Chromosome Microarray Analysis and Exome Sequencing: Implementation in Prenatal Diagnosis of Fetuses with Digestive System Malformations
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Sample Collection
2.3. Genetic Testing Tools
2.3.1. Karyotype Analysis
2.3.2. Chromosomal Microarray Analysis
2.3.3. Exome Sequencing
2.4. Intelligence and Growth Assessment
2.5. Statistical Analysis
2.6. Clinical Follow-Ups
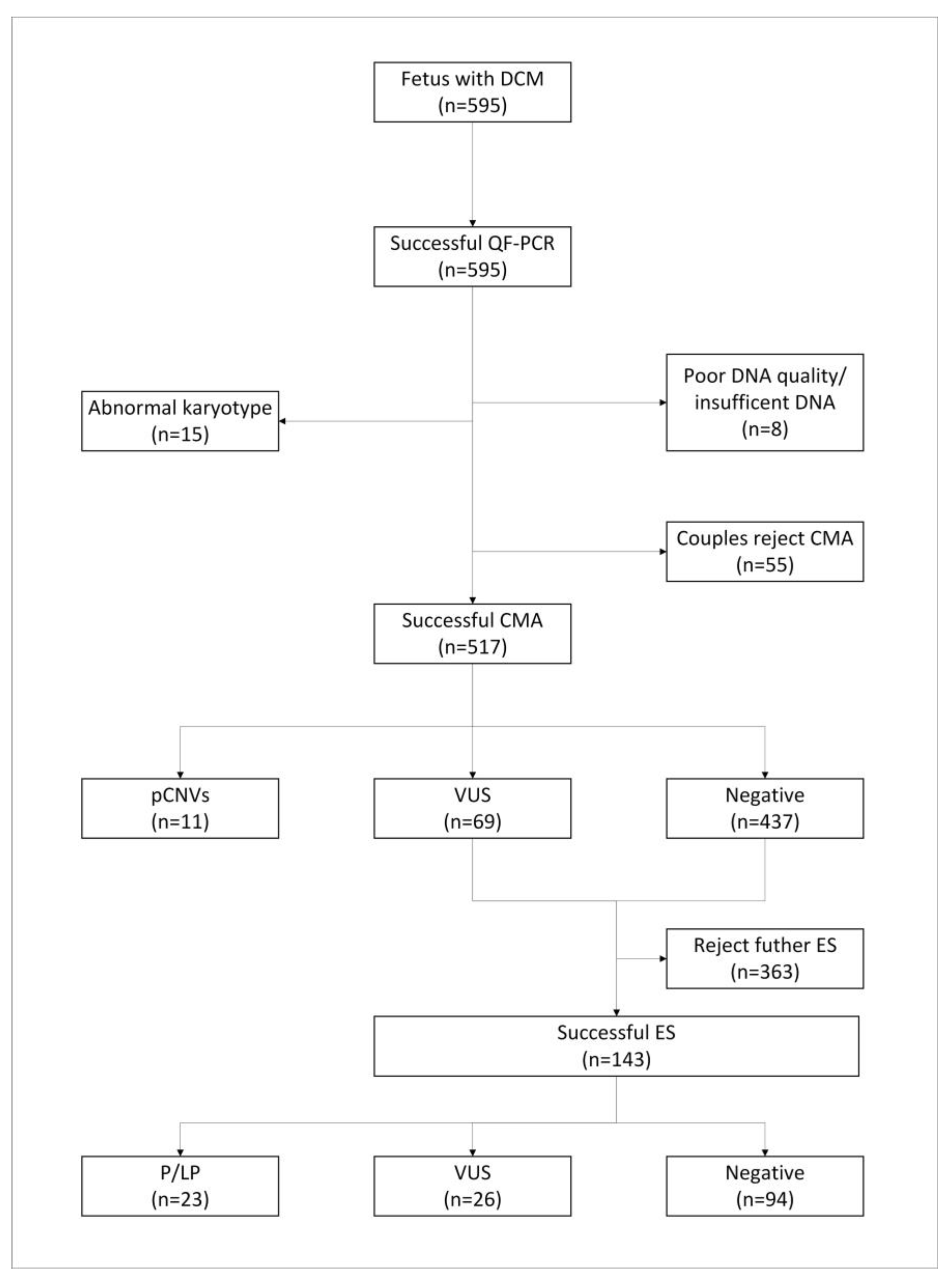
3. Results
3.1. Cohort Characteristics
3.2. Karyotype Analysis and CMA Results
3.3. Exome Sequencing Results
3.3.1. Positive Diagnostic Variations
3.3.2. Diagnostic Rate of DSMs in Different Subgroups
3.3.3. Variants of Unknown Significance
3.4. Pregnancy Outcomes
3.5. Results of Growth and Intelligence Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrera, J.M.; Torrents, M.; Mortera, C.; Cusí, V.; Muñoz, A. Routine Prenatal Ultrasound Screening for Fetal Abnormalities: 22 Years’ Experience. Ultrasound Obstet. Gynecol. 1995, 5, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Orgul, G.; Soyer, T.; Yurdakok, M.; Beksac, M.S. Evaluation of Pre- and Postnatally Diagnosed Gastrointestinal Tract Obstructions. J. Matern. Fetal Neonatal Med. 2019, 32, 3215–3220. [Google Scholar] [CrossRef] [PubMed]
- Roorda, D.; Königs, M.; Schattenkerk, L.E.; van der Steeg, L.; van Heurn, E.; Oosterlaan, J. Neurodevelopmental Outcome of Patients with Congenital Gastrointestinal Malformations: A Systematic Review and Meta-Analysis. Arch. Dis. Child.-Fetal Neonatal Ed. 2021, 106, 635–642. [Google Scholar] [CrossRef]
- Wu, J.; Lu, A.D.; Zhang, L.P.; Zuo, Y.X.; Jia, Y.P. Study of Clinical Outcome and Prognosis in Pediatric Core Binding Factor-Acute Myeloid Leukemia. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 52–57. [Google Scholar]
- Escobar, M.A.; Ladd, A.P.; Grosfeld, J.L.; West, K.W.; Rescorla, F.J.; Scherer, L.R., III; Engum, S.A.; Rouse, T.M.; Billmire, D.F. Duodenal Atresia and Stenosis: Long-Term Follow-up over 30 Years. J. Pediatr. Surg. 2004, 39, 867–871; discussion 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bethell, G.S.; Long, A.M.; Knight, M.; Hall, N.J. Congenital Duodenal Obstruction in the UK: A Population-Based Study. Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 105, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Isenburg, J.L.; Salemi, J.L.; Mai, C.T.; Liberman, R.F.; Canfield, M.A.; Copeland, G.; Haight, S.; Harpavat, S.; Hoyt, A.T.; et al. Population-Based Birth Defects Data in the United States, 2010–2014: A Focus on Gastrointestinal Defects. Birth Defects Res. 2017, 109, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed Differentiation of Human Pluripotent Stem Cells into Intestinal Tissue In Vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- Khanna, K.; Sharma, S.; Pabalan, N.; Singh, N.; Gupta, D.K. A Review of Genetic Factors Contributing to the Etiopathogenesis of Anorectal Malformations. Pediatr. Surg. Int. 2018, 34, 9–20. [Google Scholar] [CrossRef]
- Beke, A.; Papp, C.; Tóth-Pál, E.; Mezei, G.; Joó, J.G.; Csaba, A.; Papp, Z. Trisomies and Other Chromosome Abnormalities Detected after Positive Sonographic Findings. J. Reprod. Med. 2005, 50, 675–691. [Google Scholar]
- Hanna, J.S.; Neu, R.L.; Lockwood, D.H. Prenatal Cytogenetic Results from Cases Referred for 44 Different Types of Abnormal Ultrasound Findings. Prenat. Diagn. 1996, 16, 109–115. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Snijders, R.J.; Gosden, C.M.; Berry, C.; Campbell, S. Ultrasonographically Detectable Markers of Fetal Chromosomal Abnormalities. Lancet 1992, 340, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Hillman, S.C.; McMullan, D.J.; Hall, G.; Togneri, F.S.; James, N.; Maher, E.J.; Meller, C.H.; Williams, D.; Wapner, R.J.; Maher, E.R.; et al. Use of Prenatal Chromosomal Microarray: Prospective Cohort Study and Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2013, 41, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Fu, F.; Li, R.; Xie, G.E.; Zhang, Y.L.; Li, J.; Li, D.Z. Implementation of High-Resolution Snp Arrays in the Investigation of Fetuses with Ultrasound Malformations: 5 Years of Clinical Experience. Clin. Genet. 2014, 86, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Best, S.; Wou, K.; Vora, N.; Van der Veyver, I.B.; Wapner, R.; Chitty, L.S. Promises, Pitfalls and Practicalities of Prenatal Whole Exome Sequencing. Prenat. Diagn. 2018, 38, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Westerink, J.; Visseren, F.L.; Spiering, W. Diagnostic Clinical Genome and Exome Sequencing. N. Engl. J. Med. 2014, 371, 1169. [Google Scholar]
- Yang, Y.; Muzny, D.M.; Reid, J.G.; Bainbridge, M.N.; Willis, A.; Ward, P.A.; Braxton, A.; Beuten, J.; Xia, F.; Niu, Z.; et al. Clinical Whole-Exome Sequencing for the Diagnosis of Mendelian Disorders. N. Engl. J. Med. 2013, 369, 1502–1511. [Google Scholar] [CrossRef]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K.; et al. Prenatal Exome Sequencing Analysis in Fetal Structural Anomalies Detected by Ultrasonography (Page): A Cohort Study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Li, R.; Yu, Q.; Wang, D.; Deng, Q.; Li, L.; Lei, T.; Chen, G.; Nie, Z.; Yang, X.; et al. Application of Exome Sequencing for Prenatal Diagnosis of Fetal Structural Anomalies: Clinical Experience and Lessons Learned from a Cohort of 1618 Fetuses. Genome Med. 2022, 14, 123. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-Exome Sequencing in the Evaluation of Fetal Structural Anomalies: A Prospective Cohort Study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Yap, F.; Lee, Y.S.; Aw, M.M.H. Growth Assessment and Monitoring during Childhood. Ann. Acad. Med. Singap. 2018, 47, 149–155. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Leung, J.; Caviness, A.C.; Chung, W.; Flores, M.; Blum, P.; Bialek, S.R.; Miller, J.A.; Vinson, S.S.; Turcich, M.R.; et al. Long-Term Outcomes of Children with Symptomatic Congenital Cytomegalovirus Disease. J. Perinatol. 2017, 37, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Tann, C.J.; Webb, E.L.; Lassman, R.; Ssekyewa, J.; Sewegaba, M.; Musoke, M.; Burgoine, K.; Hagmann, C.; Deane-Bowers, E.; Norman, K.; et al. Early Childhood Outcomes after Neonatal Encephalopathy in Uganda: A Cohort Study. eClinicalMedicine 2018, 6, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, F.; Lei, T.; Zhen, L.; Deng, Q.; Zhou, H.; Ma, C.; Cheng, K.; Huang, R.; Li, R.; et al. Genetic Diagnosis of Fetal Microcephaly at a Single Tertiary Center in China. Front. Genet. 2023, 14, 1112153. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Bowen, J.; Gibson, F. Risk Factors for Adverse Neurodevelopment in Extremely Low Birth Weight Infants with Normal Neonatal Cranial Ultrasound. J. Perinatol. 2005, 25, 210–215. [Google Scholar] [CrossRef][Green Version]
- Hu, T.; Tian, T.; Zhang, Z.; Wang, J.; Hu, R.; Xiao, L.; Zhu, H.; Lai, Y.; Wang, H.; Liu, S. Prenatal Chromosomal Microarray Analysis in 2466 fetuses with Ultrasonographic Soft Markers: A prospective Cohort Study. Am. J. Obstet. Gynecol. 2021, 224, e1–e16. [Google Scholar] [CrossRef]
- Yi, J.L.; Zhang, W.; Meng, D.H.; Ren, L.J.; Yu, J.; Wei, Y.L. Epidemiology of Fetal Cerebral Ventriculomegaly and Evaluation of Chromosomal Microarray Analysis Versus Karyotyping for Prenatal Diagnosis in a Chinese Hospital. J. Int. Med. Res. 2019, 47, 5508–5517. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, T.; Fu, F.; Deng, Q.; Li, R.; Wang, D.; Yang, X.; Li, D.; Liao, C. Microarray Analysis in Fetuses with Duodenal Obstruction: It Is Not Just Trisomy 21. Prenat. Diagn. 2021, 41, 316–322. [Google Scholar] [CrossRef]
- Retterer, K.; Juusola, J.; Cho, M.T.; Vitazka, P.; Millan, F.; Gibellini, F.; Vertino-Bell, A.; Smaoui, N.; Neidich, J.; Monaghan, K.G.; et al. Clinical Application of Whole-Exome Sequencing across Clinical Indications. Genet. Med. 2016, 18, 696–704. [Google Scholar] [CrossRef]
- Posey, J.E.; Rosenfeld, J.A.; James, R.A.; Bainbridge, M.; Niu, Z.; Wang, X.; Dhar, S.; Wiszniewski, W.; Akdemir, Z.H.; Gambin, T.; et al. Molecular Diagnostic Experience of Whole-Exome Sequencing in Adult Patients. Genet. Med. 2016, 18, 678–685. [Google Scholar] [CrossRef]
- Fu, F.; Li, R.; Li, Y.; Nie, Z.Q.; Lei, T.; Wang, D.; Yang, X.; Han, J.; Pan, M.; Zhen, L.; et al. Whole Exome Sequencing as a Diagnostic Adjunct to Clinical Testing in Fetuses with Structural Abnormalities. Ultrasound Obstet. Gynecol. 2018, 51, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Brantberg, A.; Blaas, H.G.; Salvesen, K.A.; Haugen, S.E.; Møllerløkken, G.; Eik-Nes, S.H. Fetal Duodenal Obstructions: Increased Risk of Prenatal Sudden Death. Ultrasound Obstet. Gynecol. 2002, 20, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Martin, C.L.; Levy, B.; Ballif, B.C.; Eng, C.M.; Zachary, J.M.; Savage, M.; Platt, L.D.; Saltzman, D.; Grobman, W.A.; et al. Chromosomal Microarray Versus Karyotyping for Prenatal Diagnosis. N. Engl. J. Med. 2012, 367, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Zhou, C.; Wang, L.; Xie, H.; Xiao, Y.; Zhu, H.; Hu, T.; Zhang, Z.; Zhu, Q.; et al. Prospective Chromosome Analysis of 3429 Amniocentesis Samples in China Using Copy Number Variation Sequencing. Am. J. Obstet. Gynecol. 2018, 219, e1–e18. [Google Scholar] [CrossRef]
- Chau, M.H.K.; Cao, Y.; Kwok, Y.K.Y.; Chan, S.; Chan, Y.M.; Wang, H.; Yang, Z.; Wong, H.K.; Leung, T.Y.; Choy, K.W. Characteristics and Mode of Inheritance of Pathogenic Copy Number Variants in Prenatal Diagnosis. Am. J. Obstet. Gynecol. 2019, 221, e1–e11. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Yin, D.; Zeng, Y.; Tang, F.; Wang, J. Influence of the Detection of Parent-of-Origin on the Pregnancy Outcomes of Fetuses with Copy Number Variation of Unknown Significance. Sci. Rep. 2020, 10, 8864. [Google Scholar] [CrossRef] [PubMed]
- Hillman, S.C.; Pretlove, S.; Coomarasamy, A.; McMullan, D.J.; Davison, E.V.; Maher, E.R.; Kilby, M.D. Additional Information from Array Comparative Genomic Hybridization Technology over Conventional Karyotyping in Prenatal Diagnosis: A Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2011, 37, 6–14. [Google Scholar] [CrossRef]
- Shi, P.; Liang, H.; Hou, Y.; Chen, D.; Ren, H.; Wang, C.; Xia, Y.; Zhang, D.; Leigh, D.; Cram, D.S.; et al. The Uncertainty of Copy Number Variants: Pregnancy Decisions and Clinical Follow Up. Am. J. Obstet. Gynecol. 2023, 229, e1–e8. [Google Scholar] [CrossRef]
- Mardy, A.H.; Wiita, A.P.; Wayman, B.V.; Drexler, K.; Sparks, T.N.; Norton, M.E. Variants of Uncertain Significance in Prenatal Microarrays: A Retrospective Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 431–438. [Google Scholar] [CrossRef]
- Shi, P.; Li, R.; Wang, C.; Kong, X. Influence of Validating the Parental Origin on the Clinical Interpretation of Fetal Copy Number Variations in 141 Core Family Cases. Mol. Genet. Genom. Med. 2019, 7, e00944. [Google Scholar] [CrossRef]
- Houfflin-Debarge, V.; Bigot, J. Ultrasound and MRI Prenatal Diagnosis of Esophageal Atresia: Effect on Management. J. Pediatr. Gastroenterol. Nutr. 2011, 52 (Suppl. S1), S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation Plink: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, s13742-015-0047-8. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One Fastq Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chan, A.P. Provean Web Server: A Tool to Predict the Functional Effect of Amino Acid Substitutions and Indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [PubMed]
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An Online Bioinformatics Tool to Predict Splicing Signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
- Ghosh, R.; Harrison, S.M.; Rehm, H.L.; Plon, S.E.; Biesecker, L.G. Updated Recommendation for the Benign Stand-Alone Acmg/Amp Criterion. Hum. Mutat. 2018, 39, 1525–1530. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. Revel: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Jaganathan, K.; Panagiotopoulou, S.K.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Kalia, S.S.; Adelman, K.; Bale, S.J.; Chung, W.K.; Eng, C.; Evans, J.P.; Herman, G.E.; Hufnagel, S.B.; Klein, T.E.; Korf, B.R.; et al. Recommendations for Reporting of Secondary Findings in Clinical Exome and Genome Sequencing, 2016 Update (Acmg Sf V2.0): A Policy Statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017, 19, 249–255. [Google Scholar] [CrossRef]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A General Framework for Estimating the Relative Pathogenicity of Human Genetic Variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Manichaikul, A.; Mychaleckyj, J.C.; Rich, S.S.; Daly, K.; Sale, M.; Chen, W.M. Robust Relationship Inference in Genome-Wide Association Studies. Bioinformatics 2010, 26, 2867–2873. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A Mapreduce Framework for Analyzing Next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J.; et al. Acmg Sf V3.0 List for Reporting of Secondary Findings in Clinical Exome and Genome Sequencing: A Policy Statement of the American College of Medical Genetics and Genomics (Acmg). Genet. Med. 2021, 23, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Lin, X.; Salzberg, S.L. Genesplicer: A New Computational Method for Splice Site Prediction. Nucleic Acids Res. 2001, 29, 1185–1190. [Google Scholar] [CrossRef]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved Splice Site Detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef]
- Reva, B.; Antipin, Y.; Sander, C. Predicting the Functional Impact of Protein Mutations: Application to Cancer Genomics. Nucleic Acids Res. 2011, 39, e118. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. Mutationtaster2: Mutation Prediction for the Deep-Sequencing Age. Nat Methods 2014, 11, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. Sift Web Server: Predicting Effects of Amino Acid Substitutions on Proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Annovar: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.; Burge, C.B. Maximum Entropy Modeling of Short Sequence Motifs with Applications to RNA Splicing Signals. J. Comput. Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Subgroup Genome Project Data Processing. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
| Patient ID | MA (Years) | GA (Weeks) | Ultrasound Findings | CMA Findings | Type of CNV | Size (Mb) | Interpretation | Outcomes |
|---|---|---|---|---|---|---|---|---|
| P8245 | 25.5 | 35.1 | Upper digestive obstruction | arr17q12(34822465–36410559) × 3 | Duplication | 1.59 | P | TOP |
| A33770 | 32.4 | 25.3 | DO | arr15q25.2(83198318–84834123) × 1 | Deletion | 1.69 | P | TOP |
| A32309 | 34.0 | 25.9 | DO, polyhydramnios | arr17q12(34822,466–36397279) × 3 | Duplication | 1.57 | P | TOP |
| A29186 | 29.0 | 30.9 | DO | arr7q11.23(72718277–74143060) × 1 | Deletion | 1.42 | P | TOP |
| A26285 | 28.8 | 26.4 | DO | arr13q21.33q31.1(70986515–80427919) × 1 | Deletion | 9.44 | P | TOP |
| A12873 | 27.0 | 23.9 | DO | arr13q21.2q31.1(60952932–84894325) × 1 | Deletion | 23.94 | P | TOP |
| A30328 | 19.3 | 18.7 | Microgastria, polyhydramnios, omphalocele | arr16p13.11p12.3(15375912–18778064) × 1 | Deletion | 3.40 | P | Neonatal death |
| A29609 | 35.5 | 26.3 | gallbladder atresia, polycystic kidney | arr17q12(34822465–36418529) × 1 | Deletion | 1.60 | P | TOP |
| R4 | 28.4 | 39.0 | Peritoneal effusion | arrXq28(154952431–155233098) × 3 | Duplication | 2.52 | P | TOP |
| arrYp11.31q11.23(2650424–28799654) × 2 | 26.15 | |||||||
| A34806 | 30.9 | 28.0 | Intestinal echo enhancement, FGR | arrXp22.31(6455152–8135568) × 1 | Deletion | 1.68 | P | TOP |
| A31334 | 25.3 | 27.1 | Intestinal echo enhancement | arr(X) × 2, (Y) × 1 | Duplication | 155.07 | P | Live birth |
| Case ID | Patient ID | Digestive System Abnormality | Other Ultrasound Abnormalities | Gene | Reference Sequence | Nucleotide/Protein Change | Whether gnomAD and In-House Databases Were Documented | Inheritance and ACMG Classification | Disorders (OMIM IDs) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P8932 | Ascites | Cerebellar vermis missing, polydactyly deformity | IFT74 | NM_025103.4 | c.535C > G (p. Gln179Glu) | Yes (0.001406 *)/yes (0.003 ♯) | Het, Pat, AR, P (PVS1 + PS2 + P M2) | Joubert syndrome 40 (619582), Bardet–Biedl syndrome 22 (617119) | Live birth |
| c.909dup (p. Glu304ArgfsTer7) * | Yes (0.00005674 *)/no | Het, Mat, AR, P (PVS1 + PS2 + P M2) | ||||||||
| 2 | A35570 | Hepatomegaly | Polyhydramnios, Short femur | COL2A1 | NM_001844.5 | c.1835G > T (p.Gly612Val) * | No/no | Het, De novo, AD, LP (PVS1 + PM2) | Spondyloepiphyseal dysplasia congenita (183900) | TOP |
| 3 | A35151 | Non-visualization of the gallbladder | Short femur | SHOX | NM_000451.4 | Loss of exons 2–6 (copy number, X1) * | No/no | Het, De novo, AD, P (PVS1 + PS2 + PM2) | Leri–Weill dyschondrosteosis (127300) | Live birth |
| 4 | A33669 | Microgastria | Bilateral foot varus, duplication of kidney, polyhydramnios | SMPD4 | NM_017951.5 | c.1867del (p. Leu623TrpfsTer130) * | No/no | Het, Mat, AR, P (PVS1 + PM2 + PP4) | Neurodevelopmental disorder with microcephaly, arthrogryposis, and structural brain anomalies (618622) | TOP |
| c.2074G > T (p. Glu692Ter) | No/no | Het, Pat, AR, LP (PVS1 + PM2) | ||||||||
| 5 | A34905 | Duodenal obstruction | Omphalocele | PTPN11 | NM_002834.5 | c.867G > T (p. Arg289Ser) | Yes (0.000003978 *)/no | Het, De novo, AD, LP (PVS1 + PM2) | Noonan syndrome 1(163950)/LEOPARD syndrome 1 (151100) | Live birth |
| 6 | A34170 | Microgastria | Polyhydramnios | EFTUD2 | NM_004247.4 | c.623_624del (p. His208ArgfsTer5) * | No/no | Het, De novo, AD, P (PP1_very strong + PM1 + PP3) | Mandibulofacial dysostosis, Guion–Almeida type (610536) | Live birth |
| 7 | P9429 | Intestinal dilatation | Microcephaly | 17p13.3 | - | 3.51 Mb deletion | No/no | De novo, AD, P (PVS1 + PM2 + PP4) | Miller–Dieker lissencephaly syndrome (247200) | TOP |
| 8 | A32697 | Duodenal obstruction | Bilateral lateral ventricle widened | 17q12 | - | 1.49 Mb microduplication | No/no | Mat, AD, P (PVS1 + PM2 + PP4) | Chromosome 17q12 duplication syndrome (614526) | TOP |
| 9 | C7699 | Ascites | Abnormal foot posture, cardiovascular malformations | CPLANE1 | NM_023073.3 | c.1270C > T (p. Arg424Ter) | Yes (0.0001757 *)/no | Het, Pat, AR, P (PVS1 + PS2 + PM2) | Orofaciodigital syndrome VI (277170) Joubert syndrome 17 (614615) | TOP |
| c.4034A > G (p. Gln1345Arg) | No/no | Het, Mat, AR, LP (PVS1 + PM2) | ||||||||
| 10 | P9381 | Abdominal mass | Hydronephrosis | OTOF | NM_194248.2 | c.897 + 1G > T | Yes (0.00004619 *)/no | Het, Pat, AR, LP (PVS1 + PM2) | Deafness, autosomal recessive 9 (601071) | Live birth |
| c.4023 + 1G > A | Yes (0.01178 *)/yes (0.0089 ♯) | Het, Mat, AR, VUS (PM1 + PP3) | ||||||||
| 11 | NG1131 | Microgastria, esophagotracheal fistula | Hydronephrosis, FGR | NIPBL | NM_133433.3 | c.6983C > A (p. Thr2328Lys) * | No/no | Het, De novo, AD, LP (PS2 + PM1 + PM2 + PP3) | Cornelia de Lange syndrome 1 (122470) | TOP |
| 12 | P9325 | Ascites | No amniotic fluid | NPC1 | NM_000271.4 | c.2526T > A (p. Phe842 Leu) * | No/no | Het, Mat, AR, VUS (PVS1 + PM2_supportin) | Niemann–Pick disease, type C1 (257220) | TOP |
| c.1226T > C (p. Ile409Thr) | No/no | Het, De novo, AR, LP (PVS1 + PM2) | ||||||||
| 13 | C7430 | Ascites | NT thickening, cardiovascular malformations | FLNA | NM_001110556.1 | c.1538_ 1539delGGinsTA (p. Gly513Val) * | No/no | Hemi, Mat, XLD/XLR, LP (PVS1 + PM2) | Cardiac valvular dysplasia, X-linked (314400), congenital short bowel syndrome (300048) | TOP |
| 14 | A31257 | Ascites | - | HRAS | NM_005343.4 | c.38G > A (p. Gly13Asp) | No/no | Het, De novo, AD, P (PVS1 + PS2 + PM2) | Costello syndrome (218040), thyroid cancer, nonmedullary, 2 (188470) | TOP |
| 15 | P9203 | Abnormality of the intrahepatic bile duct | Cardiovascular malformations, polycystic kidney, hyperechogenic kidney, no amniotic fluid | PKHD1 | NM_138694.3 | c.107C > T (p. Thr36Met) | Yes (0.0005094 *)/yes (0.001 ♯) | Het, Mat, AR, P (PVS1 + PS2 + PM2) | Polycystic kidney disease 4, with or without hepatic disease (263200) | TOP |
| c.5869G > A (p. Asp1957Asn) | Yes (0.00005438 *)/ yes (0.001 ♯) | Het, Pat, AR, LP (PVS1 + PM2) | ||||||||
| 16 | W6488 | Ascites | Pleural effusion, hydrops fetalis | PTPN11 | NM_002834.5 | c.1507G > A (p. Gly503Arg) | No/no | Het, De novo, AD, P (PS2 + PM1 + P M2 + PP3) | Noonan syndrome 1(163950)/ LEOPARD syndrome 1 (151100) | TOP |
| 17 | NG800 | Ascites | FGR, hydrops fetalis, oligohydramnios, VSD | KRAS | NM_033360.4 | c.101C > T (p. Pro34Leu) | No/no | Het, De novo, AD, P (PVS1 + PS2 + PM2) | Noonan syndrome 3 (609942), cardiofaciocutaneous syndrome 2 (615278) | Live birth |
| 18 | C6196 | Hepatomegaly | Omphalocele, polyhydramnios, short long bone | CDKN1C | NM_000076.2 | c.827_ 828delinsAA (p. F276X) * | No/no | Het, Mat, AD, LP (PM2 + PM3 + PM4) | Beckwith–Wiedemann syndrome (130650), IMAGE syndrome (614732) | Live birth |
| 19 | A28929 | Hepatomegaly | Omphalocele, urachal cyst, hydrocephalus, hyperechogenic kidneys | CRB2 | NM_173689.6 | c.3548T > A (p. L1183X) * | No/no | Het, Pat, AR, P (PVS1 + PM2 + PP4) | Ventriculomegaly with cystic kidney disease (219730), focal segmental glomerulosclerosis-9 (616220) | Live birth |
| c.3307T > C (p. C1103R) * | No/no | Het, Mat, AR, LP (PM2 + PM3 + PM4) | ||||||||
| 20 | P8465 | Ascites | Multicystic kidney dysplasia | BBS2 | NM_031885 | c.534 + 1G > T | No/no | Het, Mat, AR, LP (PVS1 + PM2) | Bardet–Biedl syndrome-2 (615981) | Neonatal death |
| c.2107C > T (p.R703X) | Yes (0.0003 *)/yes (0.001 ♯) | Het, Pat, AR, P (PS2 + PM1 + PM2 + PP3) | ||||||||
| 21 | A25666 | Gastrointestinal obstruction | Situs inversus totalis, dextrocardia, abnormal vena cava morphology, ventriculomegaly | DNAH11 | NM_001277115.1 | c.11392G > T (p. Glu3798Ter) * | No/no | Het, Pat, AR, LP (PS2 + PM1 + PM2 + PP3) | Ciliary dyskinesia, primary, 7, with or without situs inversus (611884) | TOP |
| c.11374-18A > G * | No/no | Het, Mat, AR, VUS (PVS1 + PM2_supporting) | ||||||||
| 22 | A23948 | Ascites, hepatomegaly | - | PYGL | NM_002863 | c.772 + 1G > A | No/no | Hom, Mat, AR, LP (PVS1 + PM2) | Glycogen storage disease VI (232700) | Live birth |
| 23 | A33772 | Ascites, abnormal intestine morphology | Ureteropelvic junction obstruction, cloacal abnormality, renal duplication | 22q11.2 | - | 2.85 Mb deletion | No/no | De novo, AD, P (PS2 + PS3 + PM2 + PP3) | Chromosome 22q11.2 deletion syndrome (611867) | TOP |
| DSM Classification | Isolated DSM | Nonisolated DSM | Total | |||
|---|---|---|---|---|---|---|
| N | Diagnostic Rate (%) | N | Diagnostic Rate (%) | N | Diagnostic Rate (%) | |
| Abnormal duodenum morphology | 15 | 0 (0) | 26 | 2 (7.7) | 41 | 2 (4.9) |
| Absent gallbladder | 6 | 0 (0) | 8 | 1 (12.5) | 14 | 1 (7.1) |
| Ascites | 6 | 1 (16.7) | 26 | 9 (34.6) | 32 | 10 (31.25) |
| Hepatomegaly | 5 | 1 (20.0) | 13 | 3 (23.1) | 18 | 4 (22.2) |
| Abnormal stomach bubble | 5 | 0 (0) | 11 | 3 (27.3) | 16 | 3 (18.75) |
| Abdominal mass | 1 | 0 (0) | 3 | 1 (33.33) | 4 | 1 (25.0) |
| Others | 8 | 0 (0) | 10 | 2 (20) | 18 | 2 (11.1) |
| Total | 46 | 2 (4.3) | 97 | 21 (21.6) | 143 | 23 (16.1) |
| Groups | CMA | ES | ||||
|---|---|---|---|---|---|---|
| P/LP | VUS | Live Birth | P/LP | VUS | Live Birth | |
| Isolated | 7 (1.4%) | 23 (4.7%) | 354 (72.0%) | 2 (4.3%) | 1 (2.2%) | 35 (76.1%) |
| Nonisolated | 4 (3.9%) | 46 (44.7%) | 37 (35.9%) | 21 (21.6%) | 26 (26.8%) | 47 (48.5%) |
| p-value | 0.105 | 0.000 | 0.000 | 0.007 | 0.000 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, L.; Fu, F.; Li, R.; Lei, T.; Huang, R.; Li, D.; Liao, C. Chromosome Microarray Analysis and Exome Sequencing: Implementation in Prenatal Diagnosis of Fetuses with Digestive System Malformations. Genes 2023, 14, 1872. https://doi.org/10.3390/genes14101872
Wang Y, Liu L, Fu F, Li R, Lei T, Huang R, Li D, Liao C. Chromosome Microarray Analysis and Exome Sequencing: Implementation in Prenatal Diagnosis of Fetuses with Digestive System Malformations. Genes. 2023; 14(10):1872. https://doi.org/10.3390/genes14101872
Chicago/Turabian StyleWang, You, Liyuan Liu, Fang Fu, Ru Li, Tingying Lei, Ruibin Huang, Dongzhi Li, and Can Liao. 2023. "Chromosome Microarray Analysis and Exome Sequencing: Implementation in Prenatal Diagnosis of Fetuses with Digestive System Malformations" Genes 14, no. 10: 1872. https://doi.org/10.3390/genes14101872
APA StyleWang, Y., Liu, L., Fu, F., Li, R., Lei, T., Huang, R., Li, D., & Liao, C. (2023). Chromosome Microarray Analysis and Exome Sequencing: Implementation in Prenatal Diagnosis of Fetuses with Digestive System Malformations. Genes, 14(10), 1872. https://doi.org/10.3390/genes14101872






