Abstract
The model rhizobacterium Pseudomonas ogarae F113, a relevant plant growth-promoting bacterium, encodes three different Type VI secretion systems (T6SS) in its genome. In silico analysis of its genome revealed the presence of a genetic auxiliary module containing a gene encoding an orphan VgrG protein (VgrG5a) that is not genetically linked to any T6SS structural cluster, but is associated with genes encoding putative T6SS-related proteins: a possible adaptor Tap protein, followed by a putative effector, Tfe8, and its putative cognate immunity protein, Tfi8. The bioinformatic analysis of the VgrG5a auxiliary module has revealed that this cluster is only present in several subgroups of the P. fluorescens complex of species. An analysis of the mutants affecting the vgrG5a and tfe8 genes has shown that the module is involved in bacterial killing. To test whether Tfe8/Tfi8 constitute an effector–immunity pair, the genes encoding Tfe8 and Tfi8 were cloned and expressed in E. coli, showing that the ectopic expression of tfe8 affected growth. The growth defect was suppressed by tfi8 ectopic expression. These results indicate that Tfe8 is a bacterial killing effector, while Tfi8 is its cognate immunity protein. The Tfe8 protein sequence presents homology to the proteins of the MATE family involved in drug extrusion. The Tfe8 effector is a membrane protein with 10 to 12 transmembrane domains that could destabilize the membranes of target cells by the formation of pores, revealing the importance of these effectors for bacterial interaction. Tfe8 represents a novel type of a T6SS effector present in pseudomonads.
1. Introduction
The Type VI Secretion Systems (T6SSs) are nanomachine systems that translocate specific proteins directly into target cells [1]. These systems were originally described in Vibrio cholerae [2] and Pseudomonas aeruginosa [3]. T6SSs are present in more than 25% of gram-negative bacteria, mostly confined to the phylum Proteobacteria [4,5] and frequently encoding more than one T6SS in their genome [6,7]. Although it has been characterized as a classical virulence factor against eukaryotic cells [1,8,9] and plants [10,11,12], the relevance of T6SSs resides mainly with its anti-prokaryotic activity [13,14,15,16].
The common genetic organization of the T6SSs groups the structural proteins of the system with well-defined functions in genomic clusters that generally comprise 13 to 15 genes [7,17,18,19]. The genes encoding the T6SS effectors and their cognate immunity proteins are commonly linked to the hcp and/or vgrG genes within T6SS clusters [20,21,22] or in the islands/auxiliary modules harboring orphan vgrG or hcp genes [23,24]. Most T6SS effectors are antibacterial toxins that have been described in bacterial pathogens such as V. cholerae and P. aeruginosa [25,26,27]; nevertheless, T6SS antifungal effectors have been found in Serratia marcescens as growth inhibitors of pathogenic species [16]. Genes encoding effectors are frequently found adjacent to genes encoding immunity proteins, usually forming transcriptional units [13,28,29,30,31]. Genes encoding orphan VgrG proteins, not genetically linked to any T6SS structural cluster, have also been described for several T6SS-containing bacteria [24,32,33]. These orphan VgrG-encoding genes are frequently found dispersed in the genome, not being directly associated with defined T6SSs clusters. Interestingly, some of them conform to a VgrG island, auxiliary module, orphan effector island, or effector island [24,33,34,35,36] where the vgrG genes are ligated to genes encoding adaptor proteins (commonly Tap proteins) and effector–immunity pairs.
In the Pseudomonas genus, the T6SS from P. aeruginosa is a well-studied system that plays an essential role in several biological processes [13,36,37,38,39,40,41] and, to a lesser extent, in the case of P. putida as a system with activity against phytopathogens [22]. In other species from this genus, the T6SS has been implicated in siderophore production [42], bacteria colony invasion [43], and rhizosphere colonization [44].
Additionally, T6SSs have been described in species belonging to the P. fluorescens complex of species [45], playing a role in the rhizosphere and insect gut environment [46,47] or releasing anti-bacterial toxins [48,49]. We have recently described a role for the T6SS of Pseudomonas ogarae F113 in the colonization of the rhizosphere [44,50]. These findings highlight the importance of T6SSs in environmental adaption. In addition to the structural elements of T6SSs, P. ogarae F113 possesses five orphan vgrG genes across its genome [51]. In this work, we analyze one such auxiliary module which contains a vgrG gene associated with a gene encoding an adaptor Tap protein and genes encoding a putative effector–immunity pair named Tfe8-Tfi8 [44]. Our results show that this auxiliary module is functional in bacterial killing, defining a novel type of a Type 6 effector protein.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
The bacterial strains, vectors, plasmids, and derivative constructions employed in this study are listed in Supplementary Table S1, which includes relevant information associated with each strain. Pseudomonas ogarae strains were grown in Sucrose–Asparagine medium (SA) [52] at 28 °C. The Escherichia coli strains were grown in Lysogeny Broth (LB) medium [53] at 37 °C. E. coli was used for cloning purposes (DH5α cells) and protein expression [BL21(DE3) cells]. Antibiotics, inducers, and repressors were added as required at the following final concentrations: kanamycin, 50 μg mL−1 for P. ogarae F113 and insertional mutant strains, and 25 μg mL−1 for E. coli strains, and chloramphenicol, 30 μg mL−1, and ampicillin, 100 μg mL−1, for the expression plasmids, L-arabinose 0.02% v/v, isopropyl β-D-thiogalactopyronaside (IPTG) 1 mM, and glucose 0.2% v/v, according to corresponding vector.
2.2. Construction of VgrG5a Auxiliary Module Mutants
Insertional mutants in the P. ogarae F113; PSF113_0666 (VgrG5a−) and _0668 (Tfe8−) genes were obtained by introducing by electroporation the plasmid vector pCR2.1®-TOPO® (Invitrogen, Waltham, MA, USA) carrying a ca 400 bp of the internal region of each of the genes previously detailed. The single recombinant mutants obtained were selected by kanamycin resistance in SA medium and checked by PCR and Southern Blot. For the complementation of the mutations in the PSF113_0666 and PSF113_0668 genes, cosmid pBG200 was used [54]. The cosmid harbors genomic region 790,725 to 809,037 from P. ogarae F113 [55] from the sequence available in the NCBI databank, which includes the genes PSF113_0666, _0667, _0668, and _0669.
2.3. Bioinformatic Analyses
Pseudomonas gene sequences for the mutants’ design were obtained from the Pseudomonas Genome database [56]. BLASTN of the vgrG5a F113 gene was carried out against the nonredundant (nr) NCBI database to search for homologous genes in other members of the Pseudomonas genus. Adjacent genes to the hits were recovered to determine the neighborhood of the vgrG5a gene in other species. BLASTP analyses were performed on the NCBI website [57] to determine the degree of conservation and the amino acid sequence searches using SMART [58,59]. For the genome analysis of the Pseudomonas Genomes Type (Strain), Genome Server (TYGS) [60,61] was employed. Genome Blast Distance Phylogeny (GBDP), implemented in TYGS, was employed to infer genome-to-genome distances. In all cases, the parameters were used at their default values. The neighbor-joining (NJ) phylogenetic tree was constructed using MEGAX [62].
PSORTb version 3.0.2 software [63], the Protein Homology/analogy Recognition Engine (Phyre2) server [64], and PredictProtein [65] were used to perform structural-based homology prediction. Phyre2 and AlphaFold Protein Structure Database version 2.3.2 [66] were used to predict the subcellular location of proteins and transmembrane domains, and the prediction of the 3D model and interaction. ChimeraX version 1.6 [67] was employed to structure visualization.
2.4. Interbacterial Competition Assays
Competition assays were performed according to the previously reported protocol [39]. Briefly, kanamycin-resistant derivatives of P. ogarae F113 and mutants (predator) and E. coli DH5α containing a lacZ, kanamycin-resistant pK18mobsacB plasmid (prey) were grown overnight in LB medium. Each culture was adjusted to OD600 of 1.0, and 100 μL of the predator and 100 μL of the prey strains were mixed and co-cultured for 5 h at 200 rpm and 28 °C. Twenty μL of each culture was spotted onto LB-agar supplemented with 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-gal) and kanamycin and incubated at 28 °C. At least three biologically independent experiments were performed. Additionally, serial dilutions of the different assays were plated to quantify the number of CFUs in each of them.
2.5. The Toxic and Antitoxic Activity of F113 Tfe8 and Tfi8 Proteins Expressed in E. coli
The tfe8 and tfi8/tfe8 genes from P. fluorescens F113 were amplified by PCR from genomic DNA and cloned into the plasmid vector pT7-7 [68] using the NdeI and BamHI restriction sites. Cloning resulted in three plasmids, one of which was empty while the other two carried the toxin (Tfe8) and antitoxin/toxin proteins (Tfi8/Tfe8). These plasmids were transformed into E. coli BL21(DE3) expression cells. The induction of expression was performed as previously described [69]. Briefly, cultures were grown from a pre-culture adjusted to the OD600 of 1.0 at 28 °C for 4 h, and 20 μL of each culture was spotted in LB plates supplemented with ampicillin and IPTG. The plates were incubated for 24 h at 28 °C. Additionally, the PCR product of the tfe8 encoding the toxin was cloned into a pNDM220 low-copy number vector [70] named pTfe8, and the tfi8 gene encoding the antitoxin pair was cloned into a pBAD33 [71] named pTfi8, under the control of the PBAD promoter. Recombinant plasmids were checked by sequencing and then included in E. coli DH5α by transformation. The E. coli strain carrying both pTfi8 and pTfe8 plasmids was cultured overnight and then adjusted to an OD600 of 0.2. Next, the expression of Tfe8, Tfi8, or both was induced by supplementing the medium with the corresponding regulator: 1 mM IPTG for pNDM220 Tfe8 induction, 0.02% L-arabinose for Tfi8 induction, and 0.2% glucose for Tfi8 repression. Growth was recorded after 24 h by measuring the optical density at OD600. The optical density measures were relativized with the measures of E. coli containing the empty vectors.
2.6. Statistical Analyses
The normal distribution of data was checked with the Shapiro–Wilk test, and since the distribution was normal, the data were analyzed with a one-way ANOVA test, where multiple pairwise comparisons between strains were performed with a Tukey HSD test. All data were analyzed with R language version 4.1.1
3. Results
3.1. An Orphan vgrG5a-tap5a Region Is Present in Strains of the Pseudomonas fluorescens Complex of Species
The genomic sequence of P. ogarae F113 encodes an orphan vgrG gene (PSF113_0666) downstream of the speA gene (PSF113_0665), an arginine decarboxylase, in the close vicinity of the diguanylate-cyclase encoded by the gcbA gene (PSF113_0661). The vgrG gene encodes a VgrG5a protein, homologous to T6SS export proteins, and is followed by a gene encoding a DUF4123 protein which is an orthologue of several proteins that act as adaptors for type VI-secreted effectors [21,72]. We have therefore named these genes vgrG5a and tap5a (PSF113_0667) [44]. The analysis of this region revealed that downstream of these genes and in the opposite transcription direction [73], P. ogarae F113 encodes one gene which encodes a MATE/Din7 protein domain, similar to the Multidrug and Toxin Compound Extrusion transporter [74] and a small hypothetical protein. Since this genetic organization resembles a T6SS auxiliary module [75], we have named those two genes tfe8 (PSF113_0668) and tfi8 (PSF113_0669) [44], putatively encoding a T6SS effector and its cognate immunity protein. While the orthologues of the putative toxin and immunity genes are widespread within the genus Pseudomonas, the gene cluster formed by vgrG5a and tap5a is present only in a limited number of strains, all belonging to the Pseudomonas fluorescens complex of species [45,76]. We have named this region the speA-MTase locus, since synteny is maintained by the speA gene and the gene encoding a putative MTase protein (Figure 1). Figure 1 shows the genetic organization of the speA-MTAse locus in strains belonging to different subgroups of the P. fluorescens and P. putida groups.
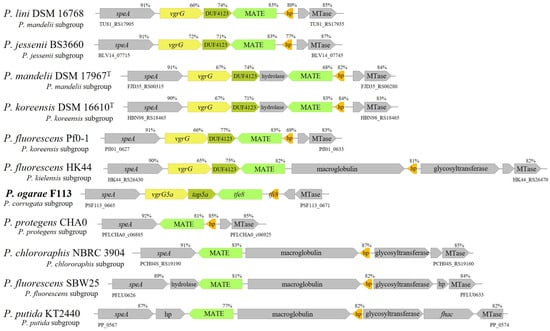
Figure 1.
Genetic organization of vgrG auxiliary module in Pseudomonas genera. The genes are at scale. Values over each gene indicate the sequence identity value in relation with the vgrG5a auxiliary module from P. ogarae F113. hp; hypothetical protein.
It can be observed that this locus shows high heterogeneity in its organization; downstream speA, the vgrG5a, and tap5a are present in strains of the Pseudomonas corrugata, P. mandelii, P. jesseni, P. koreensis, and P. kielensis subgroups. Neither of these genes are present in strains of the P. fluorescens, P. protegens, and P. chlororaphis subgroups, nor in the P. putida group. On the contrary, an orthologue of tfe8 is present in all the analyzed strains, regardless of the group or subgroup. This gene is always transcribed in the opposite direction of speA and is located in different configurations. In most strains harboring the vgrG5-tap5a genes, the tfe8 orthologue is located adjacent to this cluster. In most of the strains that do not contain the vgrG5a-tap5a, the tfe8 orthologue is adjacent to speA. However, in a few strains, another gene, putatively encoding a hydrolase, is located adjacent to the tfe8 orthologue. Regarding tfi8, its orthologues are located just upstream tfe8 and in the same sense of the transcription in most of the strains that also contain vgr5a and tap5a. In most of the other strains, both genes are separated by another gene, encoding a putative macroglobulin. These data show that the speA-MTase locus has undergone several restructurations during evolution by acquiring new genes and eliminating others. The acquisition of the vgrG5a-tapA5a pair seems to be a relevant evolutionary landmark indicating a gain of function.
In order to investigate the prevalence of this gain of function, we have investigated the presence of the vgrG5a-tap5a genes in a larger number of strains belonging to the P. corrugata, P. mandelii, P. jesseni, P. koreensis, and P. kielensis subgroups. The results have shown that the vgrG5a-tap5a are present in the vast majority of strains from these groups with genomic sequences available (Supplementary Table S2). We have not found these genes in any strains outside these subgroups. A phylogenomic tree containing all the strains tested (Figure 2 and Figure S1) shows that the presence of the vgr5a-tap5a occurs in a monophyletic group which includes the P. fluorescens subgroups P. corrugata, P. mandelii, P. jesseni, P. koreensis, and P. kielensis.
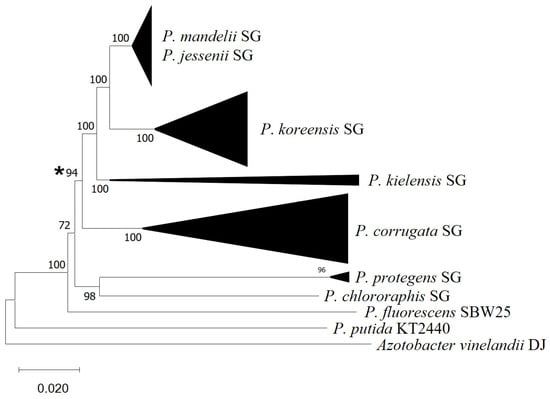
Figure 2.
GBDP-based phylogeny of fifty genomes belonging to Pseudomonas groups. Subtree composed by major subgroups has been compressed and their names indicated. Asterisk indicates the genetic acquisition event of vgrG and tap genes. MEGAX was employed for the visualization and edition of the tree. Genomes employed are listed in Table S2.
These results indicate that the acquisition of vgrG5a-tap5a has occurred once in evolution, in a common ancestor to all these subgroups. Other P. fluorescens subgroups, such as P. fluorescens, P. protegens, and P. chlororaphis, that do not contain the genes, are paraphyletic with the other subgroups (Figure 2 and Figure S1). Other restructurations within this locus are more difficult to date, but the physical separation between tfe8 and tfi8 seems to be an ancestral trait, and the loss of the intertwining gene has likely evolved with the acquisition of the vgrG5-tap5a genes. As a result of these two processes, a genetic region that appears to have genes encoding a VgrG protein, a Type VI adaptor protein, and a putative secreted effector and its cognate immunity protein has evolved in a monophyletic group of pseudomonads. Conversely to the other Tfes encoded by the P. ogarae F113 genome [44], Tfe8 does not resemble any known type VI effector or harbor any previously described T6SS-related domains, such as the PAAR or MIX domains [77].
3.2. The VgrG5a-Tap5a Cluster Is Involved in Bacterial Killing
Considering the resemblance of the P. ogarae F113 speA-MTase locus with a T6SS effector delivery system, we decided to test the relation of this locus with bacterial killing. For that, we generated insertion mutants affecting either the vgrG5a gene or the tfe8 gene, putatively encoding the type VI effector. In order to test killing, we used two approaches [5]. First, we used a non-quantitative patch method (Figure 3A) using a lacZ tagged E. coli strain as the prey and P. ogarae F113 insertional mutants as the predators. Therefore, E. coli killing was observed as a lack of blue color generated by E. coli pK18mobsacB in the presence of X-gal in LB plates. Figure 3A shows the killing ability for the F113 wild-type and an impairment in the killing for both mutants.
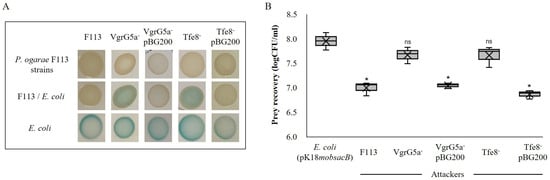
Figure 3.
Bacterial killing profile of the vgrG5a auxiliary module between P. ogarae F113 mutants and E. coli strain carrying pK18mobsacB expressing lacZ. (A) Bacterial patches obtained from the bacteria–bacteria interaction, where the blue color on LB plates (X-gal and kanamycin) indicates E. coli growth. The top row shows the growth of P. ogarae control or its insertional mutant strains. The middle row shows the growth of mixed P. ogarae/E. coli cultures after 5 h of co-incubation. Bottom row shows the E. coli reference culture. (B) Bacterial killing quantification. Box and whiskers plots indicating the amount of prey recovery from each attacker expressed as logCFU/mL. Significance was calculated using ANOVA test (* p value < 0.01); ns, not significant differences when compared to non-competing E. coli. X symbol in each box indicates the mean in each sample.
The complementation of the mutants with a cosmid (pBG200) from a F113 library, previously isolated, which spans from upstream of the gcbA gene and contains the speA-Mtase locus, fully restored the killing capacity of the mutants. In the second quantitative approach (Figure 3B), we used the same prey and predator strains, but the killing activity was quantified by the recovery of the prey cells after contact with the predator strain.
The results obtained were similar to that of the previous experiment, since prey recovery was ten times lower after contact with the wild-type strain than that after contact with either of the mutant strains. The mutants recovered their killing ability after complementation with pBG200 to the same level than the wild-type strain.
3.3. Tfe8 and Tfi8 Are a T6SS Effector and Immunity Protein
Since both the VgrG5a and Tfe8 proteins are implicated in bacterial killing, we tested the role of Tfe8 and Tfi8 by ectopic expression in E. coli cells. We also used two approaches for this test. In the first approach, we cloned either the tfe8 gene or the tfi8-tfe8 genes in the pT7-7 plasmid. Serial dilutions of E. coli cells harboring either the empty vector or the vector containing each of the two constructions were plated on a nutrient medium. Figure 4A shows that cells expressing the tfe8 gene grew two orders of magnitude less than cells with the empty vector. Furthermore, construction containing tfi8 and tfe8 grew to a level similar to that of cells containing the empty vector, indicating that cells express both tfi8 and tfe8.
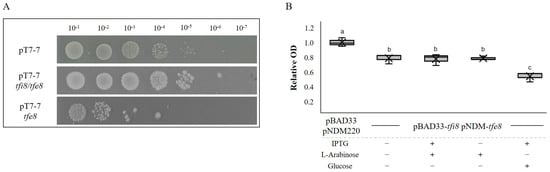
Figure 4.
Tfe8/Tfi8 constitute an effector–immunity pair. (A) Serial dilution of bacterial survival after the expression of cloned tfe8 and tfi8/tfe8 in E. coli BL21(DE3) cells. The top row corresponds to the control strain carrying an empty pT7-7 vector, the middle row corresponds to the induced cells of the cloned tfi8/tfe8 genes in pT7-7, and the lower row to the induced cells of the cloned tfe8 gene. All the strains present similar survival without induction. (B) Growth inhibition assays. Box and whiskers plot showing the growth of the E. coli DH5α-tfi8/tfe8 strain containing pBAD33-tfi8 and pNDM220-tfe8 plasmids in liquid culture. Significance was calculated using ANOVA with a Tukey test, where a and b (p value < 0.01), b and c (p value < 0.001), and between a and c (p value < 0.001). Optical density was recorded for over 24 h. Optical density was relativized to the of E. coli DH5α carrying the empty plasmids pBAD33 and pNDM220.
In the second approach, we cloned tfe8 in plasmid pNDM220 under the control of an IPTG inducible promoter and tfi8 in pBAD33 under the control of the araB promoter, that is inducible by arabinose and repressed by glucose. Both constructs were introduced in DH5α E. coli and a control strain was generated by introducing both empty vectors. As observed in Figure 4, the growth of the E. coli strain harboring both constructs was reduced in respect to the control strain under all tested conditions. These results indicate that even in the absence of induction, Tfe8 is produced, resulting in toxicity for the cells. The araB promoter seems also to be leaky in the absence of induction. However, when the araB promoter is repressed by glucose, the production of the putative immunity protein appears to be reduced, since upon induction with IPTG, the toxicity is higher, meaning that only the effector and not the immunity protein are produced.
Taken together, these results strongly suggest that tfe8 encodes a T6SS effector, probably secreted as a cargo by VgrG5a with the help of the adaptor protein Tap5a, and that Tfi8 is its putative cognate immunity protein.
The analysis of the subcellular localization of the putative immunity protein pair, Tfi8 and Tfe8, was made through a bioinformatics approach using the prediction tools PSORTb version 3.0.2, Phyre2 version 2.0, and AlphaFold version 2.3.2. These analyses indicate that both proteins are transmembrane proteins (Figure 5), where the toxin protein Tfe8 possesses between ten and twelve transmembrane domains, depending on the bioinformatics approach employed. The Tfe8 protein could form a possible pore in the target bacterial cell membrane (Figure 5A), and the analysis of the protein residues from Tfe8 revealed that 98% of this sequence has been modeled with 100% confidence by the single highest scoring template, and according to the Research Collaboratory for Structural Bioinformatics RCSB protein data bank, these template structures are related with the Multidrug and Toxin Compound Extrusion (MATE) transporter present in V. cholerae [78] and E. coli [79].
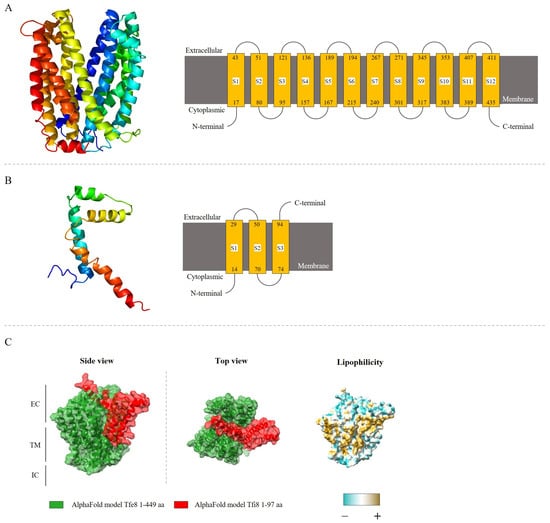
Figure 5.
Transmembrane helix prediction and topology adopted for the proteins analyzed through Phyre2 web portal for protein modeling. (A) PSF113_0668 (Tfe8). (B) PSF113_0669 (Tfi8). (C) Tfe8-Tfi8 predicted protein interaction by AlphaFold-Multimer. The complex has transmembrane, extracellular, and intracellular domains. Lateral and top view are present. Lipophilicity is indicated in the figure by colors, from more hydrophilic (blue) to more lipophilic (golden) surface. Protein structure was visualized with ChimeraX. EC; ExtraCellular, TM; TransMembrane, IC; ItraCellular. Figure S2 includes a QR code that allows the view of the interaction model in motion on the X and Y axis.
The predicted structure for the Tfi8 immunity protein presents three transmembrane domains, with an elongated tertiary structure (Figure 5B), and 61 residues of the putative immunity protein Tfi8 (63% of sequence) have been modeled with 38.1% confidence by the single highest scoring template. Model predictions employing AlphaFold predict the binding of one putative immunity Tfi8 to the pore generated by the Tfe8 protein. No signal peptides are found in any of these proteins, suggesting that they are not translocated to the cytoplasmic membrane through a general secretion pathway, where the analysis of these proteins also corroborates the idea of the transmembrane location of both proteins.
4. Discussion
Besides complete T6SSs, many bacteria encode accessory modules characterized by the presence of genes encoding T6SS proteins such as Hcp or VgrG, plus genes encoding other proteins including effectors and immunity proteins [75,80]. In the case of P. ogarae F113, its genome encodes three complete T6SSs and five accessory modules, each containing a gene encoding a different VgrG protein. We have previously shown that at least two of the T6SSs, F1-T6SS and F3-T6SS, are functional for bacterial killing [44]. Here, we analyze the role of one of the accessory modules regarding bacterial killing. The analyzed module contains a gene encoding a VgrG5a protein followed by a gene encoding a DUF4123 protein. DUF4123 proteins have been described as adaptor proteins for the secretion of cargo effectors by VgrG proteins [21]. Following this gene and in the opposite transcription direction, there are two genes encoding a MATE/DinF protein (tfe8) and a small hypothetical protein (tfi8). PSORTb predicts that both proteins are transmembrane proteins with transmembrane helices. No signal peptides are found in any of these proteins, suggesting that they are not translocated to the cytoplasmic membrane through a general secretion pathway. Phyre2 analysis of these proteins (Figure 5) also indicates the transmembrane location of both proteins. Furthermore, AlphaFold predicts an interaction of these two proteins in which Tfi8 could block the Tfe8 pore in the extracellular side, explaining its putative role as an immunity protein.
The four proteins in the auxiliary module present the structure of a typical T6SS auxiliary module, with a VgrG protein, an adaptor protein, and a putative effector and its cognate immunity protein. Similar gene arrangements have been found in other bacteria [21,35]. MATE proteins constitute a superfamily of multidrug and toxic compounds extrusion [81,82] that is divided into DinF, NorM, and eukaryotic families [83,84]. MATE/DinF-encoding genes are widespread among pseudomonads and, in many cases, are not genetically linked with genes related with secretion systems. However, a MATE-encoding gene has been found to be under the control of the Type Three Secretion system regulator HrpL in P. syringae pv. tomato DC3000, and its deletion resulted in reduced virulence [85]. This result suggests that MATE proteins can act as effectors if secreted through a specialized system.
We have shown here that this accessory module is only present within the P. fluorescens group and that it is conserved in a monophyletic branch that includes the P. corrugata, P. koreensis, P. mandelii, P. jesseni, and P. kielensis subgroups. These results suggest that the acquisition of the VgrG-Tap module, and therefore the possibility to secrete the MATE-DinF protein (Tfe8), has occurred only once in evolution, in a common ancestor of the subgroups carrying this module. The lack of close homologues of the vgrG5a and tap5a genes within the pseudomonads may indicate that its origin is from outside the genus. It is interesting to note that a limited number of strains from the P. syringae group, with genomes deposited in the Pseudomonas Genome Database [56], harbor a different VgrG-encoding gene in this locus, suggesting that these strains might also secrete a MATE/DinF protein.
We have also shown the importance of this accessory module in bacterial killing, since the mutation of either the VgrG- or the Tfe8-encoding genes resulted in a significant reduction of the E. coli killing ability in two different bacterial killing assays. In this strain, we previously showed that mutations affecting either of the two T6SSs (F1 and F3) also showed a reduction in killing [44], indicating that the auxiliary module also plays a relevant role in bacterial killing. The results presented here are also the first to show a role in the bacterial killing of a T6SS effector in this strain. Within pseudomonads, the role of auxiliary modules in bacterial killing has been previously shown in P. protegens, where two of these modules, one of them distantly located from the core T6SS genes, were shown to affect the Enterobacter population in an insect larvae gut, resulting finally in larvae death [46]. We have also shown the role of the Tfe8 protein as a putative T6SS effector by the ectopic expression of this gene in E. coli (Figure 4). The expression of tfe8 from two different vectors resulted in the reduced growth of E. coli cells, indicating Tfe8 toxicity. To our knowledge, it is the first MATE/DinF protein to be shown as a putative T6SS effector. Regarding its cognate immunity protein, the results presented here have shown that Tfi8 can be identified as a putative immunity protein, since repressing tfi8 expression while expressing tfe8 resulted in a higher impact on E. coli growth (Figure 4B).
5. Conclusions
The results presented here show that the speA-mTase locus in P. ogarae F113 harbors a T6SS accessory module formed by VgrG5a, TapA5a, Tfe8, and Tfi8. They also show that only a monophyletic group of pseudomonads harbor this accessory module that provides the ability to kill E. coli cells upon contact. A MATE/DinF protein has been shown to act as a putative type six effector that is probably secreted through T6SSs and acting as a pore-forming toxin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14111979/s1, Table S1: The bacterial strains and plasmids employed in this study; Table S2: Genomes employed in the GBDP phylogeny tree of Pseudomonas groups Figure 1 and Figure S1; Figure S1: GBDP-based phylogeny of 186 genomes belonging to Pseudomonas groups; Figure S2: QR code of Tfi8/Tfe8 interaction model.
Author Contributions
R.R. and M.M. conceived and designed the study, supervised research, and wrote the manuscript; D.D. designed and performed experiments and wrote the manuscript; E.B.-R. and D.G.-S. designed and performed experiments; M.R.-N. and D.V.-A. performed bioinformatic analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by Ministerio de Ciencia e Innovación Grant FEDER/EU Grant PID2021-125070OB-I00. DV was granted by the FPI-UAM program (SFPI/2021-00458).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplemental Material. Raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pukatzki, S.; Ma, A.T.; Revel, A.T.; Sturtevant, D.; Mekalanos, J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. USA 2007, 104, 15508–15513. [Google Scholar] [CrossRef] [PubMed]
- Pukatzki, S.; Ma, A.T.; Sturtevant, D.; Krastins, B.; Sarracino, D.; Nelson, W.C.; Heidelberg, J.F.; Mekalanos, J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 2006, 103, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordonez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Llamas, M.A.; Filloux, A. Type VI secretion systems in plant-associated bacteria. Environ. Microbiol. 2018, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.E.H.; Bailey, C.M.; Pallen, M.J. Type VI secretion: A beginner’s guide. Curr. Opin. Microbiol. 2008, 11, 3–8. [Google Scholar] [CrossRef]
- Boyer, F.; Fichant, G.; Berthod, J.; Vandenbrouck, Y.; Attree, I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: What can be learned from available microbial genomic resources? BMC Genom. 2009, 10, 104. [Google Scholar] [CrossRef]
- Robinson, J.B.; Telepnev, M.V.; Zudina, I.V.; Bouyer, D.; Montenieri, J.A.; Bearden, S.W.; Gage, K.L.; Agar, S.L.; Foltz, S.M.; Chauhan, S.; et al. Evaluation of a Yersinia pestis mutant impaired in a thermoregulated type VI-like secretion system in flea, macrophage and murine models. Microb. Pathog. 2009, 47, 243–251. [Google Scholar] [CrossRef][Green Version]
- Burtnick, M.N.; Brett, P.J.; Harding, S.V.; Ngugi, S.A.; Ribot, W.J.; Chantratita, N.; Scorpio, A.; Milne, T.S.; Dean, R.E.; Fritz, D.L.; et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 2011, 79, 1512–1525. [Google Scholar] [CrossRef]
- Lesic, B.; Starkey, M.; He, J.; Hazan, R.; Rahme, L.G. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 2009, 155, 2845–2855. [Google Scholar] [CrossRef]
- Liu, H.; Coulthurst, S.J.; Pritchard, L.; Hedley, P.E.; Ravensdale, M.; Humphris, S.; Burr, T.; Takle, G.; Brurberg, M.B.; Birch, P.R.; et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008, 4, e1000093. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Chung, P.C.; Shih, H.W.; Wen, S.R.; Lai, E.M. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J. Bacteriol. 2008, 190, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Singh, P.; Hsu, F.; Guvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.G.; Ho, B.T.; Yoder-Himes, D.R.; Mekalanos, J.J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2013, 110, 2623–2628. [Google Scholar] [CrossRef]
- Decoin, V.; Barbey, C.; Bergeau, D.; Latour, X.; Feuilloley, M.G.; Orange, N.; Merieau, A. A type VI secretion system is involved in Pseudomonas fluorescens bacterial competition. PLoS ONE 2014, 9, e89411. [Google Scholar] [CrossRef]
- Trunk, K.; Peltier, J.; Liu, Y.C.; Dill, B.D.; Walker, L.; Gow, N.A.R.; Stark, M.J.R.; Quinn, J.; Strahl, H.; Trost, M.; et al. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 2018, 3, 920–931. [Google Scholar] [CrossRef]
- Shalom, G.; Shaw, J.G.; Thomas, M.S. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology 2007, 153, 2689–2699. [Google Scholar] [CrossRef]
- Filloux, A.; Hachani, A.; Bleves, S. The bacterial type VI secretion machine: Yet another player for protein transport across membranes. Microbiology 2008, 154, 1570–1583. [Google Scholar] [CrossRef]
- Cascales, E. The type VI secretion toolkit. EMBO Rep. 2008, 9, 735–741. [Google Scholar] [CrossRef]
- Ma, L.S.; Hachani, A.; Lin, J.S.; Filloux, A.; Lai, E.M. Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 2014, 16, 94–104. [Google Scholar] [CrossRef]
- Unterweger, D.; Kostiuk, B.; Otjengerdes, R.; Wilton, A.; Diaz-Satizabal, L.; Pukatzki, S. Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae. EMBO J. 2015, 34, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Allsopp, L.P.; Filloux, A.; Llamas, M.A. The Pseudomonas putida T6SS is a plant warden against phytopathogens. ISME J. 2017, 11, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Wettstadt, S.; Wood, T.E.; Fecht, S.; Filloux, A. Delivery of the Pseudomonas aeruginosa Phospholipase Effectors PldA and PldB in a VgrG- and H2-T6SS-Dependent Manner. Front. Microbiol. 2019, 10, 1718. [Google Scholar] [CrossRef]
- De Maayer, P.; Venter, S.N.; Kamber, T.; Duffy, B.; Coutinho, T.A.; Smits, T.H. Comparative genomics of the Type VI secretion systems of Pantoea and Erwinia species reveals the presence of putative effector islands that may be translocated by the VgrG and Hcp proteins. BMC Genom. 2011, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, S.L.; Trunk, K.; English, G.; Fritsch, M.J.; Pourkarimi, E.; Coulthurst, S.J. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J. Bacteriol. 2011, 193, 6057–6069. [Google Scholar] [CrossRef] [PubMed]
- Cianfanelli, F.R.; Alcoforado Diniz, J.; Guo, M.; De Cesare, V.; Trost, M.; Coulthurst, S.J. VgrG and PAAR Proteins Define Distinct Versions of a Functional Type VI Secretion System. PLoS Pathog. 2016, 12, e1005735. [Google Scholar] [CrossRef]
- Alcoforado Diniz, J.; Coulthurst, S.J. Intraspecies Competition in Serratia marcescens Is Mediated by Type VI-Secreted Rhs Effectors and a Conserved Effector-Associated Accessory Protein. J. Bacteriol. 2015, 197, 2350–2360. [Google Scholar] [CrossRef]
- Silverman, J.M.; Brunet, Y.R.; Cascales, E.; Mougous, J.D. Structure and regulation of the type VI secretion system. Annu. Rev. Microbiol. 2012, 66, 453–472. [Google Scholar] [CrossRef]
- Russell, A.B.; Peterson, S.B.; Mougous, J.D. Type VI secretion system effectors: Poisons with a purpose. Nat. Rev. Microbiol. 2014, 12, 137–148. [Google Scholar] [CrossRef]
- Russell, A.B.; Singh, P.; Brittnacher, M.; Bui, N.K.; Hood, R.D.; Carl, M.A.; Agnello, D.M.; Schwarz, S.; Goodlett, D.R.; Vollmer, W.; et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe 2012, 11, 538–549. [Google Scholar] [CrossRef]
- Yang, X.; Long, M.; Shen, X. Effector(-)Immunity Pairs Provide the T6SS Nanomachine its Offensive and Defensive Capabilities. Molecules 2018, 23, 1009. [Google Scholar] [CrossRef]
- Abby, S.S.; Cury, J.; Guglielmini, J.; Neron, B.; Touchon, M.; Rocha, E.P. Identification of protein secretion systems in bacterial genomes. Sci. Rep. 2016, 6, 23080. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Egan, F.; Fargier, E.; Morrissey, J.P.; O’Gara, F. Genomic analysis of the type VI secretion systems in Pseudomonas spp.: Novel clusters and putative effectors uncovered. Microbiology 2011, 157, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Hachani, A.; Allsopp, L.P.; Oduko, Y.; Filloux, A. The VgrG proteins are “a la carte” delivery systems for bacterial type VI effectors. J. Biol. Chem. 2014, 289, 17872–17884. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, D.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Mullins, T.; Kostiuk, B.; Provenzano, D.; Pukatzki, S. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat. Commun. 2014, 5, 3549. [Google Scholar] [CrossRef]
- Allsopp, L.P.; Wood, T.E.; Howard, S.A.; Maggiorelli, F.; Nolan, L.M.; Wettstadt, S.; Filloux, A. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, 7707–7712. [Google Scholar] [CrossRef]
- Sana, T.G.; Berni, B.; Bleves, S. The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting. Front. Cell. Infect. Microbiol. 2016, 6, 61. [Google Scholar] [CrossRef]
- Silverman, J.M.; Agnello, D.M.; Zheng, H.; Andrews, B.T.; Li, M.; Catalano, C.E.; Gonen, T.; Mougous, J.D. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell 2013, 51, 584–593. [Google Scholar] [CrossRef]
- Hachani, A.; Lossi, N.S.; Filloux, A. A visual assay to monitor T6SS-mediated bacterial competition. J. Vis. Exp. JoVE 2013, 73, e50103. [Google Scholar] [CrossRef]
- Pissaridou, P.; Allsopp, L.P.; Wettstadt, S.; Howard, S.A.; Mavridou, D.A.I.; Filloux, A. The Pseudomonas aeruginosa T6SS-VgrG1b spike is topped by a PAAR protein eliciting DNA damage to bacterial competitors. Proc. Natl. Acad. Sci. USA 2018, 115, 12519–12524. [Google Scholar] [CrossRef]
- Whitney, J.C.; Quentin, D.; Sawai, S.; LeRoux, M.; Harding, B.N.; Ledvina, H.E.; Tran, B.Q.; Robinson, H.; Goo, Y.A.; Goodlett, D.R.; et al. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 2015, 163, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Kuo, T.Y.; Hsieh, F.C.; Chen, P.Y.; Wang, C.S.; Shih, Y.L.; Lai, Y.M.; Liu, J.R.; Yang, Y.L.; Shih, M.C. Involvement of type VI secretion system in secretion of iron chelator pyoverdine in Pseudomonas taiwanensis. Sci. Rep. 2016, 6, 32950. [Google Scholar] [CrossRef] [PubMed]
- Molina-Santiago, C.; Pearson, J.R.; Navarro, Y.; Berlanga-Clavero, M.V.; Caraballo-Rodriguez, A.M.; Petras, D.; Garcia-Martin, M.L.; Lamon, G.; Haberstein, B.; Cazorla, F.M.; et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun. 2019, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Duran, D.; Bernal, P.; Vazquez-Arias, D.; Blanco-Romero, E.; Garrido-Sanz, D.; Redondo-Nieto, M.; Rivilla, R.; Martin, M. Pseudomonas fluorescens F113 type VI secretion systems mediate bacterial killing and adaption to the rhizosphere microbiome. Sci. Rep. 2021, 11, 5772. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Sanz, D.; Meier-Kolthoff, J.P.; Göker, M.; Martín, M.; Rivilla, R.; Redondo-Nieto, M. Genomic and Genetic Diversity within the Pseudomonas fluorescens Complex. PLoS ONE 2016, 11, e0150183. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Pechy-Tarr, M.; Brochet, S.; Heiman, C.M.; Stojiljkovic, M.; Maurhofer, M.; Keel, C. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. ISME J. 2019, 13, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Marchi, M.; Boutin, M.; Gazengel, K.; Rispe, C.; Gauthier, J.P.; Guillerm-Erckelboudt, A.Y.; Lebreton, L.; Barret, M.; Daval, S.; Sarniguet, A. Genomic analysis of the biocontrol strain Pseudomonas fluorescens Pf29Arp with evidence of T3SS and T6SS gene expression on plant roots. Environ. Microbiol. Rep. 2013, 5, 393–403. [Google Scholar] [CrossRef]
- Tang, J.Y.; Bullen, N.P.; Ahmad, S.; Whitney, J.C. Diverse NADase effector families mediate interbacterial antagonism via the type VI secretion system. J. Biol. Chem. 2018, 293, 1504–1514. [Google Scholar] [CrossRef]
- Whitney, J.C.; Chou, S.; Russell, A.B.; Biboy, J.; Gardiner, T.E.; Ferrin, M.A.; Brittnacher, M.; Vollmer, W.; Mougous, J.D. Identification, structure, and function of a novel type VI secretion peptidoglycan glycoside hydrolase effector-immunity pair. J. Biol. Chem. 2013, 288, 26616–26624. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Redondo-Nieto, M.; Martin, M.; Rivilla, R. Comparative genomics of the Pseudomonas corrugata subgroup reveals high species diversity and allows the description of Pseudomonas ogarae sp. nov. Microb. Genom 2021, 7, 000593. [Google Scholar] [CrossRef]
- Redondo-Nieto, M.; Barret, M.; Morrisey, J.P.; Germaine, K.; Martinez-Granero, F.; Barahona, E.; Navazo, A.; Sanchez-Contreras, M.; Moynihan, J.A.; Giddens, S.R.; et al. Genome sequence of the biocontrol strain Pseudomonas fluorescens F113. J. Bacteriol. 2012, 194, 1273–1274. [Google Scholar] [CrossRef]
- Scher, F.; Baker, R. Effect of Pseudomonas putida and a Synthetic Iron Chelator on Induction of Soil Suppressiveness to Fusarium Wilt Pathogens. Phytopathology 1982, 72, 1567–1573. [Google Scholar] [CrossRef]
- Sambrook, J.R.; David, W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Staskawicz, B.; Dahlbeck, D.; Keen, N.; Napoli, C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 1987, 169, 5789–5794. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nieto, M.; Barret, M.; Morrissey, J.; Germaine, K.; Martinez-Granero, F.; Barahona, E.; Navazo, A.; Sanchez-Contreras, M.; Moynihan, J.A.; Muriel, C.; et al. Genome sequence reveals that Pseudomonas fluorescens F113 possesses a large and diverse array of systems for rhizosphere function and host interaction. BMC Genom. 2013, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Winsor, G.L.; Griffiths, E.J.; Lo, R.; Dhillon, B.K.; Shay, J.A.; Brinkman, F.S. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016, 44, D646–D653. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Bernhofer, M.; Dallago, C.; Karl, T.; Satagopam, V.; Heinzinger, M.; Littmann, M.; Olenyi, T.; Qiu, J.; Schutze, K.; Yachdav, G.; et al. PredictProtein—Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021, 49, W535–W540. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.; Richardson, C.C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 1985, 82, 1074–1078. [Google Scholar] [CrossRef]
- Wenzel, M.; Friedrich, L.; Gottfert, M.; Zehner, S. The type III-secreted protein NopE1 affects symbiosis and exhibits a calcium-dependent autocleavage activity. Mol. Plant Microbe Interact. 2010, 23, 124–129. [Google Scholar] [CrossRef]
- Gotfredsen, M.; Gerdes, K. The Escherichia coli relBE genes belong to a new toxin–antitoxin gene family. Mol. Microbiol. 1998, 29, 1065–1076. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Liang, X.; Moore, R.; Wilton, M.; Wong, M.J.; Lam, L.; Dong, T.G. Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc. Natl. Acad. Sci. USA 2015, 112, 9106–9111. [Google Scholar] [CrossRef]
- Blanco-Romero, E.; Duran, D.; Garrido-Sanz, D.; Rivilla, R.; Martin, M.; Redondo-Nieto, M. Transcriptomic analysis of Pseudomonas ogarae F113 reveals the antagonistic roles of AmrZ and FleQ during rhizosphere adaption. Microb. Genom. 2022, 8, 000750. [Google Scholar] [CrossRef] [PubMed]
- Lu, M. Structures of multidrug and toxic compound extrusion transporters and their mechanistic implications. Channels 2016, 10, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Keppel, K.; Fridman, C.M.; Bosis, E.; Salomon, D. Multiple T6SSs, Mobile Auxiliary Modules, and Effectors Revealed in a Systematic Analysis of the Vibrio parahaemolyticus Pan-Genome. mSystems 2022, 7, e0072322. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Arrebola, E.; Martinez-Granero, F.; Garcia-Mendez, S.; Muriel, C.; Blanco-Romero, E.; Martin, M.; Rivilla, R.; Redondo-Nieto, M. Classification of Isolates from the Pseudomonas fluorescens Complex into Phylogenomic Groups Based in Group-Specific Markers. Front. Microbiol. 2017, 8, 413. [Google Scholar] [CrossRef]
- Wood, T.E.; Howard, S.A.; Wettstadt, S.; Filloux, A. PAAR proteins act as the ‘sorting hat’ of the type VI secretion system. Microbiol. 2019, 165, 1203–1218. [Google Scholar] [CrossRef]
- He, X.; Szewczyk, P.; Karyakin, A.; Evin, M.; Hong, W.-X.; Zhang, Q.; Chang, G. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 2010, 467, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Mousa, J.J.; Yang, Y.; Tomkovich, S.; Shima, A.; Newsome, R.C.; Tripathi, P.; Oswald, E.; Bruner, S.D.; Jobin, C. MATE transport of the E. coli-derived genotoxin colibactin. Nat. Microbiol. 2016, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Fridman, C.M.; Bosis, E.; Salomon, D. A modular effector with a DNase domain and a marker for T6SS substrates. Nat. Commun. 2019, 10, 3595. [Google Scholar] [CrossRef]
- Hvorup, R.N.; Winnen, B.; Chang, A.B.; Jiang, Y.; Zhou, X.F.; Saier, M.H., Jr. The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) exporter superfamily. Eur. J. Biochem. 2003, 270, 799–813. [Google Scholar] [CrossRef]
- Kusakizako, T.; Miyauchi, H.; Ishitani, R.; Nureki, O. Structural biology of the multidrug and toxic compound extrusion superfamily transporters. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183154. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H.; Paulsen, I.T.; Skurray, R.A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 1999, 31, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006, 27, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lizardo, M.G.; Aragón, I.M.; Carvajal, V.; Matas, I.M.; Pérez-Bueno, M.L.; Gallegos, M.T.; Barón, M.; Ramos, C. Contribution of the non-effector members of the HrpL regulon, iaaL and matE, to the virulence of Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Microbiol. 2015, 15, 165. [Google Scholar] [CrossRef]
- Brown, W.C.; Campbell, J.L. A new cloning vector and expression strategy for genes encoding proteins toxic to Escherichia coli. Gene 1993, 127, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Schafer, A.; Tauch, A.; Jager, W.; Kalinowski, J.; Thierbach, G.; Puhler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Shanahan, J.; O’Sullivan, D.; Simpson, P.; Glennon, J.D.; O’Gara, F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 1992, 58, 353–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).