Abstract
In higher plants, WRKY transcription factors are broadly involved in a variety of life activities and play an important role in both biotic and abiotic stress responses. However, little is known about the functions of WRKY genes in the popular species, such as Cucurbita maxima (pumpkin), which is planted worldwide. In the present study, 102 CmWRKY genes were identified in the C. maxima genome. Chromosome location, multiple sequence alignment, phylogenetic analysis, and synteny analysis of the CmWRKYs were performed. Notably, we found that silencing CmWRKY22 promoted cucumber mosaic virus (CMV) infection, whereas overexpression of CmWRKY22 inhibited the CMV infection. Subsequently, an electrophoretic mobility shift assay (EMSA) confirmed that CmWRKY22 was able to bind to the W-box at the promoter of CmPR1b, which is a responsive gene of the salicylic acid (SA) signaling pathway. In summary, this study has provided a foundation for the antiviral functions of WRKY transcription factors in C. maxima.
1. Introduction
Transcription factors (TFs) play an important role in plants, as evidenced by the sequence-specific DNA binding and the activation or repression of downstream target gene expression [1,2]. Such properties are not limited to higher plants but are present in other living organisms. Because of its important biological functions, TFs are also commonly used as targets for molecular breeding, a process that involves not only the control of gene expression but also a large number of cellular processes [3,4]. Considering the WRKY family being one of the largest classes of transcription factor families in plants, it contains multiple family members, such as basic helix–loop–helix (bHLH), ethylene responsive factor (ERF), CUC2 (cup-shaped cotyledon) (NAC), basic leucine zipper (bZIP), myeloblastosis (MYB), NAM (no apical meristem), ATAF1/2, and C2H2 families [5]. The potential of the WRKY family to participate in a diversity of biological processes (e.g., stress tolerance, growth and development) has recently attracted considerable attention [6,7].
Generally, WRKY TFs contain one or two highly conserved domains of approximately 60 amino acids in size. They have a conserved seven amino acid–peptide (WRKYGQK) signature at the N-terminus of the protein, and a zinc finger motif (C2HC or C2H2) at the C-terminus of the protein [8]. This highly conserved domain can bind to the W-box region of downstream target genes to regulate their expression. For example, the conserved WRKYGQK domain in AtWRKY4 has been found to be directly involved in DNA binding, by using nuclear magnetic resonance [9,10]. Considering that research on Arabidopsis thaliana WRKY genes is relatively early and comprehensive, the existing classification of the WRKY gene for other species is also guided by the classification guidelines for the Arabidopsis WRKY genes. As a matter of fact, WRKY genes can be divided into three groups according to their sequence characteristics and motifs: Groups I, II and III [8]. Group II is further divided into five subgroups: IIa, IIb, IIc, IId, and IIe [8]. The difference between Group I, and Groups II and III is that the WRKY genes in Group I contain two WRKY domains, whereas those in Groups II and III contain only one WRKY domain. The difference between Groups II and III is that they have different zinc finger motifs, C2H2 (C-X4-5-C-X22-23-H-X1-H) and C2HC (C-X7-C-X23-H-X1-C), respectively [11]. Recently, several WRKY genes with important functional significance were characterized in different WRKY groups. For example, GhWRKY25, a Group I family member cloned from cotton, was found to have a lower expression level under abiotic stresses, whereas overexpression of GhWRKY25 could promote resistance to the pathogen Botrytis cinerea and tolerance to drought in Nicotiana benthamiana [12]. Following exposure to external environmental stress factors, the expression levels of VfWRKY1 and VfWRKY2 in Vicia faba L. [13] and CsWRKY2 in Camellia sinensis [14] become abnormal. A similar situation was observed in Groups II and III. For example, four WRKY genes (AtWRKY6, AtWRKY11, AtWRKY54, and AtWRKY70) identified in A. thaliana, GhWRKY17 identified in Gossypium hirsutum, and MtWRKY76 identified in Medicago truncatula could enhance leaf senescence, and drought and salt tolerances [14,15,16,17]. In addition, the abnormal expression of WRKY genes can be induced by hormone stimulation in plants [18]. Taken together, WRKY genes elicit a wide range of responses when plants are subjected to external environmental stress, indicating the functional diversity of WRKY genes.
Pumpkin (C. maxima) belongs to the Cucurbitaceae family. It has a high yield and is an important cultivar that has brought significant economic benefits to the world [19]. It is used in a variety of ways, such as soups, purees, jams, and pies, for human consumption [20]. WRKY TFs have been identified in several species but have been less studied in the Cucurbitaceae family. In the present study, we identified 102 CmWRKY genes by searching the C. maxima genome. We clarified the classification of CmWRKY genes by the comparison with the WRKY family of A. thaliana (AtWRKYs). Multiple sequence alignments of amino acids, conserved motifs, gene structure, and synteny analysis were also performed in this study. Interestingly, the WRKY gene CmWRKY22 was found to play a positive regulatory role in plant resistance to cucumber mosaic virus (CMV) infection. Electrophoretic mobility shift assay (EMSA) experiments indicated that CmWRKY22 may bind to the W-BOX of the CmPR1b promoter region to exert its antiviral effects. These studies provide greater insight into the participation of plant WRKY TFs in virus–host interactions.
2. Materials and Methods
2.1. Identification of WRKY Proteins in C. maxima
In order to obtain the full set of C. maxima WRKY genes, we downloaded the C. maxima genome, including gene prediction information and predicted protein sequences from the Cucurbit Genomics Database (http://cucurbitgenomics.org/, accessed on 15 July 2022). The WRKY protein sequences of A. thaliana was downloaded from PlantTFDB (http://planttfdb.gao-lab.org, accessed on 19 July 2022). The Hidden Markov Model (HMM) profile of the WRKY domain (PF03106) was downloaded from the Pfam database (http://pfam.xfam.org/, accessed on 30 July 2022), and the HMMER software (http://hmmer.org/, accessed on 30 July 2022) was used to search for WRKY proteins in the full C. maxima protein. We used the Batch Web CD-Search Tool in NCBI (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi, accessed on 1 August 2022) and the Search Pfam tool in the Pfam database to verify the presence of the WRKY domain in the candidate genes. Genes without intact WRKY domains were excluded. We then renamed CmWRKYs according to their chromosomal location. The Molecular weight (MW), Open reading frame (ORF), Aliphatic index (Ai), Isoelectric point (PI), and Grand average of hydropathicity (GRAVY) were were computed for WRKY proteins in C. maxima using the Expasy online software (https://web.expasy.org/protparam/, accessed on 1 August 2022). Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, accessed on 25 July 2022) was used to predict the subcellular localization of CmWRKY proteins.
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
All 102 predicted CmWRKY proteins with amino acids spanning the WRKY core domain were used to create multiple alignments using MUSCLE [21]. A phylogenetic tree was constructed based on the N-terminal WRKY domain of each CmWRKY protein alignment using the maximum likelihood in IQ-TREE [22], and with 1000 ultrafast bootstraps. The groups and subgroups of the WRKY family in C. maxima were divided according to the previously reported classification of the WRKY family in A. thaliana.
2.3. Conserved Motifs, Protein Structure, Synteny Analysis, and Chromosomal Location
To reveal the conserved motif of all CmWRKY proteins, we used the MEME software (https://meme-suite.org/meme/tools/meme, accessed on accessed on 1 August 2022). The motif location, gene structure information (such as introns and exons) and chromosomal localization of the CmWRKY genes were then visualized using TBtools [23]. Likewise, we performed a synteny analysis using MCSCcanX and TBtools to study the synteny events of C. maxima [24]. At the same time, to determine the chromosomal location of the CmWRKY genes, we downloaded the genes from the Cucurbit Genomics Database (http://cucurbitgenomics.org/, accessed on 15 July 2022), mapped them to the C. maxima chromosomes, and renamed these WRKY genes CmWRKY1-102 according to the position of the gene on the chromosome using TBtools [23]. In addition, previous studies have shown that C. maxima has 20 chromosomes [25]. They were named chr1-20 in this study.
2.4. Plant Growth and Virus Inoculation
C. maxima and N. benthamiana seeds were germinated in plots containing vermiculite and substrate (W/W, 1:3). The temperature of the greenhouse was maintained at 25 °C with 60% humidity, and in a 16 h/8 h (light/dark) cycle. Three-week-old C. maxima plants were used for agrobacterial infiltration of TRV RNA1 and RNA2 for the TRV assay. For virus inoculation, agrobacterium strain harboring CMV RNA1, RNA2 and RNA3 were mixed at 1:1:1 ratio and co-transformed into N. benthamiana, P19 as the silencing suppressor was also co-infiltrated at an OD600 of 0.5–0.8. After inoculation, the leaves were inoculated in greenhouse for 3–5 days and then collected for quantitative RT-PCR analysis.
2.5. RNA Isolation and Quantitative RT-PCR Analysis
To examine gene expression during biotic stress, total RNA extraction and reverse transcription were conducted according to the manual protocol. Briefly, 1.5 μg total RNA was reverse transcribed under the following conditions: 5 min at 25 °C, 45 min at 37 ℃, 5 s at 8 °C, 5 min at 4 °C. The cDNA was used for qRT-PCR. Specific primers were designed using the Primer-BLAST software (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 30 November 2022). Quantitative RT-PCR analysis was carried out using AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China) and the ABI Q5 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) software 1.4.1. All the data were calculated using the 2−ΔΔCT method [26]. At least three biological replicates were used for the analysis. The β-actin gene and N. benthamiana actin gene (GenBank No. JQ256516) were used as internal reference genes in the analysis.
2.6. Plasmid Construction and Agrobacterium Transformation
For the TRV-based virus-induced gene silencing assay, the full coding sequence (CDS) of CmWRKY22 was amplified with the specific primer CmWRKY22. The product was digested into the TRV vector after digestion with the corresponding restriction enzymes BamHⅠ/SmaⅠ (Thermo Fisher Scientific, Beijing, China) to generate TRV RNA2 (CmWRKY22). TRV RNA2 (CmWRKY22) plasmid was then individually transformed into Agrobacterium Tumefaciens GV3101. In addition, the coding sequence of CmWRKY22 was amplified, cloned and inserted into expression vectors pGWB5C to generate pGWB5C: CmWRKY22-GFP and transformed into Agrobacterium Tumefaciens GV3101.
2.7. Electrophoretic Mobility Shift Assay (EMSA)
The ability of CmWRKY22 to bind CmPR1b was analyzed using EMSA. The W-box of the CmPR1b promoter was used to synthesize biotin end labels. Unlabeled (competitor) and labeled (mut competitor) W-box oligonucleotides were used as controls. The EMSA kit was purchased from Thermo Scientific (Waltham, MA, USA) and was used following the manufacturer’s protocol.
3. Results
3.1. Identification of the WRKY Gene Family in C. maxima
A total of 102 genes encoding putative WRKY proteins were identified in the C. maxima genome. Among them, 100 WRKY genes were mapped on chromosomes 1–20 and were named CmWRKY1 to CmWRKY100 according to their chromosomal locations and two WRKY genes could not be mapped to any chromosome and were named CmWRKY 101 and 102 (Figure 1). Furthermore, chromosomes 11 and 14 had the largest number of CmWRKY genes (9, 9%), whereas chromosome 15 had the smallest (2, 2%). Seven chromosomes had four genes localized on them, which implies that genetic variations in CmWRKY genes might exist in the evolution process. Several parameters of CmWRKY genes are listed in Table S1, including the deduced protein length, MW, PI, predicted subcellular localization, AI, and GRAVY. The deduced lengths of the CmWRKY proteins range from 145 (CmWRKY64) to 1305 amino acids (CmWRKY27), whereas the MW range from 16.79 kDa to 145.74 kDa. In addition, the isoelectric point ranges from 4.78 (CmWRKY78) to 9.8 (CmWRKY50), whereas the AI range from 43.49 (CmWRKY39) to 76.95 (CmWRKY41). The different features of these proteins indicate their functional diversities.
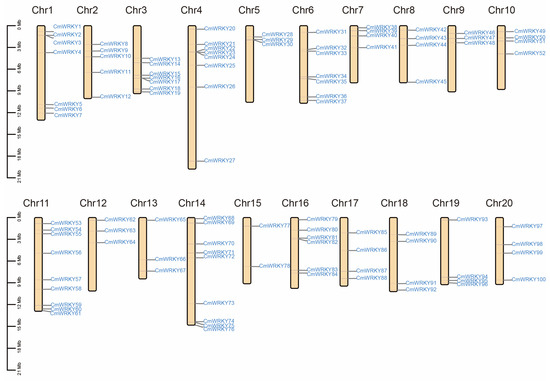
Figure 1.
Mapping of the WRKY gene family on C. maxima genome. The size of a chromosome is indicated by its relative length. The 100 putative WRKY genes were renamed from CmWRKY1 to CmWRKY100 according to their chromosome location. Two putative WRKY genes (CmWRKY101 and CmWRKY102) could not be localized on a specific chromosome.
3.2. Phylogenetic Analysis of CmWRKY Gene
To examine the phylogenetic relationship of the WRKY gene in the C. maxima family, 102 CmWRKY genes from C. maxima and 75 AtWRKY genes from A. thaliana were selected for phylogenetic analysis. A previous study has shown that the WRKY gene family is highly conserved in monocots and eudicots and is divided into three groups [27]. The WRKY proteins in C. maxima were classified into three groups: I, II, and III. The largest of these groups, Group II, contained 72 CmWRKY proteins and accounted for 70% of all the CmWRKY proteins in C. maxima. Groups I and III contained 18 and 12 CmWRKY proteins, accounting for 18% and 12% of all CmWRKY proteins in C. maxima, respectively. In addition, Group II was further divided into five subgroups (IIa, IIb, IIc, IId, and IIe) containing 6, 9, 28, 14, and 15 CmWRKY proteins, respectively (Figure 2A). Similar to AtWRKYs, most CmWRKY proteins are located in Group IIc. Group I contains two conserved WRKY domains, one with the zinc finger motifs C-X4-C-X22-H-X-H in the N-terminus and the other with the X4-C-X23-H-X-H in the C-termini (Table S1, Figure 2B), while Groups II and III contain only one WRKY domain. Notably, among the five subgroups of Group II, the zinc finger motifs of subgroup IIc are different from those of the other subgroups. Specifically, the motifs of IIc are C-X4-C-X23-H-X-H, and those of IIa, IIb, IId, and IIe are C-X5-C-X23-H-X-H (Table S1, Figure 2B). Moreover, the motif of Group III is C-X7-C-X23-H-X-H (Table S1, Figure 2B).
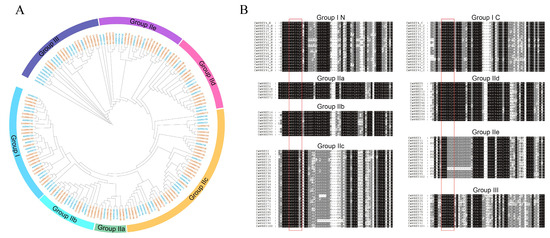
Figure 2.
Unrooted Phylogenetic tree and multiple sequence alignments of CmWRKY domains. (A) The phylogenetic tree was constructed using maximum likelihood in IQ-TREE based on N-terminal WRKY domains. Orange represents the WRKY gene in C. maxima and blue represents the WRKY gene in A. thaliana. The different colors on the outer ring represent different groups. (B) The N-terminal WRKY domains and C-terminal WRKY domains of Group I WRKYs are shown by the “Group IN” or “Group IC”, respectively. The conserved 7-peptide amino acids are marked with a red box, and the alignments were performed using MUSCLE.
3.3. Conserved Motifs and Gene Structure of CmWRKY Proteins
To further understand the conservation of CmWRKYs, we surveyed the gene structure and conserved motif of all CmWRKY genes identified using the Multiple EM for Motif Elicitation (MEME) software 5.5.4. Eleven motifs, named 1–11, were identified (Figure 3). The number of conserved motifs for each CmWRKY gene ranged from two (CmWRKY13 and CmWRKY62) to nine (CmWRKY27 and CmWRKY77) (Figure 3). All CmWRKYs contained motif 1 and motif 2. The conserved motifs of CmWRKYs categorized into the same group or subgroup had highly similar compositions. For example, in subgroup IIc, most CmWRKYs are composed of motifs 4-1-2, except for CmWRKY97 and CmWRKY62, which are composed of 4-1-13 and 4-2, respectively. At the same time, Group IIc has the lowest number of motifs. Subgroup IIa has the same motif composition (6-1-2-7). Groups I and IId has rich motif compositions, and all groups contain motifs 1 and 2. In contrast, motifs 12 and 15 are only present in subgroups IId and IIe, respectively. Motif 6 was found only in subgroups IIa and IIb, and motif 3 was only found in Group I.
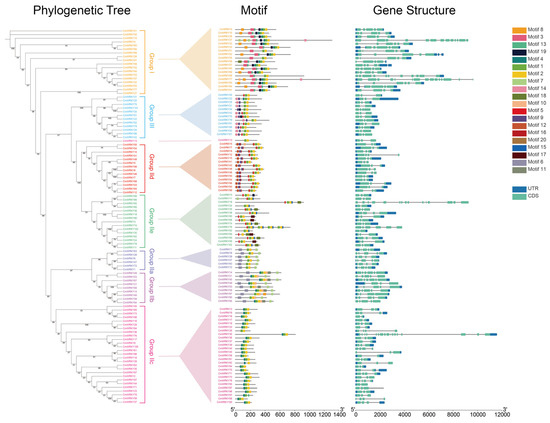
Figure 3.
Phylogenetic relationship, motif compositions, and intron–exon structures. The unrooted phylogenetic tree was constructed using IQ-TREE by the maximum likelihood method with 1000 ultrafast bootstrap replicates. The middle figure displays the motif compositions of CmWRKY proteins as determined by MEME software 5.5.4 analysis, with various colored boxes serving as representations. An intron–exon structure of the 102 CmWRKY genes is shown in the right panel. The scale at the bottom can be used to assess length.
The structural features and evolution of CmWRKY genes were further analyzed based on the intron/exon distribution and intron number. The number of introns in the CmWRKY family ranges from one to 16 (Figure 3). A total of 46 CmWRKY genes contain two introns, which is the largest proportion, 18 CmWRKY genes contain four introns, 16 CmWRKY genes contain three introns, seven CmWRKY genes contain five introns, and two CmWRKY genes contain 14 introns. In addition, nine CmWRKY genes contain only one intron, and only one CmWRKY gene contains 6, 10, 12, and 16 introns, respectively. CmWRKY genes, categorized into the same group and subgroup, have similar intron numbers. For example, in subgroup IId, only one CmWRKY gene had three introns, while the others had two. Most CmWRKY genes in subgroups IIc, IIe, and III also had two introns. In contrast, CmWRKY genes in Group I, and subgroups IIa and IIb all have more than two introns, some have four or five introns, and others have even more than six introns.
Hence, the similar motif and gene structure of proteins in the same group or subgroups suggests that these proteins may have similarities in the role of these proteins.
3.4. Synteny Analysis of CmWRKYs
As a model plant, the WRKY family of A. thaliana has been systematically studied for many years [28]. Thus, to understand the evolution of the C. maxima WRKY family members, a synteny analysis was performed between C. maxima and A. thaliana (Figure 4, Table S2). The differently colored lines in the background highlight syntenic WRKY gene pairs with different chromosomes of C. maxima and A. thaliana. A total of 90 pairs of syntenic relationships were identified, including 48 AtWRKY genes and 63 CmWRKY genes. Among these WRKY genes, five CmWRKY genes (CmWRKY11, CmWRKY14, CmWRKY18, CmWRKY39, and CmWRKY40) and three AtWRKY genes (AtWRKY61, AtWRKY70, and AtWRKY29) were found to be associated with three synteny events. Three AtWRKY genes (AtWRKY15, AtWRKY23, and AtWRKY48) were associated with four synteny events, and one AtWRKY gene (AtWRKY46) was associated with five synteny events. Two synteny events accounted for the largest proportion of 90 pairs of synteny relations.
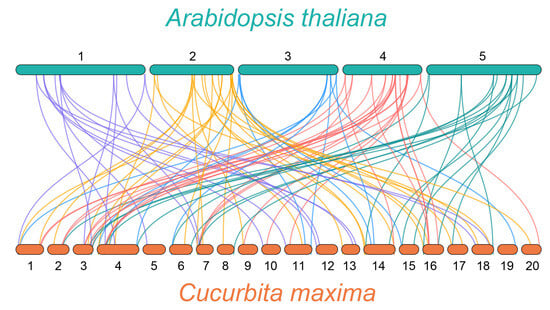
Figure 4.
Synteny analyses between the WRKY genes of C. maxima and A. thaliana. The collinear gene pair with A. thaliana genes are highlighted with different color lines.
3.5. CmWRKY22 Negatively Regulated CMV Infection
WRKY TFs are involved in plant resistance to viruses The initial homology analysis in our study indicated that six CmWRKY genes might be involved in the process of virus resistance using homology analysis [29,30,31,32,33,34] (Table S3). To confirm the virus resistance function of these genes, the relative gene expression levels were analyzed after CMV infection. The results showed that all six CmWRKY genes were induced following CMV infection, with CmWRKY22 showing the highest expression (Figure 5A). Therefore, we focused our investigation on the role of CmWRKY22 in CMV infection. We used tobacco rattle virus (TRV)-based virus-induced gene silencing in C. maxima plants. Notably, the full coding sequence of CmWRKY22 was cloned into TRV RNA2 to generate TRV: CmWRKY22 and co-inoculated with TRV RNA1. Compared with the control plants, TRV: CmWRKY22 exhibited more severe viral disease symptoms on leaves after CMV infection (Figure 5B). Meanwhile, the relative expression level of CmWRKY22 in TRV:00 (control) was significantly higher, while the expression level of CMV Coat Protein (CP) was lower (Figure 5C,D). In addition, we examined the CmWRKY22 and CMV CP expression levels in CmWRKY22 transient overexpression plants. As shown in (Figure 5E–G), the symptoms and virus accumulation were significantly lower in the CmWRKY22 overexpression plants than in the control plants. These results indicate that CmWRKY22 may have a negative effect on CMV infection.
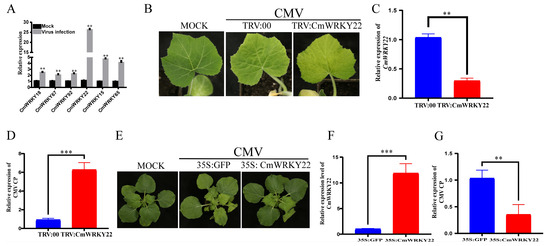
Figure 5.
CmWRKY22 plays a negative role in CMV infection. (A) The relative expression level of six CmWRKY genes after CMV infection. (B) Tobacco Rattle Virus (TRV)-based virus-induced gene silencing of CmWRKY22 promoted CMV infection and exhibits more mosaic leaf symptoms. (C) The relative expression level of CmWRKY22 in TRV:00 and TRV: CmWRKY22 plants. (D) The relative expression level of CMV CP in C. maxima plants (TRV: CmWRKY22) compared with control (TRV:00) plants. (E) CmWRKY22 inhibited CMV infection in N. benthamiana. (F) The relative expression level of CmWRKY22 in 35S: GFP and 35S: CmWRKY22 in N. benthamiana. (G) The relative expression of CMV CP in 35S: CmWRKY22 compared with control plants. These qRT-PCR results suggested that CmWRKY22 plays a negative role in CMV infection. The asterisks represent significant differences of treatment group and control group (Student’s t-test, ‘***’, p < 0.001 ‘**’, p < 0.01), all data were analyzed using 2−ΔΔCT method.
3.6. CmWRKY22 Regulated the Expression of Salicylic Acid (SA)-Responsive Gene PR1b in Plants
Since SA plays a crucial role in plant resistance to pathogens and viral infections [35,36], we have further investigated if CmWRKY22 is involved in the SA signaling pathway. Using the TRV assay, we found that the expression of SA synthesis-related genes (CmICS1, CmPAL, and CmATM1) was not significantly different between TRV:00 and TRV:CmWRKY22, whereas the expression of SA-responsive genes (pathogenesis-related proteins b, PR1b) was significantly higher (Figure 6A). In addition, only the expression of NbPR1b was significantly higher in CmWRKY22 transient overexpression plants. (Figure 6B). WRKY TFs were known to be able to bind to the W-box in the promoter region of a gene to regulate its expression. Thus, we performed an EMSA analysis of CmWRKY22 with the W-box region of the CmPR1b promoter, and the results showed that CmWRKY22 was able to bind the W-box motif of the CmPR1b promoter (Figure 6C).
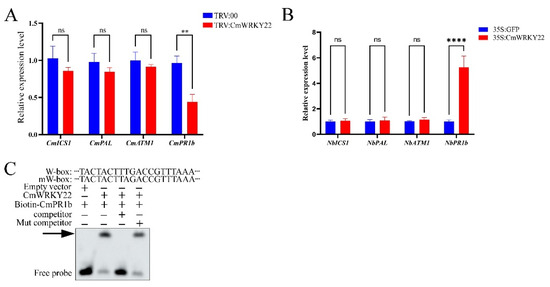
Figure 6.
CmPR1b was significantly induced and CmWRKY22 could regulated the expression of SA-responsive gene CmPR1b. (A) Relative expression level of SA biosynthesis and responsive genes between TRV:00 and TRV: CmWRKY22. (B) Relative expression level of SA biosynthesis and responsive genes. The asterisks in A and B represent significant differences of treatment group and control group (Student’s t-test, ‘**’, p < 0.01, ‘****’, p < 0.0001, ns, no significance), all data were analyzed using 2−ΔΔCT method. (C) EMSA results confirmed the CmWRKY22 binds to the W-box of CmPR1b promoter. The arrow indicated the binding of CmWRKY22 to the biotin-labled W-box promoter. The “+” and “−” indicated the correspond component.
4. Discussion
The WRKY TF family is engaged fundamentally in the entire plant growth and development process and plays a pivotal role in the exposure of both biotic and abiotic stresses [7,37]. It has been studied extensively in sereral monocot and dicot species, including A. thaliana [38], Rice [38] and Wheat [39]. However, the identification of WRKY TFs in the Cucurbitaceae family pales in comparison to other large families, such as only 63 WRKY genes were identified in Citrullus lanatus [40] and 55 WRKY genes in Cucumis sativus [41]. However, we identified 102 WRKY genes in the C. maxima, which is much more than the above C. lanatus and C. sativus, suggesting that the WRKY TFs in the Cucurbitaceae family still has great potential for exploitation.
Gene duplication has a vital part in biological evolution as it helps to increase genetic and functional diversity [42,43,44] and can result in the expansion of WRKY gene family members [42]. In this study, we identified 102 WRKY genes in the C. maxima genome that were distributed in a diverse manner across 20 chromosomes (Figure 1) and were extremely similar in their number to A. thaliana (102). However, the number of WRKY genes is significantly larger as compared to the number of WRKY genes on some other species, such as Isatis indigotica (64) [45] and Salvia miltiorrhiza (61) [46]. Therefore, we speculate that gene duplication events may also occur in C. maxima. A syntenic relationship analysis based on CmWRKY family with AtWRKY family has shown a total of 90 pairs of syntenic relationships between C. maxima and A. thaliana, suggesting that the evolution of C. maxima and A. thaliana are quite closely related.
CMV infection is a major plant viral disease that affects a variety of plants and causes severe yield losses. WRKY transcription factors are involved in various biological processes in plants and play a broad-spectrum regulatory role in plant defense responses [36,47,48]. Therefore, to gain a better understanding of the role of WRKY transcription in CMV infection of C. maxima, we have selected several genes that may be involved in the response to CMV infection. These are homologs of genes that have been reported to play important roles in viral infection in other species, including A. thaliana [29,30,31], Solanum lycopersicum [32], G. hirsutum [33], and Capsicum annuum [34]. Six candidate genes were identified, among which CmWRKY22 showed the highest expression, reaching >25-fold higher levels after CMV infection. Therefore, we conducted subsequent experiments using CmWRKY22 as a target. The other five genes, which may also be involved in the process of CMV–host interactions, will be the targets of our future studies.
C. maxima WRKY TFs have been divided into three groups: I, II, and III, while CmWRKY22 belongs to Group III. Several studies have been conducted on Group III members in different species, including A. thaliana [49], rice [50,51], grape [50], and S. lycopersicum [31]. Prior studies have shown that Group III members contribute to the pathogen defense efforts, for example by participating in the SA pathway in plants [31,49]. It has been reported that SA also plays an important role in plant resistance to biotic stress, including viruses and pathogens [36,52,53,54,55]. Meanwhile, AtWRKY22 can regulate SA and jasmonic acid (JA) signaling [56], which leads us to speculate that CmWRKY22 is also involved in this process.
Therefore, we used TRV-based, virus-induced gene silencing and overexpression to explore the functions of CmWRKY22. As shown in Figure 5, CmWRKY22 plays a negative regulatory function in CMV infection. Then, we analyzed the expression levels of three SA synthesis-related genes and one SA-responsive gene in the TRV assay and overexpression plants. The results showed that the SA-responsive gene PR1b was significantly upregulated (Figure 6A,B). As one of the largest TF families, the WRKY TF family can bind to the W-box (TTGACC/T) of gene promoters and regulate gene expression [57,58]. The EMSA also showed that CmWRKY22 could bind to the promoter of CmPR1b (Figure 6C). Therefore, we speculate that CmWRKY22 may bind to the W-box of CmPR1b to regulate its expression and, thus, protect against CMV infection.
5. Conclusions
One-hundred-and-two WRKY genes were identified in C. maxima. Their chromosomes’ location, phylogenetic relationship, conserved motifs, and gene structure were examined. Moving a step further, we identified a gene, CmWRKY22, that plays a positive regulatory role in plant resistance to cucumber mosaic virus (CMV) infection, and subsequently confirmed the ability of CmWRKY22 to bind to the promoter region of the SA-responsive gene, CmPR1b, via EMSA experiments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14112030/s1, Table S1. Identified WRKY genes in C. maxima. Abbreviations: Ai, aliphatic index; Chr, chromosome numbers; GRAVY, grand average of hydropathicity; MW, molecular weight; NA, not Available; ORF, opening reading frame; pI, isoelectric point; UN, unknow chromosome. Table S2. The synteny regions between C. maxima WRKYgenes. Table S3. List of CmWRKY genes may be involved in the process of virus resistance.
Author Contributions
Conceptualization, Q.Z. and P.Z.; methodology, Z.G.; software, X.Z. and D.W.; validation, L.Z. and K.B.; writing—original draft preparation, Q.Z. and P.Z.; writing—review and editing, Q.Z. and P.Z.; visualization, Q.Z.; supervision, P.Z.; project administration, P.Z.; funding acquisition, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Zhejiang Province, China (LY23C140003), Research and demonstration of key technologies for ensuring supply of vegetable industry (2020-J-005-ydhz).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study are available in the article.
Acknowledgments
We would like to thank Xu Chen for providing C. maxima plants and improving the language of our manuscripts.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, X.; Kumar, D.; Chen, H.; Wu, S.; Kim, J.Y. Transcription factor-mediated cell-to-cell signalling in plants. J. Exp. Bot. 2014, 65, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Ratcliffe, O.J. A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 2000, 3, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Century, K.; Reuber, T.L.; Ratcliffe, O.J. Regulating the regulators: The future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol. 2008, 147, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014, 42, D1182–D1187. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Liu, S.; Somssich, I.E. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Ma, S.H.; Ye, N.H.; Jiang, M.; Cao, J.S.; Zhang, J.H. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Maeo, K.; Hayashi, S.; Kojima-Suzuki, H.; Morikami, A.; Nakamura, K. Role of conserved residues of the WRKY domain in the DNA-binding of tobacco WRKY family proteins. Biosci. Biotechnol. Biochem. 2001, 65, 2428–2436. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, I.; Matsuda, T.; Tomo, Y.; et al. Solution structure of an arabidopsis WRKY DNA binding domain. Plant Cell 2005, 17, 944–956. [Google Scholar] [CrossRef]
- Bakshi, M.; Oelmüller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav. 2014, 9, e27700. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Xing, F.; Wang, N.; Wen, F.; Zhu, C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Abid, G.; Muhovski, Y.; Mingeot, D.; Saidi, M.N.; Aouida, M.; Aroua, I.; M’hamdi, M.; Barhoumi, F.; Rezgui, S.; Jebara, M. Identification and characterization of two faba bean (Vicia faba L.) WRKY transcription factors and their expression analysis during salt and drought stress. J. Agric. Sci. 2017, 155, 791–803. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Z.; Wang, W.; Jiang, X.; Li, D.; Pan, J.; Li, X. CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol. Plant. 2016, 60, 443–451. [Google Scholar] [CrossRef]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in arabidopsis. Plant Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Zhang, Z.Q.; Dong, J.L.; Wang, T. Overexpression of MtWRKY76 increases both salt and drought tolerance in Medicago truncatula. Environ. Exp. Bot. 2016, 123, 50–58. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Jiang, S.Y.; Kumar, N.; Venkatesh, P.N.; Ramachandran, S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008, 49, 865–879. [Google Scholar] [CrossRef]
- Robinson, R.; Decker-Walters, D. Cucurbits; CAB International: Wallingford, NY, USA, 1997. [Google Scholar]
- Alfawaz, M.A. Chemical composition and oil characteristics of pumpkin (Cucurbita maxima) seed kernels. Food Sci. Agric. 2004, 2, 5–18. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype Stability and Unbiased Fractionation in the Paleo-Allotetraploid Cucurbita Genomes. Mol. Plant 2017, 10, 1293–1306. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, E1963–E1971. [Google Scholar] [CrossRef]
- Gao, R.; Liu, P.; Yong, Y.; Wong, S.M. Genome-wide transcriptomic analysis reveals correlation between higher WRKY61 expression and reduced symptom severity in Turnip crinkle virus infected Arabidopsis thaliana. Sci. Rep. 2016, 6, 24604. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.Y.; Wu, P.; Xu, Z.S.; Que, F.; Wang, F.; Xiong, A.S. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 788. [Google Scholar] [CrossRef]
- Huh, S.U.; Choi, L.M.; Lee, G.J.; Kim, Y.J.; Paek, K.H. Capsicum annuum WRKY transcription factor d (CaWRKYd) regulates hypersensitive response and defense response upon Tobacco mosaic virus infection. Plant Sci. 2012, 197, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Huaxia, Y.; Lu, W.; Wu, C.; Cao, X.; Guo, X. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biol. 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Yang, F.; Ma, Y.; Wu, Q.; Yi, K.; Zhang, D. Transcription factor WRKY30 mediates resistance to Cucumber mosaic virus in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 517, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, Y.; Liu, Y.; Meng, D.; Jin, T.; Zhou, X. HC-Pro viral suppressor from tobacco vein banding mosaic virus interferes with DNA methylation and activates the salicylic acid pathway. Virology 2016, 497, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Murphy, A.M.; Lewsey, M.G.; Westwood, J.H.; Zhang, H.M.; González, I.; Canto, T.; Carr, J.P. Domains of the cucumber mosaic virus 2b silencing suppressor protein affecting inhibition of salicylic acid-induced resistance and priming of salicylic acid accumulation during infection. J. Gen. Virol. 2014, 95, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Han, H.; Lee, S. The role of WRKY transcription factors, FaWRKY29 and FaWRKY64, for regulating Botrytis fruit rot resistance in strawberry (Fragaria × ananassa Duch.). BMC Plant Biol. 2023, 23, 420. [Google Scholar] [CrossRef]
- Abdullah-Zawawi, M.R.; Ahmad-Nizammuddin, N.F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef]
- Ye, H.; Qiao, L.; Guo, H.; Guo, L.; Ren, F.; Bai, J.; Wang, Y. Genome-Wide Identification of Wheat WRKY Gene Family Reveals That TaWRKY75-A Is Referred to Drought and Salt Resistances. Front. Plant Sci. 2021, 12, 663118. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Ling, J.; Jiang, W.; Zhang, Y.; Yu, H.; Mao, Z.; Gu, X.; Huang, S.; Xie, B. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genom. 2011, 12, 471. [Google Scholar] [CrossRef]
- Chang, D.; Duda, T.F. Extensive and Continuous Duplication Facilitates Rapid Evolution and Diversification of Gene Families. Mol. Biol. Evol. 2012, 29, 2019–2029. [Google Scholar] [CrossRef]
- Zhang, J.Z. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Hughes, A.L. The evolution of functionally novel proteins after gene duplication. Proc. Biol. Sci. 1994, 256, 119–124. [Google Scholar] [PubMed]
- Qu, R.J.; Cao, Y.W.; Tang, X.Q.; Sun, L.Q.; Wei, L.; Wang, K.C. Identification and expression analysis of the WRKY gene family in Isatis indigotica. Gene 2021, 783, 145561. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, D.; Shao, F.; Lu, S. Molecular cloning and expression analysis of WRKY transcription factor genes in Salvia miltiorrhiza. BMC Genom. 2015, 16, 200. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yue, C.; Chen, D.; Zheng, Y.; Zhang, Q.; Yang, J.; Ye, N. Genome-wide identification of WRKY family genes and their response to abiotic stresses in tea plant (Camellia sinensis). Genes Genom. 2019, 41, 17–33. [Google Scholar] [CrossRef]
- Kalde, M.; Barth, M.; Somssich, I.E.; Lippok, B. Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Mol. Plant-Microbe Interact. 2003, 16, 295–305. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Zhu, Y.; Li, Y.; Yan, H.; Xiang, Y. Comparative genomic analysis of the WRKY III gene family in populus, grape, arabidopsis and rice. Biol. Direct 2015, 10, 48. [Google Scholar] [CrossRef]
- Nakayama, A.; Fukushima, S.; Goto, S.; Matsushita, A.; Shimono, M.; Sugano, S.; Jiang, C.-J.; Akagi, A.; Yamazaki, M.; Inoue, H. Genome-wide identification of WRKY45-regulated genes that mediate benzothiadiazole-induced defense responses in rice. BMC Plant Biol. 2013, 13, 150. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- White, R.F. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 1979, 99, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Salicylic acid-induced differential resistance to the Tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Yu, C.L.; Makhotenko, A.V.; Makarova, S.S.; Love, A.J.; Kalinina, N.O.; MacFarlane, S.; Chen, J.P.; Taliansky, M.E. Interaction of a plant virus protein with the signature Cajal body protein coilin facilitates salicylic acid-mediated plant defence responses. New Phytol. 2019, 224, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Kloth, K.J.; Wiegers, G.L.; Busscher-Lange, J.; van Haarst, J.C.; Kruijer, W.; Bouwmeester, H.J.; Dicke, M.; Jongsma, M.A. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J. Exp. Bot. 2016, 67, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; Taylor, I.A.; Peng, Y.-L.; Wang, D.; Liu, J. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Y.; Wang, L.; Liu, X.; Liu, Y.; Phillips, J.; Deng, X. A WRKY transcription factor participates in dehydration tolerance in Boea hygrometrica by binding to the W-box elements of the galactinol synthase (BhGolS1) promoter. Planta 2009, 230, 1155–1166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).