Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
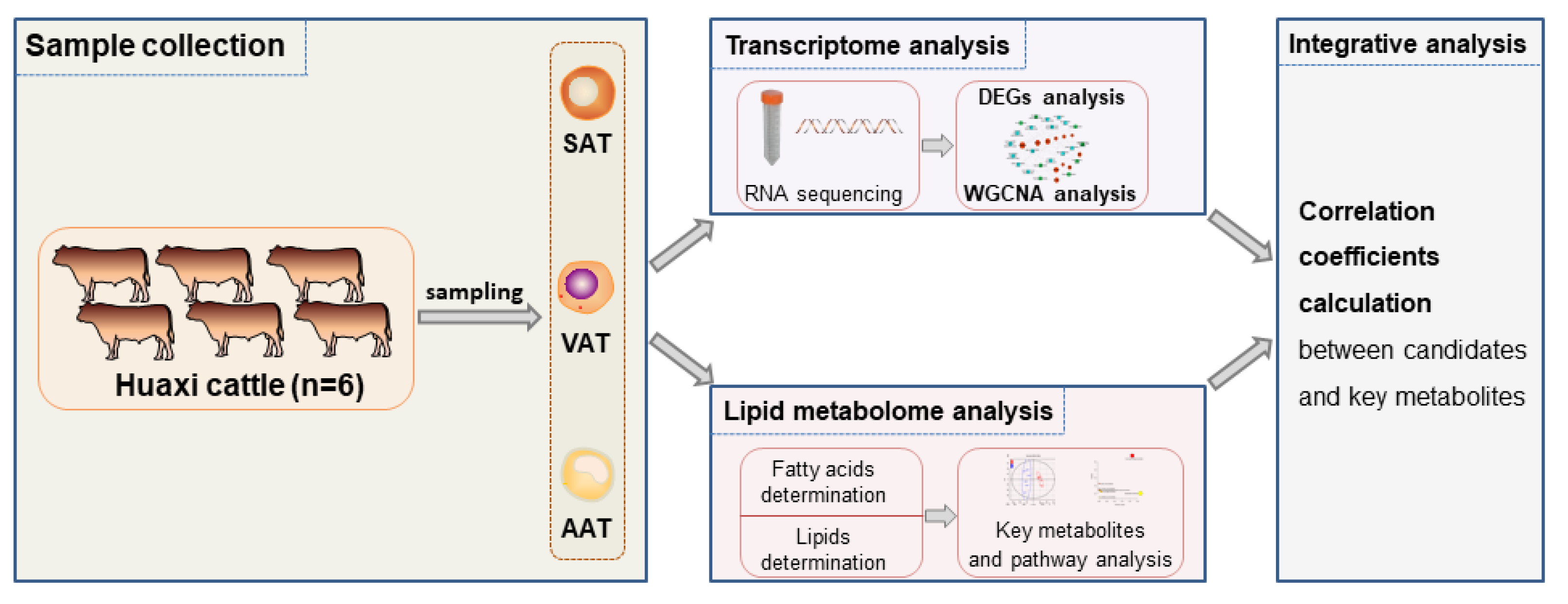
2.2. Animals and Sampling
2.3. Transcriptomic Analysis
2.4. Functional Enrichment Analysis
2.5. Metabolite Content Determination
2.5.1. Fatty Acid Species and Content Determination
2.5.2. Lipid Species and Content Determination
2.6. Lipidomics Raw Data Preprocessing
2.7. Lipid Metabolomic Profiling Analysis
2.8. Transcriptomic and Lipid Metabolomic Integration Analysis
3. Results
3.1. The Evaluation of Sequencing Data
3.2. Principal Component Analysis among Three Groups
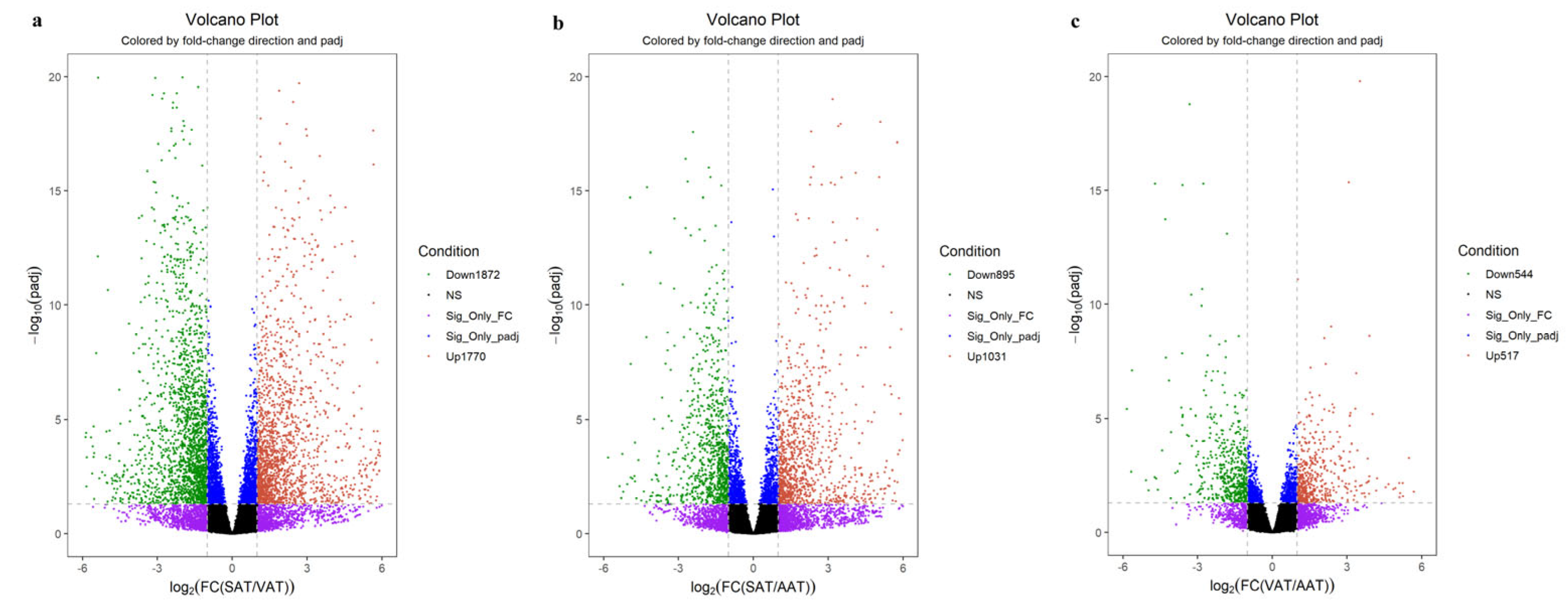
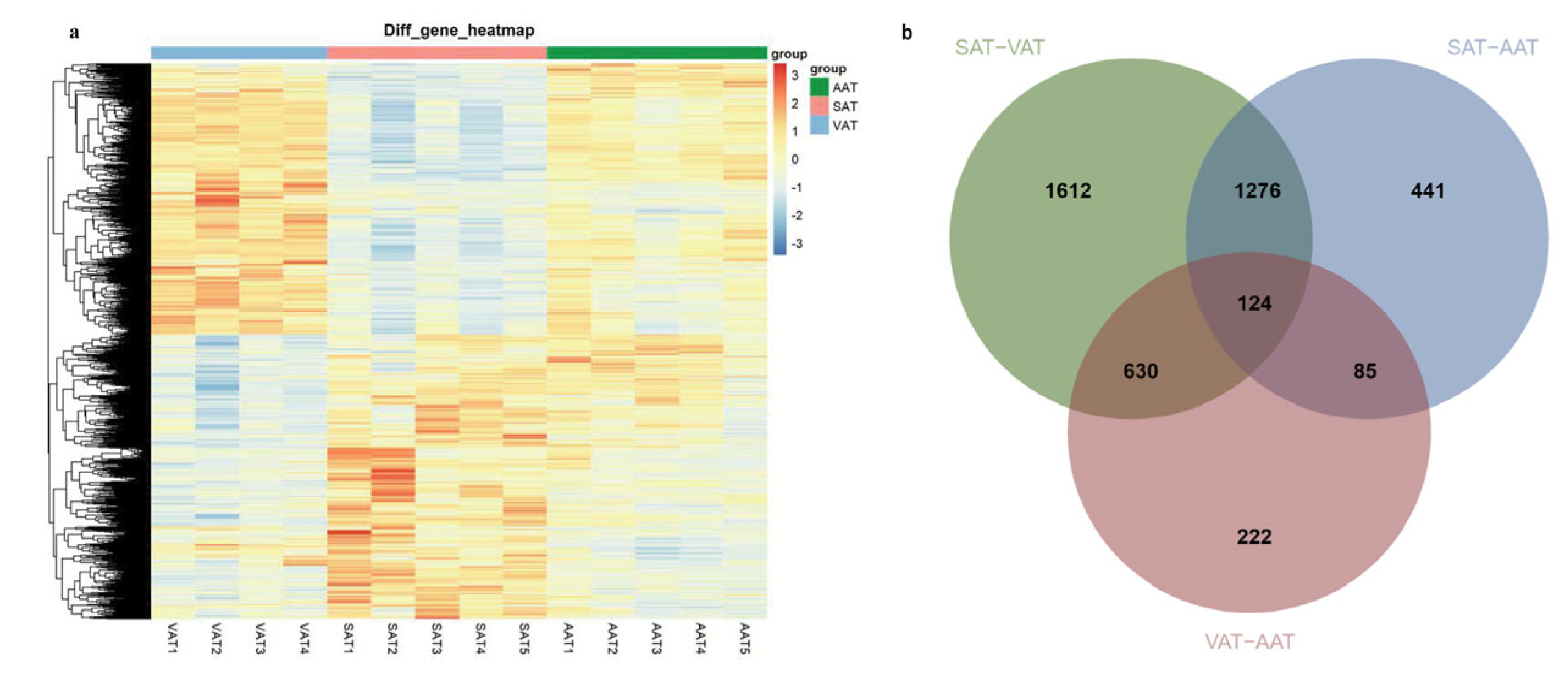
3.3. Identification of Differentially Expressed Genes among Three Groups
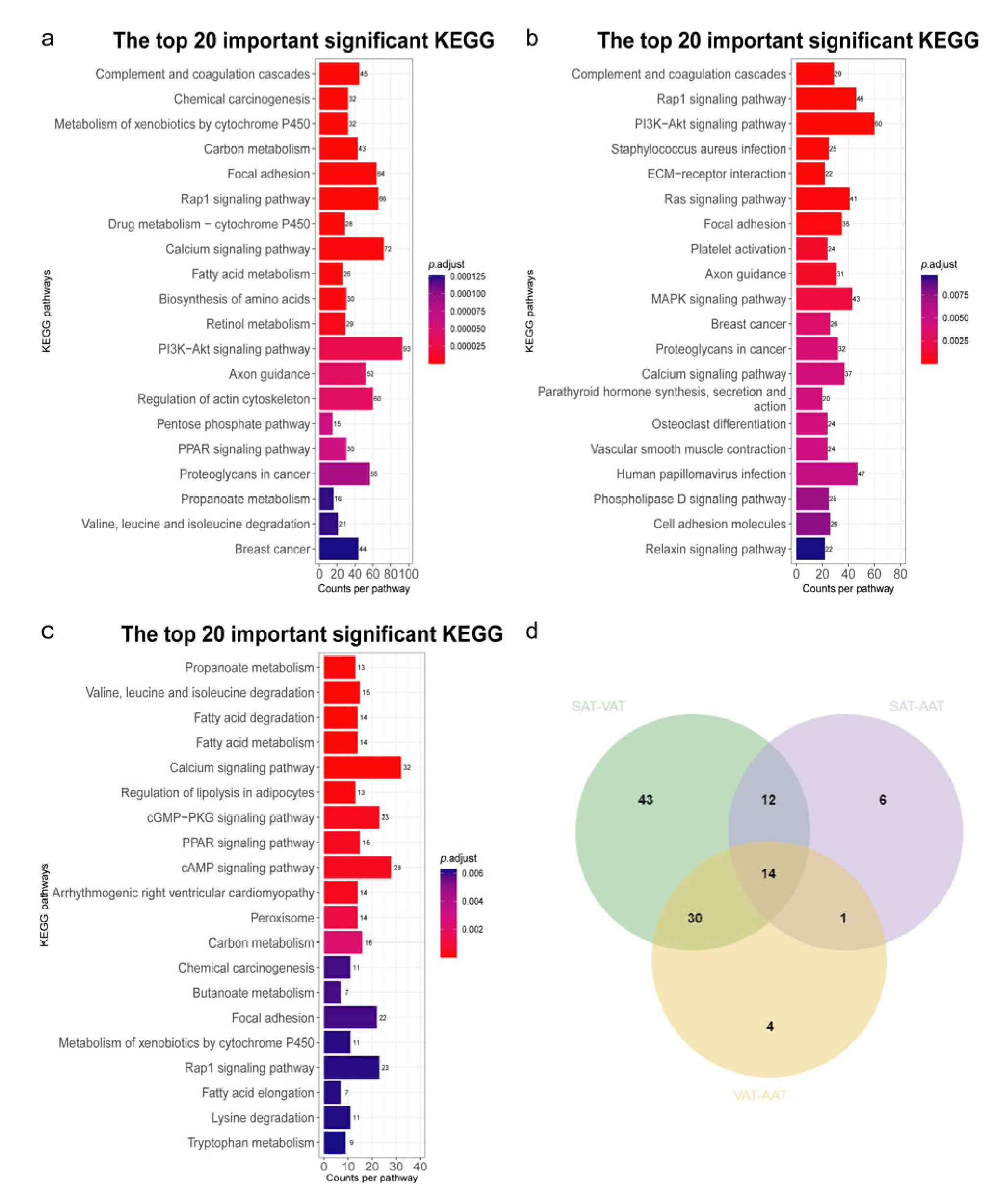
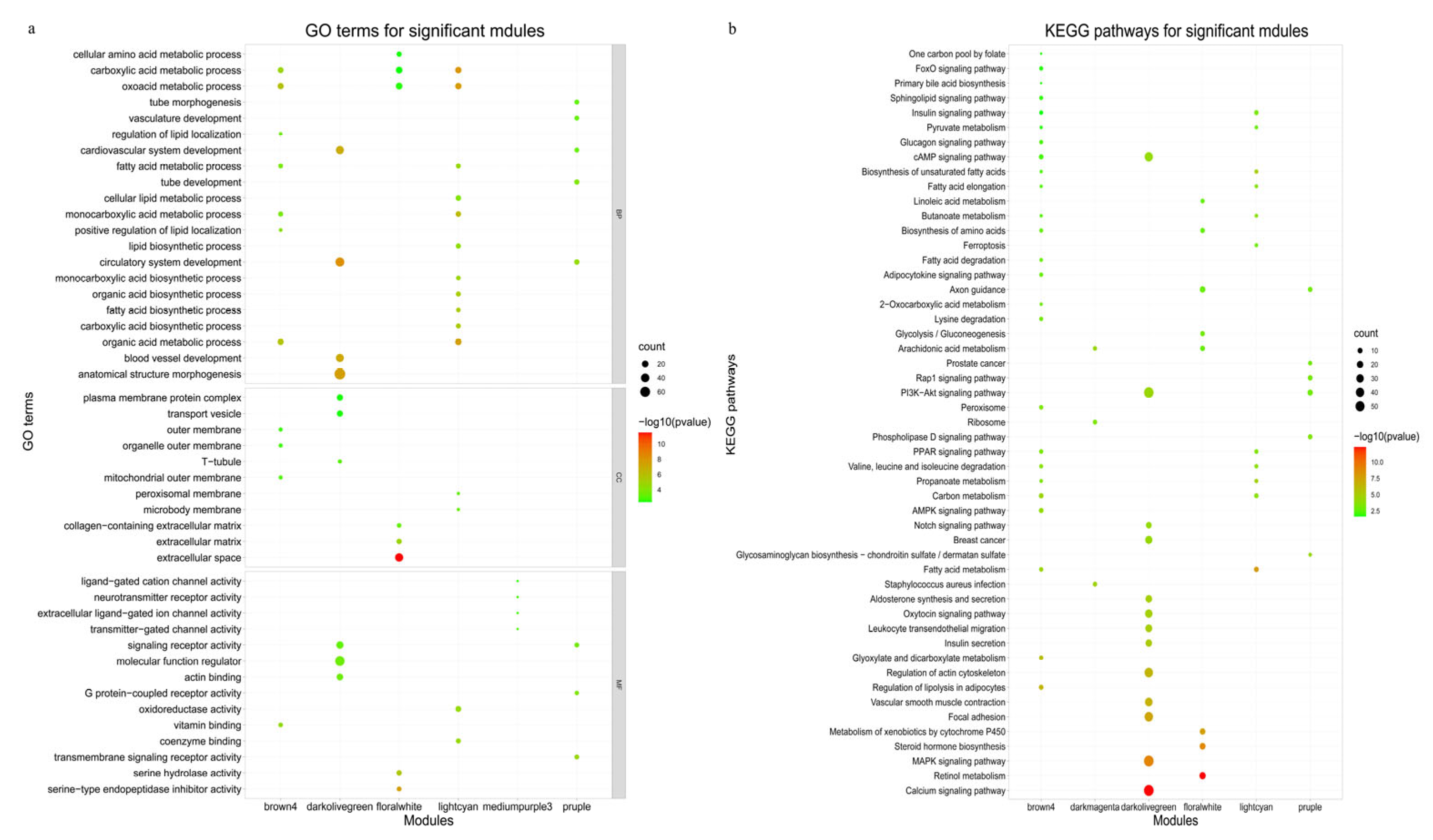
3.4. Functional Enrichment Analysis of Differentially Expressed Genes
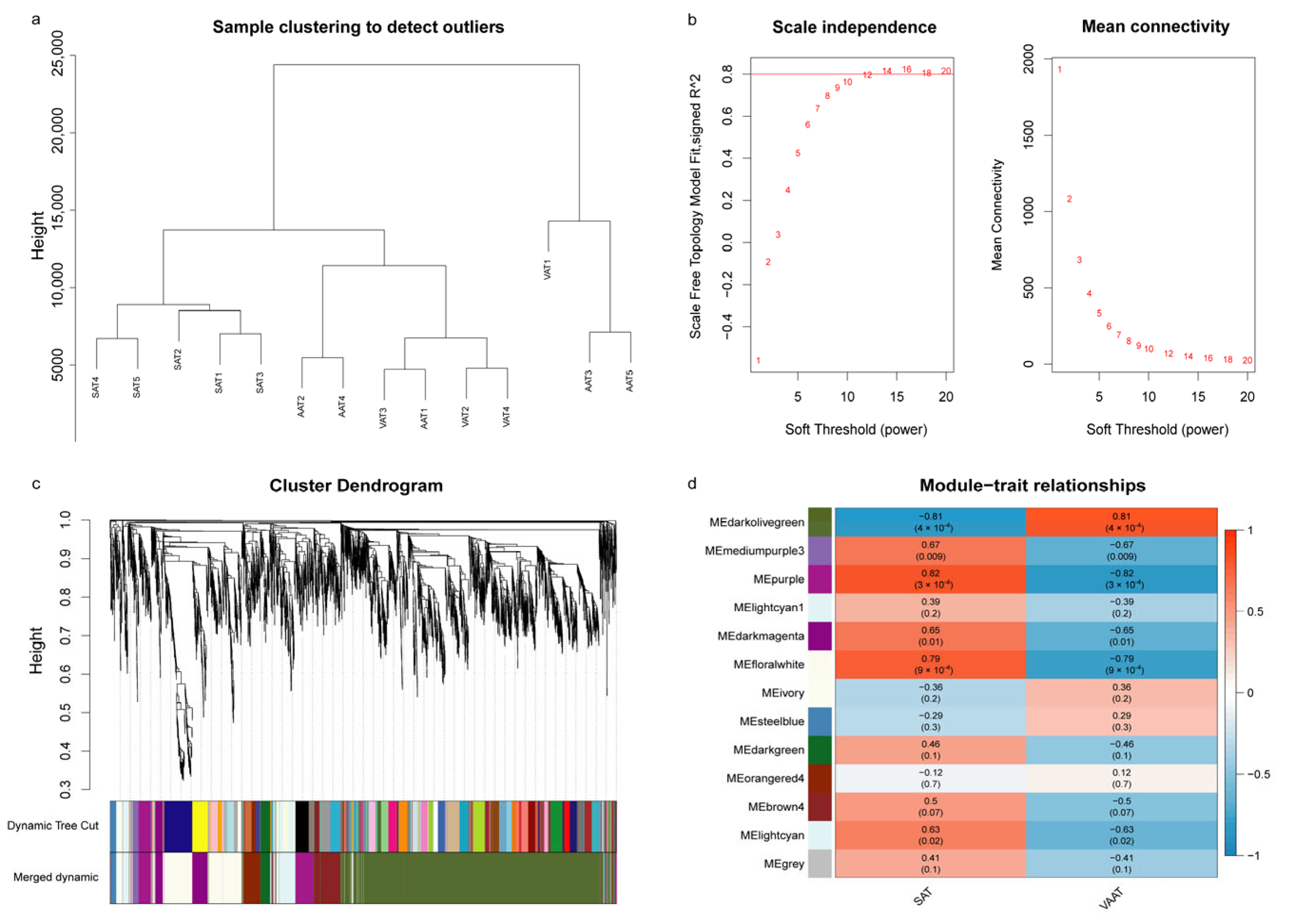
3.5. Weighted Gene Co-Expression Network Analysis
3.5.1. Adipose Tissue-Module Relationship Analysis
3.5.2. Significant Module Genes Analysis
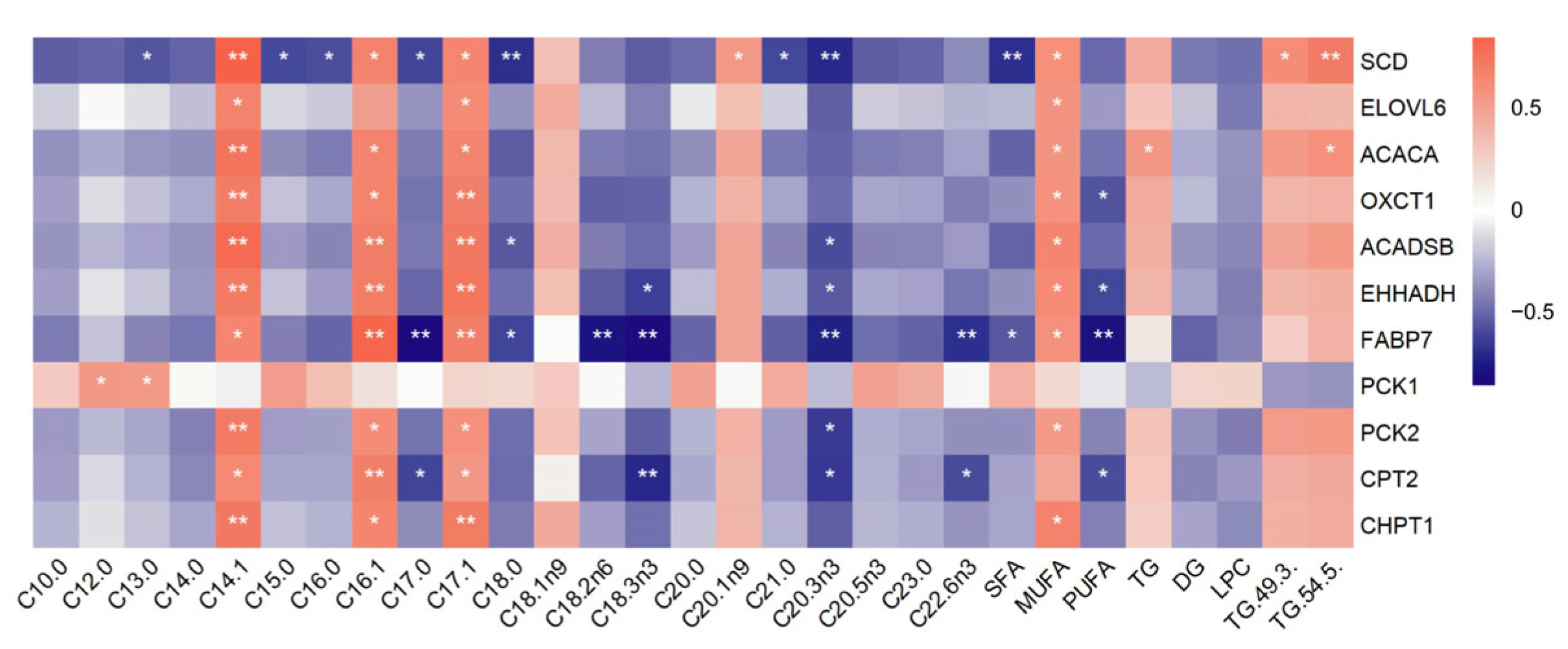
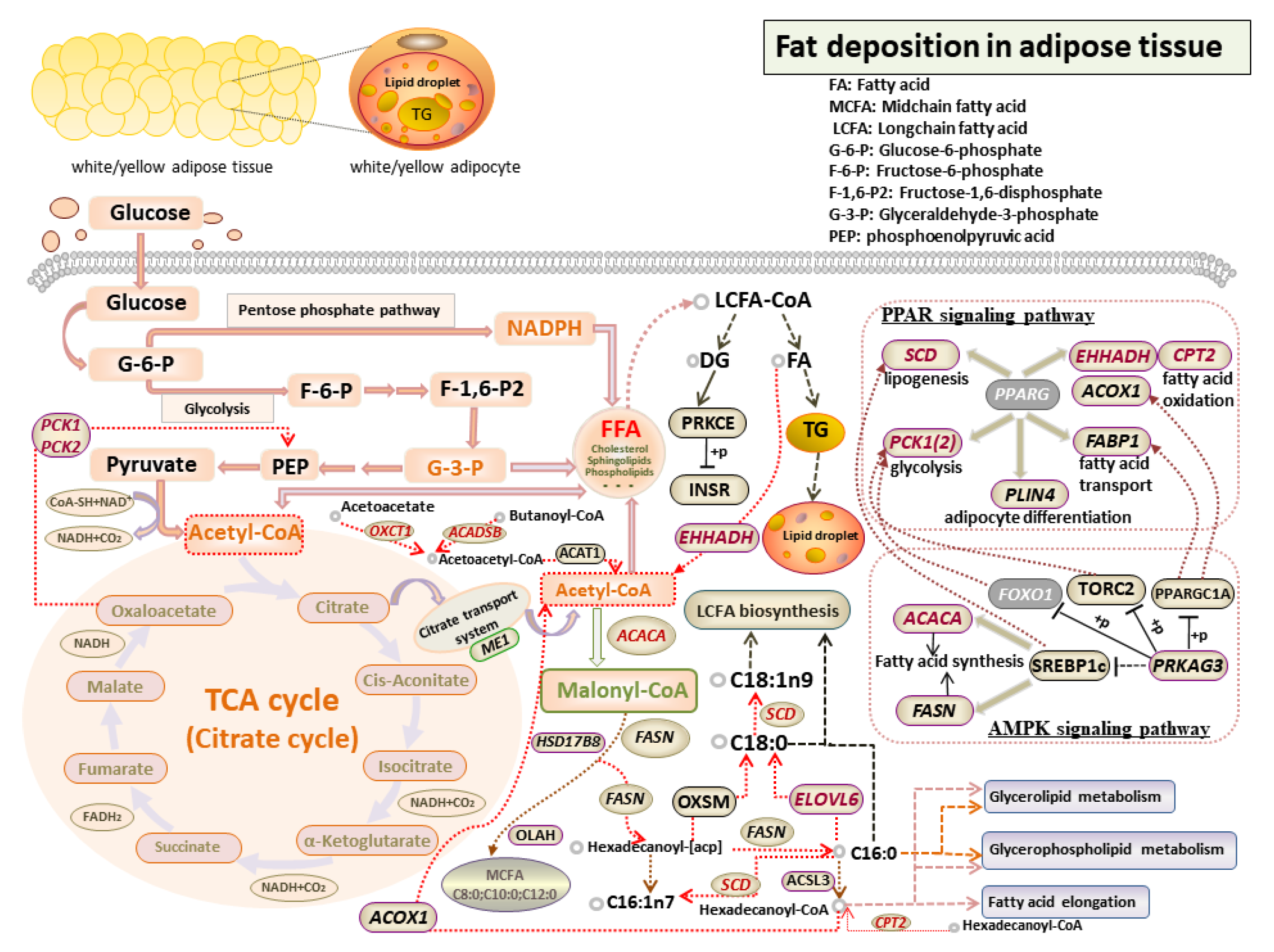
3.6. Identification of Candidate Genes Affecting Fat Deposition
3.7. Metabolites Determination
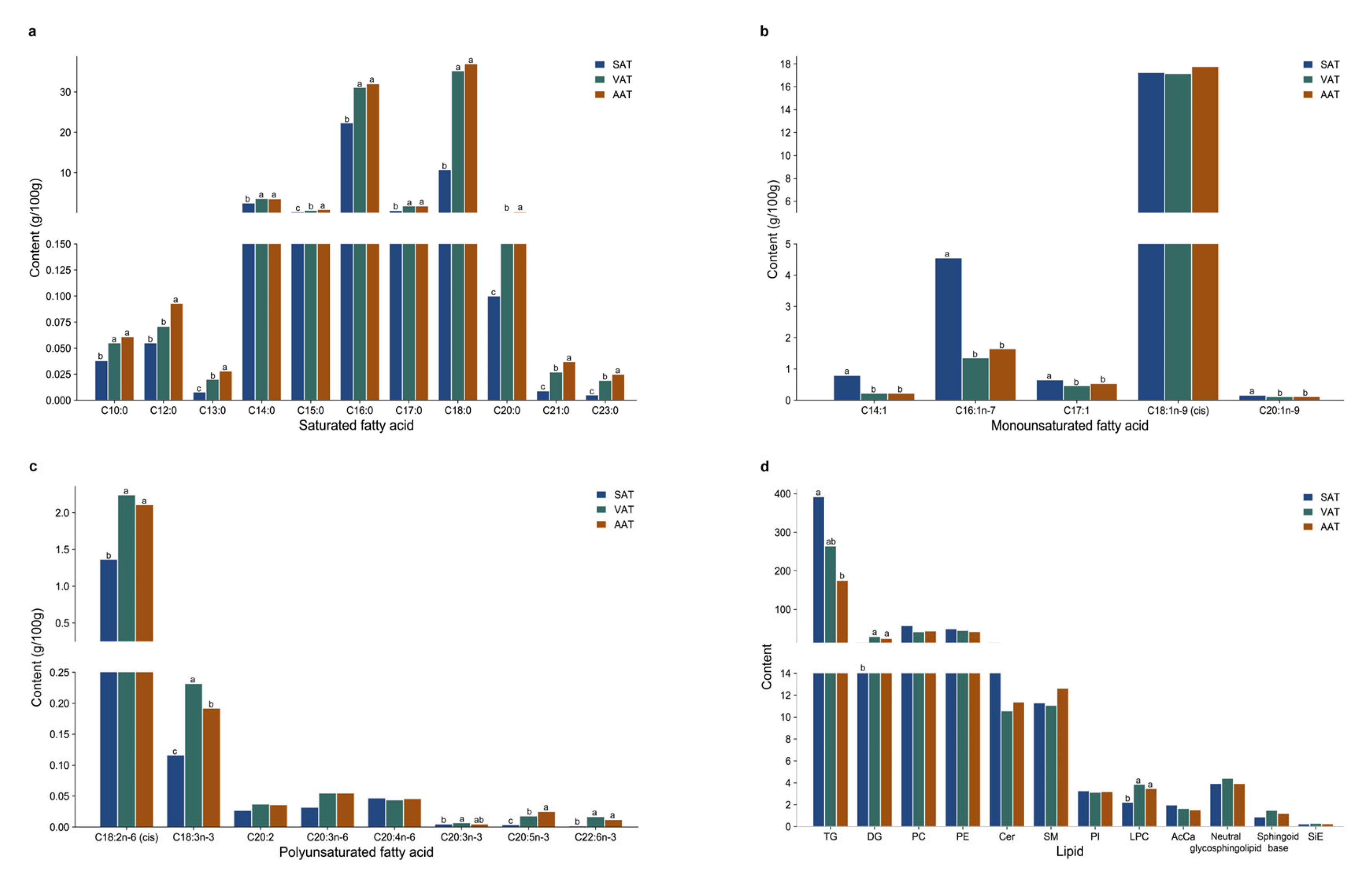
3.7.1. Fatty Acid Composition and Content
3.7.2. Lipid Composition and Content
3.8. Lipid Data Analysis
3.8.1. Assessment of Lipid Data Quality
3.8.2. Screening of Differentially Expressed Lipids among Three Groups
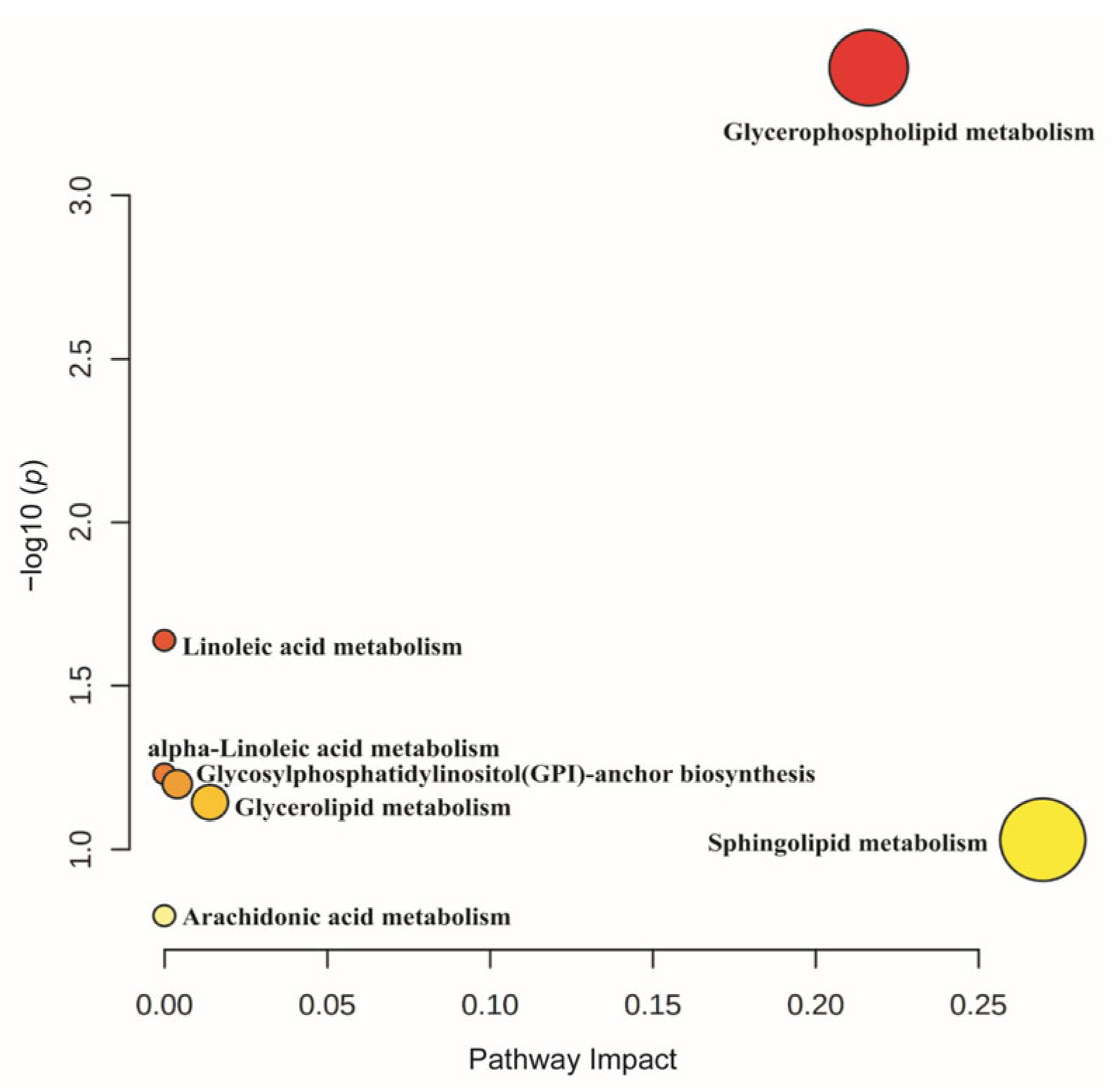
3.8.3. Pathway Enrichment of Differentially Expressed Lipids
3.9. Transcriptomic and Lipid Metabolomic Integration Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantovani, G.; Bondioni, S.; Alberti, L.; Gilardini, L.; Invitti, C.; Corbetta, S.; Zappa, M.A.; Ferrero, S.; Lania, A.G.; Bosari, S.; et al. Protein kinase A regulatory subunits in human adipose tissue: Decreased R2B expression and activity in adipocytes from obese subjects. Diabetes 2009, 58, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, S.; Yan, P. The meat quality, muscle fiber characteristics and fatty acid profile in Jinjiang and F1 Simmental×Jinjiang yellow cattle. Asian-Australas. J. Anim. Sci. 2018, 31, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Desnoyers, F.; Pascal, G.; Etienne, M.; Vodovar, N. Cellularity of adipose tissue in fetal pig. J. Lipid Res. 1980, 21, 301–308. [Google Scholar] [CrossRef]
- Faty, A.; Ferré, P.; Commans, S. The acute phase protein Serum Amyloid A induces lipolysis and inflammation in human adipocytes through distinct pathways. PLoS ONE 2012, 7, e34031. [Google Scholar] [CrossRef]
- Perrini, S.; Laviola, L.; Cignarelli, A.; Melchiorre, M.; De Stefano, F.; Caccioppoli, C.; Natalicchio, A.; Orlando, M.R.; Garruti, G.; De Fazio, M.; et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia 2008, 51, 155–164. [Google Scholar] [CrossRef]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Bonin, M.N.; Ferraz, J.B.; Eler, J.P.; Rezende, F.M.; Cucco, D.C.; Carvalho, M.E.; Silva, R.C.; Gomes, R.C.; Oliveira, E.C. Sire effects on carcass and meat quality traits of young Nellore bulls. Genet. Mol. Res. GMR 2014, 13, 3250–3264. [Google Scholar] [CrossRef]
- Jiang, M.; Fan, W.L.; Xing, S.Y.; Wang, J.; Li, P.; Liu, R.R.; Li, Q.H.; Zheng, M.Q.; Cui, H.X.; Wen, J.; et al. Effects of balanced selection for intramuscular fat and abdominal fat percentage and estimates of genetic parameters. Poult. Sci. 2017, 96, 282–287. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Li, R.W.; Li, C.J.; Thomson, J.M.; Bequette, B.J. Characterization of the longissimus lumborum transcriptome response to adding propionate to the diet of growing Angus beef steers. Physiol. Genom. 2012, 44, 543–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cesar, A.S.; Regitano, L.C.; Poleti, M.D.; Andrade, S.C.; Tizioto, P.C.; Oliveira, P.S.; Felício, A.M.; do Nascimento, M.L.; Chaves, A.S.; Lanna, D.P.; et al. Differences in the skeletal muscle transcriptome profile associated with extreme values of fatty acids content. BMC Genom. 2016, 17, 961. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Guan, L.L.; Zhang, B.; Dodson, M.V.; Okine, E.; Moore, S.S. Gene expression patterns of bovine perimuscular preadipocytes during adipogenesis. Biochem. Biophys. Res. Commun. 2008, 366, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Byrne, K.A.; Reverter, A.; Harper, G.S.; Taniguchi, M.; McWilliam, S.M.; Mannen, H.; Oyama, K.; Lehnert, S.A. Transcriptional profiling of skeletal muscle tissue from two breeds of cattle. Mamm. Genome 2005, 16, 201–210. [Google Scholar] [CrossRef]
- Midelfart, A. Metabonomics--a new approach in ophthalmology. Acta Ophthalmol. 2009, 87, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Shajahan-Haq, A.N.; Cheema, M.S.; Clarke, R. Application of metabolomics in drug resistant breast cancer research. Metabolites 2015, 5, 100–118. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 16–23. [Google Scholar] [CrossRef]
- Mahdavi, V.; Ghanati, F.; Ghassempour, A. Integrated pathway-based and network-based analysis of GC-MS rice metabolomics data under diazinon stress to infer affected biological pathways. Anal. Biochem. 2016, 494, 31–36. [Google Scholar] [CrossRef]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K. Modern plant metabolomics: Advanced natural product gene discoveries, improved technologies, and future prospects. Nat. Prod. Rep. 2015, 32, 212–229. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Quiles-Zafra, R. Lipidomics: An omics discipline with a key role in nutrition. Talanta 2020, 219, 121197. [Google Scholar] [CrossRef]
- May, F.J.; Baer, L.A.; Lehnig, A.C.; So, K.; Chen, E.Y.; Gao, F.; Narain, N.R.; Gushchina, L.; Rose, A.; Doseff, A.I.; et al. Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep. 2017, 18, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Saraf, M.K.; Annamalai, P.; Yfanti, C.; Chao, T.; Wong, D.; Shinoda, K.; et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab. 2016, 23, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography-mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.J.; Reverter, A.; Griffiths, W.J.; Yutuc, E.; Wang, Y.; Jeanes, A.; McWilliam, S.; Pethick, D.W.; Greenwood, P.L. Gene expression identifies metabolic and functional differences between intramuscular and subcutaneous adipocytes in cattle. BMC Genom. 2020, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.; Dona, A.; Hamblin, D.; D’Occhio, M.J.; González, L.A. Changes in the blood metabolome of Wagyu crossbred steers with time in the feedlot and relationships with marbling. Sci. Rep. 2020, 10, 18987. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; He, Y.; Li, Y.; Liu, J.; Erdman, R.A.; Sonstegard, T.S.; Song, J. Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci. Rep. 2016, 6, 25948. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Fan, W.; Zhang, X.; Zhan, S.; Zhong, T.; Guo, J.; Cao, J.; Li, L.; Zhang, H.; et al. Integrated application of metabolomics and RNA-seq reveals thermogenic regulation in goat brown adipose tissues. FASEB J. 2021, 35, e21868. [Google Scholar] [CrossRef]
- Lachmann, A.; Clarke, D.J.B.; Torre, D.; Xie, Z.; Ma’ayan, A. Interoperable RNA-Seq analysis in the cloud. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194521. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H.; et al. Transcriptome, proteome, and metabolome reveal the mechanism of tolerance to selenate toxicity in Cardamine violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, K.; Chang, T.; An, B.; Liang, M.; Deng, T.; Cao, S.; Du, Y.; Cai, W.; Gao, X.; et al. Integrating genomics and transcriptomics to identify candidate genes for subcutaneous fat deposition in beef cattle. Genomics 2022, 114, 110406. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Chen, X.; Yu, S.; Xu, J.; Su, Y.; Zhu, W. Transcriptomic and metabolomic responses induced in the livers of growing pigs by a short-term intravenous infusion of sodium butyrate. Animal 2018, 12, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yin, M.; Gu, J.; Hou, Y.; Tian, F.; Sun, F. Metabolomic Profiling of Plasma Samples from Women with Recurrent Spontaneous Abortion. Med. Sci. Monit. 2018, 24, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Xiao, C.; Sun, T.; Yang, Z.; Xu, W.; Wang, J.; Zeng, L.; Deng, J.; Yang, X. Transcriptome landscapes of differentially expressed genes related to fat deposits in Nandan-Yao chicken. Funct. Integr. Genom. 2021, 21, 113–124. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability-A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Mannen, H. Identification and utilization of genes associated with beef qualities. Anim. Sci. J. 2011, 82, 1–7. [Google Scholar] [CrossRef]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D. PPAR-γ and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr. Stem Cell Res. Ther. 2018, 13, 185–192. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Wang, L.; Zhang, W.; Wang, Y.; Gui, L.; Zan, L.; Zhao, C. The Effect of FATP1 on Adipocyte Differentiation in Qinchuan Beef Cattle. Animals 2021, 11, 2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bin, Y.; Zhang, J.; Cui, L.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Ren, J.; Huang, L. Genome-wide association studies for fatty acid metabolic traits in five divergent pig populations. Sci. Rep. 2016, 6, 24718. [Google Scholar] [CrossRef] [PubMed]
- Sheashea, M.; Xiao, J.; Farag, M.A. MUFA in metabolic syndrome and associated risk factors: Is MUFA the opposite side of the PUFA coin? Food Funct. 2021, 12, 12221–12234. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R.; Hemingway, C.J. The regulation of acetyl-CoA carboxylase--a potential target for the action of hypolipidemic agents. Adv. Enzyme Regul. 1999, 39, 205–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Knight, T.J.; Reecy, J.M.; Wheeler, T.L.; Shackelford, S.D.; Cundiff, L.V.; Beitz, D.C. Associations of polymorphisms in the promoter I of bovine acetyl-CoA carboxylase-alpha gene with beef fatty acid composition. Anim. Genet. 2010, 41, 417–420. [Google Scholar]
- Da Costa, A.S.; Pires, V.M.; Fontes, C.M.; Mestre Prates, J.A. Expression of genes controlling fat deposition in two genetically diverse beef cattle breeds fed high or low silage diets. BMC Vet. Res. 2013, 9, 118. [Google Scholar] [CrossRef]
- Lengi, A.J.; Corl, B.A. Identification and characterization of a novel bovine stearoyl-CoA desaturase isoform with homology to human SCD5. Lipids 2007, 42, 499–508. [Google Scholar] [CrossRef]
- Lehnert, S.A.; Reverter, A.; Byrne, K.A.; Wang, Y.; Nattrass, G.S.; Hudson, N.J.; Greenwood, P.L. Gene expression studies of developing bovine longissimus muscle from two different beef cattle breeds. BMC Dev. Biol. 2007, 7, 95. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xu, L.; Zhao, X.; Pan, K.; Yi, Z.; Bai, J.; Qi, X.; Xin, J.; Li, M.; Ouyang, K.; et al. RNA-Seq analysis reveals the potential molecular mechanisms of daidzein on adipogenesis in subcutaneous adipose tissue of finishing Xianan beef cattle. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Larsen, T.W.; Smith, S.B.; Tume, R.K. Delta9 desaturase activity in bovine subcutaneous adipose tissue of different fatty acid composition. Lipids 1999, 34, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Siebert, B.D.; Pitchford, W.S.; Kruk, Z.A.; Kuchel, H.; Deland, M.P.; Bottema, C.D. Differences in delta9 desaturase activity between Jersey- and Limousin-sired cattle. Lipids 2003, 38, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T. Role of fatty acid elongase Elovl6 in the regulation of energy metabolism and pathophysiological significance in diabetes. Diabetol. Int. 2021, 12, 68–73. [Google Scholar] [CrossRef]
- Sunaga, H.; Matsui, H.; Anjo, S.; Syamsunarno, M.R.; Koitabashi, N.; Iso, T.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; Kurabayashi, M. Elongation of Long-Chain Fatty Acid Family Member 6 (Elovl6)-Driven Fatty Acid Metabolism Regulates Vascular Smooth Muscle Cell Phenotype Through AMP-Activated Protein Kinase/Krüppel-Like Factor 4 (AMPK/KLF4) Signaling. J. Am. Heart Assoc. 2016, 5, e004014. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Yoshikawa, T.; Amemiya-Kudo, M.; Hasty, A.H.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; et al. Cloning and characterization of a mammalian fatty acyl-CoA elongase as a lipogenic enzyme regulated by SREBPs. J. Lipid Res. 2002, 43, 911–920. [Google Scholar] [CrossRef]
- Junjvlieke, Z.; Khan, R.; Mei, C.; Cheng, G.; Wang, S.; Raza, S.H.A.; Hong, J.; Wang, X.; Yang, W.; Zan, L. Effect of ELOVL6 on the lipid metabolism of bovine adipocytes. Genomics 2020, 112, 2282–2290. [Google Scholar] [CrossRef]
- Moisá, S.J.; Shike, D.W.; Faulkner, D.B.; Meteer, W.T.; Keisler, D.; Loor, J.J. Central Role of the PPARγ Gene Network in Coordinating Beef Cattle Intramuscular Adipogenesis in Response to Weaning Age and Nutrition. Gene Regul. Syst. Biol. 2014, 8, 17–32. [Google Scholar] [CrossRef]
- Ji, P.; Osorio, J.S.; Drackley, J.K.; Loor, J.J. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression. J. Dairy Sci. 2012, 95, 4333–4351. [Google Scholar] [CrossRef]
- Junjvlieke, Z.; Mei, C.G.; Khan, R.; Zhang, W.Z.; Hong, J.Y.; Wang, L.; Li, S.J.; Zan, L.S. Transcriptional regulation of bovine elongation of very long chain fatty acids protein 6 in lipid metabolism and adipocyte proliferation. J. Cell. Biochem. 2019, 120, 13932–13943. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Tacuma, D.; Parales-Giron, J.; Prom, C.; Chirivi, M.; Laguna, J.; Lock, A.L.; Contreras, G.A. Transcriptomic profiling of adipose tissue inflammation, remodeling, and lipid metabolism in periparturient dairy cows (Bos taurus). BMC Genom. 2020, 21, 824. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pei, J.; Chu, M.; Wu, X.; Kalwar, Q.; Yan, P.; Guo, X. Fat Deposition in the Muscle of Female and Male Yak and the Correlation of Yak Meat Quality with Fat. Animals 2021, 11, 2142. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont, J.P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Aspects Med. 2004, 25, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sun, X.; Rafikov, R.; Kumar, S.; Hou, Y.; Oishi, P.E.; Datar, S.A.; Raff, G.; Fineman, J.R.; Black, S.M. PPAR-γ regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow. PLoS ONE 2012, 7, e41555. [Google Scholar] [CrossRef]
- Houten, S.M.; Denis, S.; Argmann, C.A.; Jia, Y.; Ferdinandusse, S.; Reddy, J.K.; Wanders, R.J. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 2012, 53, 1296–1303. [Google Scholar] [CrossRef]
- Ranea-Robles, P.; Violante, S.; Argmann, C.; Dodatko, T.; Bhattacharya, D.; Chen, H.; Yu, C.; Friedman, S.L.; Puchowicz, M.; Houten, S.M. Murine deficiency of peroxisomal L-bifunctional protein (EHHADH) causes medium-chain 3-hydroxydicarboxylic aciduria and perturbs hepatic cholesterol homeostasis. Cell. Mol. Life Sci. 2021, 78, 5631–5646. [Google Scholar] [CrossRef]
- Juge-Aubry, C.E.; Kuenzli, S.; Sanchez, J.C.; Hochstrasser, D.; Meier, C.A. Peroxisomal bifunctional enzyme binds and activates the activation function-1 region of the peroxisome proliferator-activated receptor alpha. Biochem. J. 2001, 353 Pt 2, 253–258. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, C. The role of OXCT1 in the pathogenesis of cancer as a rate-limiting enzyme of ketone body metabolism. Life Sci. 2017, 183, 110–115. [Google Scholar] [CrossRef]
- Kumar, H.; Srikanth, K.; Park, W.; Lee, S.H.; Choi, B.H.; Kim, H.; Kim, Y.M.; Cho, E.S.; Kim, J.H.; Lee, J.H.; et al. Transcriptome analysis to identify long non coding RNA (lncRNA) and characterize their functional role in back fat tissue of pig. Gene 2019, 703, 71–82. [Google Scholar] [CrossRef]
- Zeng, J.; Zhou, S.W.; Zhao, J.; Jin, M.H.; Kang, D.J.; Yang, Y.X.; Wang, X.L.; Chen, Y.L. Role of OXCT1 in ovine adipose and preadipocyte differentiation. Biochem. Biophys. Res. Commun. 2019, 512, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Badoud, F.; Lam, K.P.; DiBattista, A.; Perreault, M.; Zulyniak, M.A.; Cattrysse, B.; Stephenson, S.; Britz-McKibbin, P.; Mutch, D.M. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J. Proteome Res. 2014, 13, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.C.; Viars, C.S.; Fu, K.; Rozen, R. Localization of short/branched chain acyl-CoA dehydrogenase (ACADSB) to human chromosome 10. Genomics 1995, 25, 743–745. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Wang, H.; Zhu, L.; Xu, K. Decreased Expression of ACADSB Predicts Poor Prognosis in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 762629. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Fang, X.; Zhao, Z.; Yu, X.; Yang, R. The effect of short/branched chain acyl-coenzymeA dehydrogenase gene on triglyceride synthesis of bovine mammary epithelial cells. Arch. Anim. Breed. 2018, 61, 115–122. [Google Scholar]
- Jiang, P.; Iqbal, A.; Wang, M.; Li, X.; Fang, X.; Yu, H.; Zhao, Z. Transcriptomic Analysis of Short/Branched-Chain Acyl-Coenzyme a Dehydrogenase Knocked Out bMECs Revealed Its Regulatory Effect on Lipid Metabolism. Front. Vet. Sci. 2021, 8, 744287. [Google Scholar] [CrossRef]
- Beale, E.G.; Harvey, B.J.; Forest, C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem. Biophys. 2007, 48, 89–95. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, D.; Peng, Z.; Zhu, Y.; Li, R.; Wu, Q.; Li, Y.; Li, H.; Xu, W.; Zhang, M.; et al. Identification of Differentially Expressed Genes and Lipid Metabolism Signaling Pathways between Muscle and Fat Tissues in Broiler Chickens. J. Poult. Sci. 2021, 58, 131–137. [Google Scholar] [CrossRef]
- Hakimi, P.; Yang, J.; Casadesus, G.; Massillon, D.; Tolentino-Silva, F.; Nye, C.K.; Cabrera, M.E.; Hagen, D.R.; Utter, C.B.; Baghdy, Y.; et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J. Biol. Chem. 2007, 282, 32844–32855. [Google Scholar] [CrossRef]
- Semakova, J.; Hyroššová, P.; Méndez-Lucas, A.; Cutz, E.; Bermudez, J.; Burgess, S.; Alcántara, S.; Perales, J.C. PEPCK-C reexpression in the liver counters neonatal hypoglycemia in Pck1 (del/del) mice, unmasking role in non-gluconeogenic tissues. J. Physiol. Biochem. 2017, 73, 89–98. [Google Scholar] [CrossRef]
- Forest, C.; Tordjman, J.; Glorian, M.; Duplus, E.; Chauvet, G.; Quette, J.; Beale, E.G.; Antoine, B. Fatty acid recycling in adipocytes: A role for glyceroneogenesis and phosphoenolpyruvate carboxykinase. Biochem. Soc. Trans. 2003, 31 Pt 6, 1125–1129. [Google Scholar] [CrossRef]
- Beale, E.G.; Forest, C.; Hammer, R.E. Regulation of cytosolic phosphoenolpyruvate carboxykinase gene expression in adipocytes. Biochimie 2003, 85, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Wang, K.; Ao, H.; Chen, S.; Tan, Z.; Wang, Y.; Xitong, Z.; Yang, T.; Zhang, F.; Liu, Y.; et al. Comparative adipose transcriptome analysis digs out genes related to fat deposition in two pig breeds. Sci. Rep. 2019, 9, 12925. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Furuhashi, M.; Sugaya, T.; Oikawa, T.; Matsumoto, M.; Funahashi, Y.; Matsukawa, Y.; Gotoh, M.; Miura, T. Transcriptome and Metabolome Analyses in Exogenous FABP4- and FABP5-Treated Adipose-Derived Stem Cells. PLoS ONE 2016, 11, e0167825. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shen, L.; Zhou, L.; Sha, W.; Yang, J.; Lu, G. Upregulation of FABP7 inhibits acute kidney injury-induced TCMK-1 cell apoptosis via activating the PPAR gamma signalling pathway. Mol. Omics 2020, 16, 533–542. [Google Scholar] [CrossRef]
- Bartoň, L.; Bureš, D.; Kott, T.; Řehák, D. Associations of polymorphisms in bovine DGAT1, FABP4, FASN, and PPARGC1A genes with intramuscular fat content and the fatty acid composition of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 2016, 114, 18–23. [Google Scholar] [CrossRef]
- Hoashi, S.; Hinenoya, T.; Tanaka, A.; Ohsaki, H.; Sasazaki, S.; Taniguchi, M.; Oyama, K.; Mukai, F.; Mannen, H. Association between fatty acid compositions and genotypes of FABP4 and LXR-alpha in Japanese black cattle. BMC Genet. 2008, 9, 84. [Google Scholar] [CrossRef]
- Shan, T.Z.; Ren, Y.; Wu, T.; Liu, C.X.; Wang, Y.Z. Regulatory role of Sirt1 on the gene expression of fatty acid-binding protein 3 in cultured porcine adipocytes. J. Cell. Biochem. 2009, 107, 984–991. [Google Scholar] [CrossRef]
- Wen, S.; He, Y.; Wang, L.; Zhang, J.; Quan, C.; Niu, Y.; Huang, H. Aberrant activation of super enhancer and choline metabolism drive antiandrogen therapy resistance in prostate cancer. Oncogene 2020, 39, 6556–6571. [Google Scholar] [CrossRef]
- Ko, M.; Hattori, T.; Abdullah, M.; Gong, J.S.; Yamane, T.; Michikawa, M. Phosphatidylcholine protects neurons from toxic effects of amyloid β-protein in culture. Brain Res. 2016, 1642, 376–383. [Google Scholar] [CrossRef]
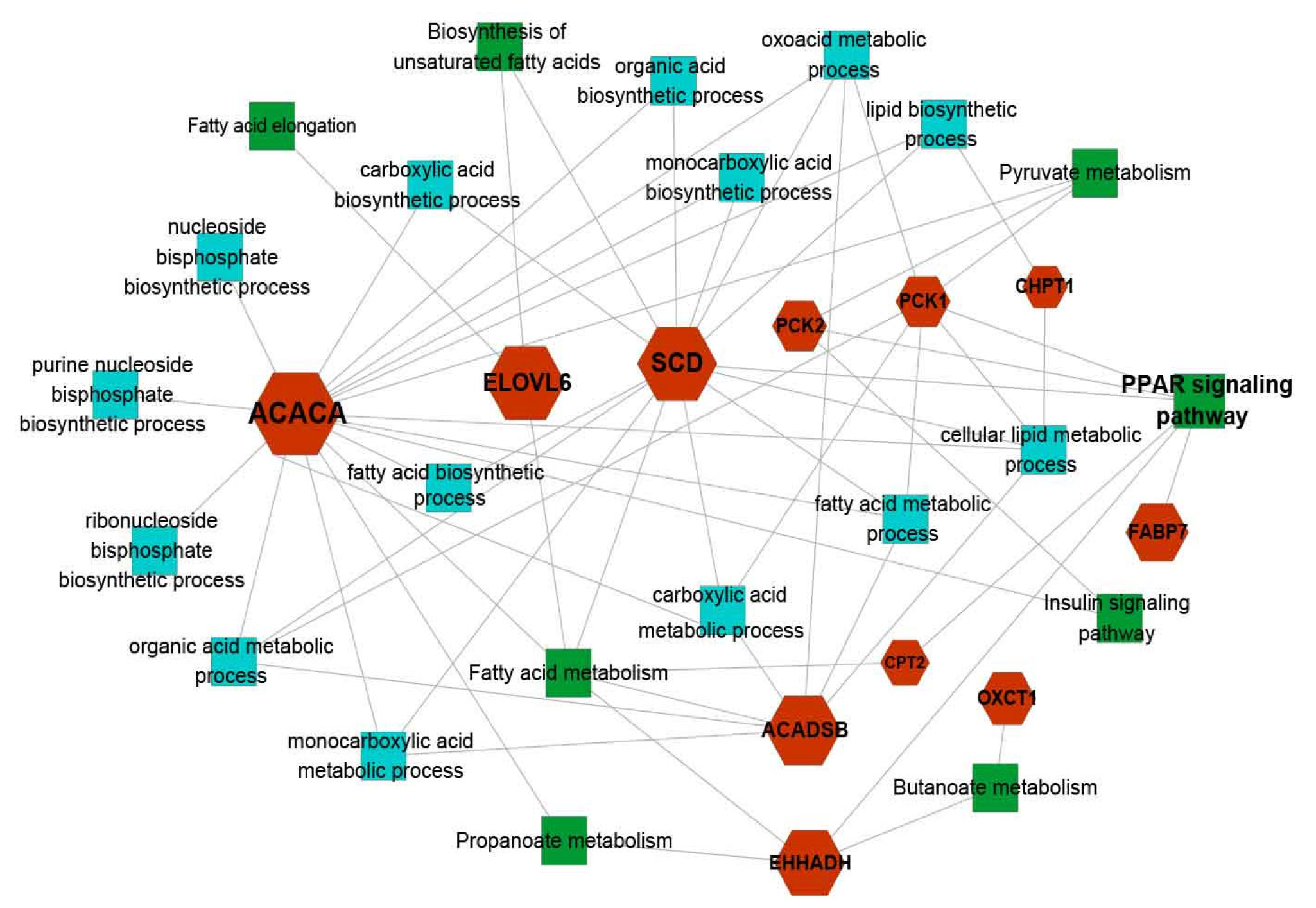

| ID | Description | Overlapped Genes 1 |
|---|---|---|
| bta03320 | PPAR signaling pathway | PLIN4/FABP7/PCK1 |
| bta04911 | Insulin secretion | CACNA1C |
| bta04010 | MAPK signaling pathway | KIT/CACNA1C/CACNA1H/RPS6KA6 |
| bta04151 | PI3K-Akt signaling pathway | PCK1/KIT/COMP/ITGB4 |
| bta04020 | Calcium signaling pathway | CACNA1C/CACNA1H |
| bta04014 | Ras signaling pathway | KIT/PLA2G4B/PLD2 |
| bta04015 | Rap1 signaling pathway | KIT |
| bta04512 | ECM-receptor interaction | COMP/ITGB4 |
| bta04510 | Focal adhesion | COMP/ITGB4 |
| bta04360 | Axon guidance | NGEF/NTNG2/BMP7/ROBO3 |
| bta05205 | Proteoglycans in cancer | TWIST1 |
| bta05224 | Breast cancer | KIT/HEY2 |
| bta05414 | Dilated cardiomyopathy | CACNA1C/ITGB4 |
| bta04934 | Cushing syndrome | CACNA1C/CACNA1H |
| ID | Gene Symbol | BTA 1 | GS 2 | MM 3 |
|---|---|---|---|---|
| ENSBTAG00000019625 | EHHADH | 1 | 0.59 (0.03) | 0.96 (2.67 × 10−8) |
| ENSBTAG00000014649 | CPT2 | 3 | 0.59 (0.03) | 0.84 (1.91 × 10−4) |
| ENSBTAG00000002490 | CHPT1 | 5 | 0.56 (0.04) | 0.84 (1.77 × 10−4) |
| ENSBTAG00000010564 | ELOVL6 | 6 | 0.52 (0.05) | 0.98 (3.51 × 10−10) |
| ENSBTAG00000000011 | TDH | 8 | 0.57 (0.03) | 0.91 (5.54 × 10−6) |
| ENSBTAG00000011934 | PCK2 | 10 | 0.58 (0.03) | 0.92 (3.90 × 10−6) |
| ENSBTAG00000022583 | SHC4 | 10 | 0.74 (0.002) | 0.89 (1.76 × 10−5) |
| ENSBTAG00000008734 | PM20D1 | 16 | 0.69 (0.01) | 0.92 (3.44 × 10−6) |
| ENSBTAG00000015908 | MBOAT7 | 18 | 0.76 (0.002) | 0.93 (1.67 × 10−6) |
| ENSBTAG00000017567 | ACACA | 19 | 0.51 (0.05) | 0.96 (7.79 × 10−8) |
| ENSBTAG00000033186 | OXCT1 | 20 | 0.56 (0.04) | 0.99 (2.50 × 10−11) |
| ENSBTAG00000014205 | PRKAR2A | 22 | 0.53 (0.05) | 0.88 (2.93 × 10−5) |
| ENSBTAG00000046753 | LOC107131843 | 25 | 0.61 (0.02) | 0.91 (5.93 × 10−6) |
| ENSBTAG00000055207 | SCD | 26 | 0.60 (0.02) | 0.97 (9.99 × 10−9) |
| ENSBTAG00000018041 | ACADSB | 26 | 0.61 (0.02) | 0.95 (1.74 × 10−7) |
| ENSBTAG00000046155 | RGN | X | 0.61 (0.02) | 0.81 (4.20 × 10−4) |
| ENSBTAG00000019852 | PDHA1 | X | 0.61 (0.02) | 0.96 (5.01 × 10−8) |
| Node 1 | Node 2 | Confidence Score |
|---|---|---|
| ACACA | ACADSB | 0.463 |
| CPT2 | 0.619 | |
| EHHADH | 0.608 | |
| ELOVL6 | 0.838 | |
| PCK1 | 0.543 | |
| PCK2 | 0.407 | |
| SCD | 0.527 | |
| ACADSB | CPT2 | 0.670 |
| EHHADH | 0.995 | |
| OXCT1 | 0.426 | |
| CHPT1 | SCD | 0.523 |
| CPT2 | EHHADH | 0.765 |
| PCK1 | 0.553 | |
| EHHADH | ELOVL6 | 0.486 |
| FABP7 | 0.533 | |
| OXCT1 | 0.944 | |
| PCK1 | 0.572 | |
| PCK1 | PCK2 | 0.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Chang, T.; An, B.; Liang, M.; Deng, T.; Li, K.; Cao, S.; Du, Y.; Gao, X.; Xu, L.; et al. Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle. Genes 2023, 14, 37. https://doi.org/10.3390/genes14010037
Du L, Chang T, An B, Liang M, Deng T, Li K, Cao S, Du Y, Gao X, Xu L, et al. Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle. Genes. 2023; 14(1):37. https://doi.org/10.3390/genes14010037
Chicago/Turabian StyleDu, Lili, Tianpeng Chang, Bingxing An, Mang Liang, Tianyu Deng, Keanning Li, Sheng Cao, Yueying Du, Xue Gao, Lingyang Xu, and et al. 2023. "Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle" Genes 14, no. 1: 37. https://doi.org/10.3390/genes14010037
APA StyleDu, L., Chang, T., An, B., Liang, M., Deng, T., Li, K., Cao, S., Du, Y., Gao, X., Xu, L., Zhang, L., Li, J., & Gao, H. (2023). Transcriptomics and Lipid Metabolomics Analysis of Subcutaneous, Visceral, and Abdominal Adipose Tissues of Beef Cattle. Genes, 14(1), 37. https://doi.org/10.3390/genes14010037





