the mean ± SEM. Variance analysis was operated using GLM procedures, based on at least three replicates for each treatment. * p < 0.05 compared between the two indicated constructions.
4. Discussion
Under natural conditions, the growth rate of the Reeves’ turtle is relatively slow, being slow at 1–2 years, accelerating at 3–4 years, and reaching sexual maturity at 5–6 years. Female turtles grow faster than male turtles. In the 6th year of sexual maturity, female turtles weigh about 400 g, while male turtles weigh approximately 250 g [35]. Although the growth speed of turtles is slower than that of other vertebrates, their growth is also controlled by the growth hormone (GH)—insulin-like growth factor-1 (IGF-I) system. SS mainly inhibits the growth of animals by reducing the release of hypophysis growth hormone [10]. As a type of inhibiting hormone required for regulating the stable growth of animals, SS can prevent animals from being damaged when they grow too fast. For example, because of the over-secretion of GH, the growth and development of patients with gigantism will be reduced, causing side effects such as low resistance and a short lifespan [36]. Therefore, SS plays an indispensable role in the growth axis of animals. In this study, β-actin was used as the internal reference gene of RT-PCR. The expression levels of the SS gene mRNA in the brain, hypophysis, liver, spleen, kidney, stomach, and intestine were analyzed. SS gene mRNA was highly expressed in the nerve and digestive tissues of the Reeves’ turtle, with the highest expression found in the brain. This is consistent with the research results regarding SS gene expression in humans, rats, fish [37,38,39], and other animals, but the expression amount in other tissues was not found to be the same. In this experiment, no expression of SS mRNA was found in the spleen or kidney of the Reeves’ turtle; however, the expression of SS mRNA has been detected in the spleen of fish [38], and also in the kidney of Chinese sturgeon [39]. According to a comprehensive analysis of the above results, the expression of SS gene mRNA is different among different species, but the expression of SS gene mRNA in the brain of different organisms is the highest, indicating that SS gene mRNA is expressed in different animals mainly in the brain, which is the main regulatory organ governing the release of the SS gene to control the growth and development of animals. However, due to the different expression characteristics, growth rules, and lifestyles of various animals, there may be differences in the expression of SS gene mRNA among the different organs of different animals. In this study, the relative expression of SS gene mRNA in the nervous system of male turtles was higher than that of female turtles. Combined with the above results, it is suggested that the growth speed of male and female turtles in the natural state is inconsistent, which may be caused by the difference in SS gene expression between male and female individuals. It was found that the expression abundance of growth hormone can affect the expression of SS. After Kamegai et al. excised the hypophysis gland of mice so that they could not secrete growth hormone by themselves, the concentration of SS decreased. When exogenous growth hormone was then injected, the concentration of SS in the mice began to increase again [40]. In this experiment, through a continuous injection of growth hormone, a large amount of the SS gene was expressed in both female and male turtles to balance their growth speed. Male turtles had strong adaptability to the growth hormone. After two weeks of injection, the turtles adapted to the injection amount of exogenous growth hormone, and the expression of SS gene mRNA in the brain began to decrease, which was similar to the natural state. However, the adaptability of female turtles to exogenous growth hormone was weak, and the expression of SS gene mRNA in the brain was higher and lasted longer than that in males, which indicates that the female is more sensitive to growth hormone. Therefore, it is speculated that the reason female turtles grow faster than male turtles under natural conditions may be due to the sensitivity of the female growth hormone.
Through experiments, we found that the GHR gene can be expressed in all tissues of the Reeves’ turtle, which is consistent with the expression in chickens, fishes, rats [41,42,43], etc. The expression of the GHR gene seriously influences the effect of growth hormone. When the expression of the GHR gene is high, it can combine with growth hormone to promote the growth of the organism. However, when the expression of the GHR gene is inadequate, growth hormone cannot combine with GHR, resulting in the organism producing negative feedback regulation, and inhibiting the growth and development of the organism. The GHR protein encoded by chickens with dwarfism is abnormal because of the mutation of the GHR gene, which cannot combine with growth hormone, resulting in growth retardation [44]. It was found that the expression of the GHR gene was different in different organs of animals at different periods. The expression of the GHR gene in the kidney of rabbits was the highest in the fetal period, while the expression of the GHR gene in the heart, liver, muscle, and other tissues was low. However, the expression of the GHR gene in the kidney was unchanged in the second to sixth month, but it was largely expressed in the heart, liver, muscle, and other tissues [45]. According to the above results, the developmental changes in GHR gene mRNA in different species and tissues will vary, but the highest expression level in the liver suggests that there may be differences in the expression regulation mechanism in different tissues. Combined with the results of this study, GHR gene mRNA expression in the brain of the male Reeves’ turtle was found to be the highest, followed by that in the liver. In the female, the expression of GHR gene mRNA was the highest in the liver, followed by that in the brain. This indicates that the male and female development of the Reeves’ turtle is not synchronous; the male development is slower than that of the female. The highest level of mRNA expression indicates that all the organs needed for survival are mature and begin to concentrate on the growth stage. Meanwhile, the highest level of GHR gene mRNA expression occurring in the male brain indicates that the brain development of the male is not perfect at this stage, so it is necessary to concentrate on the development of the brain to control the release of various hormones. This difference under natural conditions may be one of the reasons for the asynchronous growth of the male and female turtle. In this study, GHR gene mRNA expression in the liver of male and female turtles changed after the injection of human growth hormone. In the first week, the mRNA expression of the GHR gene in the livers of males and females increased rapidly, and was significantly higher than that in the control group. In the second week, the mRNA expression of the GHR gene in the livers of both turtles decreased to a level similar to that in the control group, and continued to increase at the third and fourth weeks, suggesting that the sensitivity of the livers of the Reeves’ turtle to exogenous growth hormone was higher at the first injection of growth hormone. The expression of mRNA increased rapidly. When injected twice, the body had adapted to the exogenous substance, so the expression of GHR gene mRNA in the liver was self-regulated to be consistent with that found under natural conditions. However, with the continuous injection of growth hormone, the concentration of growth hormone in the body increased, so that the mRNA expression of the GHR gene in the liver was increased, and began to promote the growth of the Reeves’ turtle.
IGF-I is one of the important hormones regulating the growth and development of animals. It is generally believed that IGF-I plays a leading role in the growth of animals. IGF-I can be synthesized by many tissues and organs in animals, while the main source of IGF-I in circulation is the liver [46]. In this experiment, we found that the IGF-I gene is widely distributed in various tissues of the Reeves’ turtle, and the IGF-I gene content in the liver is the highest. This result is consistent with that found in pigs, sheep, and Oreochromis mossambicus [47,48,49]. Although the expression level of the IGF-I gene is the same among all types of animals, the expression level of IGF-I in different tissues of different sexes of the same animal also varies. Combined with the results of this study, it was found that the expression level of IGF-I gene mRNA in the liver of the male and female Reeves’ turtle was the highest among all the tissues, but the expression level of the IGF-I gene was different between them. The expression of mRNA in the female turtle liver, spleen, and kidney was higher than that in the male, suggesting that the growth rate of females in the first year may be faster than that in males. Further, the tissues related to detoxification and metabolism, such as the spleen and kidney, develop better, and females in the first year can adapt to harsh environments better than the males. The effect of growth hormone on the expression of the IGF-I gene has been proved in various animals. López-Fernández et al. injected recombinant human growth hormone into aging rats for 7 days continuously, and the expression of the IGF-I gene was restored to the level of adult rats [50]. After Pedroso et al. injected recombinant growth hormone into salmon, the expression level of IGF-I gene mRNA in the liver increased significantly [51]. In this experiment, the expression trend of IGF-I gene mRNA in the liver of male and female turtles was similar with the continuous injection of growth hormone. In the first week, the expression of IGF-I gene mRNA in the liver of turtles was upregulated. The turtles then adapted to the injection amount of exogenous growth hormone. The expression amount of IGF-I gene mRNA in the liver required a lot of SS to balance its growth rate; the male turtles adapted to the growth hormone, and after two weeks, the turtles adapted to the injection amount of exogenous growth hormone, the IGF-I gene in the liver. The continuous upregulation of mRNA may be due to the accumulation of growth hormone in the body after the third week, the sensitivity of the turtles to growth hormone being reduced, and the turtles adapting to the injection amount of exogenous growth hormone. According to the analysis of the changing trend of the mRNA of SS, GHR, and the IGF-I gene in the four weeks after injection, when the expression of SS gene mRNA in the brain was downregulated, the expression of GHR and IGF-I gene mRNA in the liver was upregulated. When the expression of SS gene mRNA in the brain was upregulated, the expression of GHR and IGF-I gene mRNA in the liver was downregulated at the same time, suggesting that the expression level of the SS gene in the brain was related to the expression level of GHR and IGF-I. After the injection of growth hormone, although the increase in SS gene expression inhibited the secretion of GH, the exogenous growth hormone was able to take the place of GH synthesized in vivo and GHR, which accelerated the growth of the turtles. Although the expression of the SS gene increased in the early stage of injection, the expression of the SS gene decreased slowly in the late stage of injection, which may indicate that the adaptability of turtles to exogenous growth hormones improved. Our laboratory measured the concentration of IGF-I in the blood of the Reeves’ turtle. The concentration of IGF-I in the blood of the yellow-feathered broiler is approximately three times that of the Reeves’ turtle, and the content of IGF-I in the blood of the blue pond pig is about ten times that of the Reeves’ turtle [3]. In combination with this experiment, it was found that the IGF-I gene can be expressed in a large amount in turtles, so it is suggested that the slow growth of the Reeves’ turtle may be related to its low expression level of growth hormone.
5. Conclusions
Before this study was performed, there was no data available on SS in turtles, and no data regarding the growth-axis-related genes of turtles injected with exogenous growth hormone. In this study, we found that exogenous growth hormones can effectively stimulate the changes in genes related to the growth axis, which may help us to understand the molecular mechanism of the growth of turtles. The results in this study indicated that SS gene mRNA expression was the highest in the brain and the lowest in the liver. GHR gene mRNA and IGF-I gene mRNA are expressed in the brain, hypophysis, liver, kidney, spleen, stomach, intestine, and other tissues. In males, GHR gene mRNA expression is the highest in the brain, while in females, IGF-I gene mRNA expression is the highest in the liver. In females, IGF-I gene mRNA expression is higher in the liver, kidney, and spleen than that in the male. At the early stage of the injection, SS gene expression increased, but it later slowly reduced. This may suggest the adaptability of the Reeves’ turtle to exogenous growth hormone. At the same time, exogenous growth hormone can be recognized by the GHR gene and can promote the expression of the IGF-I gene. The experiments showed that the injection of recombinant human growth hormone affected the expression of SS, GHR, and IGF-1 genes, and promoted the growth of the Reeves’ turtle. This provides a theoretical basis for further research on the molecular biology of tortoises and turtles, and guidance on breeding production.
Author Contributions
Validation, Y.-F.L.; formal analysis, Y.-Y.L.; resources, Z.-H.R.; data curation, R.L.; writing—original draft, writing—review and editing, R.-L.X.; R.L.; supervision, W.-S.L.; project administration, W.-S.L.; funding acquisition, W.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Special Fund for Science and Technology of Guangdong Province (grant 210715146901539).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of South China Agricultural University (protocol code 2022g027, 11 September 2022).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Omeyer, L.C.M.; Fuller, W.J.; Godley, B.J.; Snape, R.T.E. The effect of biologging systems on reproduction, growth and survival of adult sea turtles. Mov. Ecol. 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Congdon, J.D.; Nagle, R.D.; Kinney, O.M.; van Loben Sels, R.C.; Quinter, T.; Tinkle, D.W. Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp. Gerontol. 2003, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Congdon, J.D.; Nagle, R.D.; Kinney, O.M.; van Loben Sels, R.C. Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Exp. Gerontol. 2001, 36, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Omeyer, L.C.M.; Fuller, W.J.; Godley, B.J.; Snape, R.T.E. Determinate or indeterminate growth? Revisiting the growth strategy of sea turtles. Mar. Ecol. Prog. Ser. 2018, 596, 199–211. [Google Scholar] [CrossRef]
- Frýdlová, P.; Mrzílková, J.; Šeremeta, M.; Křemen, J. Determinate growth is predominant and likely ancestral in squamate reptiles. Proc. R. Soc. B Biolpgical Sci. 2021, 287, 20202737. [Google Scholar] [CrossRef]
- Frýdlová, P.; Mrzílková, J.; Šeremeta, M.; Křemen, J. Universality of indeterminate growth in lizards rejected: The micro-ct reveals contrasting timing of growth cartilage persistence in iguanas, agamas, and chameleons. Sci. Rep. 2019, 9, 18913. [Google Scholar] [CrossRef]
- Frýdlová, P.; Nutilová, V.; Dudák, J.; Žemlička, J.; Němec, P.; Velenský, P.; Jirásek, T. Patterns of growth in monitor lizards (Varanidae) as revealed by computed tomography of femoral growth plates. Zoomorphology 2017, 136, 95–106. [Google Scholar] [CrossRef]
- Zhu, W.; He, Y.; Ruan, Z.; Zhang, X.; Liao, L.; Gao, Y.; Lin, N.; Chen, X.; Liang, R.; Liu, W. Identification of the cDNA Encoding the Growth Hormone Receptor (GHR) and the Regulation of GHR and IGF-I Gene Expression by Nutritional Status in Reeves’ Turtle (Chinemys reevesii). Front. Genet. 2020, 11, 587. [Google Scholar] [CrossRef]
- Liu, W.-S.; Ma, J.-E.; Li, W.-X.; Zhang, J.-G.; Wang, J.; Nie, Q.-H.; Qiu, F.-F.; Fang, M.-X.; Zeng, F.; Wang, X.; et al. The Long Intron 1 of Growth Hormone Gene from Reeves’ Turtle (Chinemys reevesii) Correlates with Negatively Regulated GH Expression in Four Cell Lines. Int. J. Mol. Sci. 2016, 17, 543. [Google Scholar] [CrossRef]
- Very, N.M.; Kittilson, J.D.; Klein, S.E.; Sheridan, M.A. Somatostatin inhibits basal and growth hormone-stimulated hepatic insulin-like growth factor-I production. Mol. Cell. Endocrinol. 2008, 281, 19–26. [Google Scholar] [CrossRef]
- Liu, W.S.; Li, Y. Studies on the biochemical indices of blood in Chinemys reevesii. Acta Hydrobiol. Sin. 2006, 30, 499–501. [Google Scholar]
- Liu, W.S.; Li, Y. Studies on the micro-structure of hypophysis gland and ultrastructure of adenohypophysis in Chinemys reevesii. Acta Hydrobiol. Sin. 2005, 29, 661–666. [Google Scholar]
- Krulich, L.; Dhariwal, A.P.S.; Mccann, S.M. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology 1968, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic Polypeptide That Inhibits the Secretion of Immunoreactive Hypophysis Growth Hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Pradayrol, L.; Chayvialle, J.A.; Carlquist, M.; Mutt, V. Isolation of a porcine intestinal peptide with C-terminal somatostatin. Biochem. Biophys. Res. Commun. 1978, 85, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-W.; Otto, C.J.; Peter, R.E. Evolution of neuroendocrine peptide systems: Hormone and somatostatin. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 119, 375–388. [Google Scholar] [CrossRef]
- Barnett, P. Somatostatin and somatostatin receptor physiology. Endocrine 2003, 20, 25–64. [Google Scholar] [CrossRef]
- Brockway, D.F.; Griffith, K.R.; Aloimonos, C.M.; Clarity, T.T.; Moyer, J.B.; Smith, G.C.; Dao, N.C.; Hossain, M.S.; Drew, P.J.; Gordon, J.A.; et al. Somatostatin peptide signaling dampens cortical circuits and promotes exploratory behavior. Cell Rep. 2023, 42, 112976. [Google Scholar] [CrossRef]
- Nagaeva, E.; Schäfer, A.; Linden, A.M.; Elsilä, L.V.; Egorova, K.; Umemori, J.; Ryazantseva, M.; Korpi, E.R. Somatostatin-Expressing Neurons in the Ventral Tegmental Area Innervate Specific Forebrain Regions and Are Involved in Stress Response. eNeuro 2023, 10, ENEURO.0149-23.2023. [Google Scholar] [CrossRef]
- Tang, M.; Liu, C.; Li, R.; Lin, H.; Peng, Y.; Lang, Y.; Su, K.; Xie, Z.; Li, M.; Yang, X.; et al. Spatial and temporal expression pattern of somatostatin receptor 2 in mouse. Chin. J. Biotechnol. 2023, 39, 2656–2668. [Google Scholar] [CrossRef]
- Peluso, G.; Petillo, O.; Melone, M.A.; Mazzarella, G.; Ranieri, M.; Tajana, G.F. Modulation of cytokine production in activated human monocytes by somatostatin. Neuropeptides 1996, 30, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Li, J.; Blum, A.M.; Metwali, A.; Patel, Y.C.; Weinstock, J.V. SSTR2A is the dominant somatostatin receptor subtype expressed by inflammatory cells, is widely expressed and directly regulates T cell IFN-γ release. Eur. J. Immunol. 1999, 29, 2454–2463. [Google Scholar] [CrossRef]
- Herszényi, L.; Mihály, E.; Tulassay, Z. Somatostatin and gastrointestinal tract. Clinical experiences. Orvosi Hetil. 2013, 154, 1535–1540. [Google Scholar] [CrossRef]
- Kappeler, L.; Zizzari, P.; Grouselle, D.; Epelbaum, J.; Bluet-Pajot, M.T. Plasma and Hypothalamic Peptide-Hormone Levels Regulating Somatotroph Function and Energy Balance in Fed and Fasted States: A Comparative Study in Four Strains of Rats. J. Neuroendocrinol. 2004, 16, 980–988. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Melmed, S. Pituitary somatostatin receptor signaling. Trends Endocrinol. Metab. 2010, 21, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, S.; Eberle, A.N. Somatostatin analogs and radiopeptides in cancer therapy. Biopolymers 2002, 66, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Kvols, L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 2963–2970. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Bronstein, M.D.; Brue, T.; Coculescu, M.; Fleseriu, M.; Guitelman, M.; Pronin, V.; Raverot, G.; Shimon, I.; Lievre, K.K.; et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): A randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014, 2, 875–884. [Google Scholar] [CrossRef]
- McLean, E.; Donaldson, E.M. The Role of Growth Hormone in the Growth of Poikilotherms. Endocrinol. Growth Dev. Metab. Vertebr. 1993, 248, 43–71. [Google Scholar]
- Reindl, K.; Sheridan, M.A. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 231–245. [Google Scholar] [CrossRef]
- Shafik, B.M.; Kamel, E.R.; Mamdouh, M.; Elrafaay, S.; Nassan, M.A.; El-Bahy, S.M.; El-Tarabany, M.S.; Manaa, E.A. Performance, Blood Lipid Profile, and the Expression of Growth Hormone Receptor (GHR) and Insulin-Like Growth Factor-1 (IGF-1) Genes in Purebred and Crossbred Quail Lines. Animals 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.L.; Fox, B.K.; Davis, L.K.; Visitacion, N. Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: Tissue specific expression and differential regulation by salinity and fasting. Gen. Comp. Endocrinol. 2007, 154, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, A.M.; Schwartz, T.S.; Schwartz, T.S.; Madden, J.A.; Boyken, S.E.; Ford, N.B.; Serb, J.M.; Bronikowski, A.M. Rates of molecular evolution vary in vertebrates for insulin-like growth factor-1 (IGF-1), a pleiotropic locus that regulates life history traits. Gen. Comp. Endocrinol. 2012, 178, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Denley, A.; Cosgrove, L.J.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005, 16, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, X.; Chen, Y. Age and growth in reeves’ turtle Chinemys reebesii Gray. J. Dalian Fish. Univ. 2005, 20, 73–76. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Z.Y.; Su, C.B.; Wang, R.Z.; Ma, W.B. Diagnosis and treatment of pituitary growth hormone adenoma. Chin. J. Neurosurg. Dis. Res. 2004, 04, 254–255. [Google Scholar]
- Jiang, Q.; Lian, A.; He, Q. Dopamine inhibits somatolactin gene expression in tilapia pituitary cells through the dopamine D2 receptors. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 197, 35–42. [Google Scholar] [CrossRef]
- Ye, X.; Li, W.S.; Lin, H.R. Progress of research on fish somatostatin and receptor. J. Fish. China 2007, 31, 264–272. [Google Scholar]
- Li, C.J.; Wei, Q.W.; Zhou, L.; Cao, H.; Zhang, Y.; Gui, J.F. Molecular and expression characterization of two somatostatin genes in the Chinese sturgeon, Acipenser sinensis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 127–134. [Google Scholar] [CrossRef]
- Kamegai, J.; Unterman, T.G.; Frohman, L.A.; Kineman, R.D. Hypothalamic/hypophysis-axis of the spontaneous dwarf rat: Autofeedback regulation of growth hormone(GH) includes suppression of GH releasing-hormone receptor messenger ribonucleic acid. Endocrinology 1998, 139, 3554–3560. [Google Scholar] [CrossRef][Green Version]
- Hu, B.; Hu, S.; Yang, M.; Liao, Z.; Zhang, D.; Luo, Q.; Zhang, X.; Li, H. Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 1608. [Google Scholar] [CrossRef] [PubMed]
- Won, E.T.; Douros, J.D.; Hurt, D.A.; Borski, R.J. Leptin stimulates hepatic growth hormone receptor and insulin-like growth factor gene expression in a teleost fish, the hybrid striped bass. Gen. Comp. Endocrinol. 2016, 229, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Mertani, H.C.; Morel, G. In situ gene expression of growth hormone (GH) receptor and GH binding protein in adult male rat tissues. Mol. Cell. Endocrinol. 1995, 109, 47–61. [Google Scholar] [CrossRef]
- Burnside, J.; Liou, S.S.; Zhong, C.; Cogburn, L.A. Abnormal growth hormone receptor gene expression in the sex-linked dwarf chicken. Gen. Comp. Endocrinol. 1992, 88, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ymer, S.I.; Herington, A.C. Developmental expression of the growth hormone receptor gene in rabbit tissues. Mol. Cell. Endocrinol. 1992, 83, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Setser, J.; Zhao, H.; Stannard, B.; Haluzik, M.; Glatt, V.; Bouxsein, M.L.; Kopchick, J.J.; LeRoith, D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1–deficient mice. J. Clin. Investig. 2004, 113, 96–105. [Google Scholar] [CrossRef]
- Kim, I.; Jin, E.J.; Baik, K.; Park, C.H.; Kim, W.K.; Kang, C.W.; Ko, Y.; Jang, I.; Choi, W.S.; Lee, C.Y. Expression and secretion of the insulin-like growth factor system components by pig liver cells. Asian Australas. J. Anim. Sci. 2008, 21, 1244–1251. [Google Scholar] [CrossRef]
- de Brun, V.; Meikle, A.; Casal, A.; Carriquiry, M.; Menezes, C.; Forcada, F.; Sosa, C.; Abecia, J.A. Hepatic Expression of Insulin-Like Growth Factor-1 in Underfed Pregnant Ewes. J. Agric. Ence Technol. A 2016, 6, 355–362. [Google Scholar]
- Schmid, A.C.; Lutz, I.; Kloas, W.; Reinecke, M. Thyroid hormone stimulates hepatic IGF-I mRNA expression in a bony fish, the tilapia Oreochromis mossambicus, in vitro and in vivo. Gen. Comp. Endocrinol. 2003, 130, 129–134. [Google Scholar] [CrossRef]
- López-Fernández, J.; Sánchez-Franco, F.; Velasco, B.; Tolón, R.M.; Pazos, F.; Cacicedo, L. Growth hormone induces somatostatin and insulin-like growth factor I gene expression in the cerebral hemispheres of aging rats. Endocrinology 1996, 137, 4384–4391. [Google Scholar] [CrossRef]
- Pedroso, F.L.; Fukada, H.; Masumoto, T. In vivo and in vitro effect of recombinant salmon growth hormone treatment on IGF-I and IGFBPs in yellowtail Seriola quinqueradiata. Fish. Sci. 2009, 75, 887–894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).