Differential Gene Expression of Innate Immune Response Genes Consequent to Solenopsis invicta Virus-3 Infection
Abstract
1. Introduction
2. Methods
2.1. Field Collection of Fire Ants
2.2. Laboratory Colony Establishment
2.3. Infection Inoculum Preparation
2.4. Sample Preparation of Uninfected Ants
2.5. Infection Methodology and Infected RNA Sample Preparation
2.6. RNA Preparation and mRNA Sequencing
3. Results
3.1. Colony Level Outcomes
3.2. Transcriptome Sequencing Results
3.2.1. Differential Expression Following SINV-3 Exposure
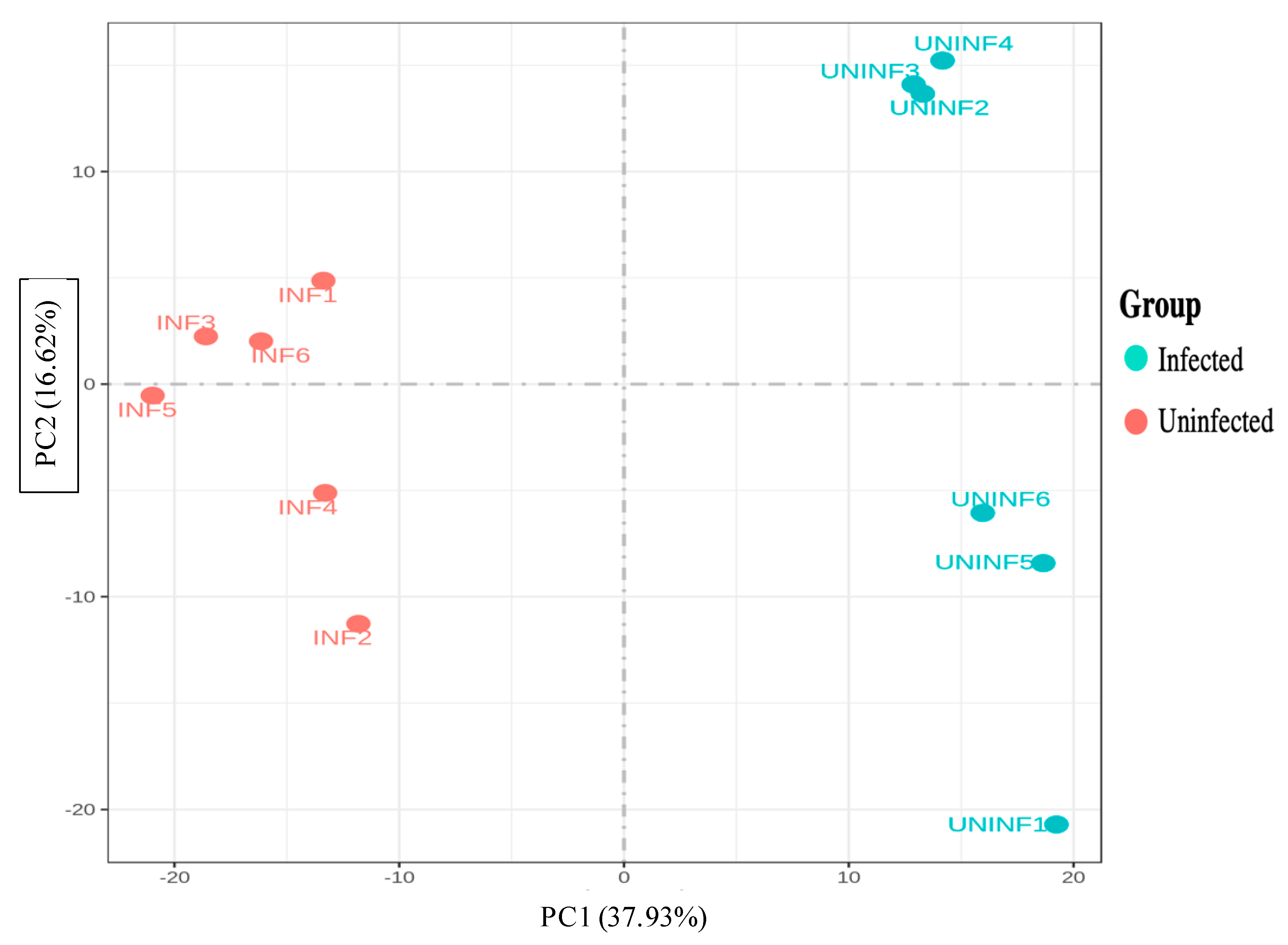
3.2.2. Principal Component Analysis
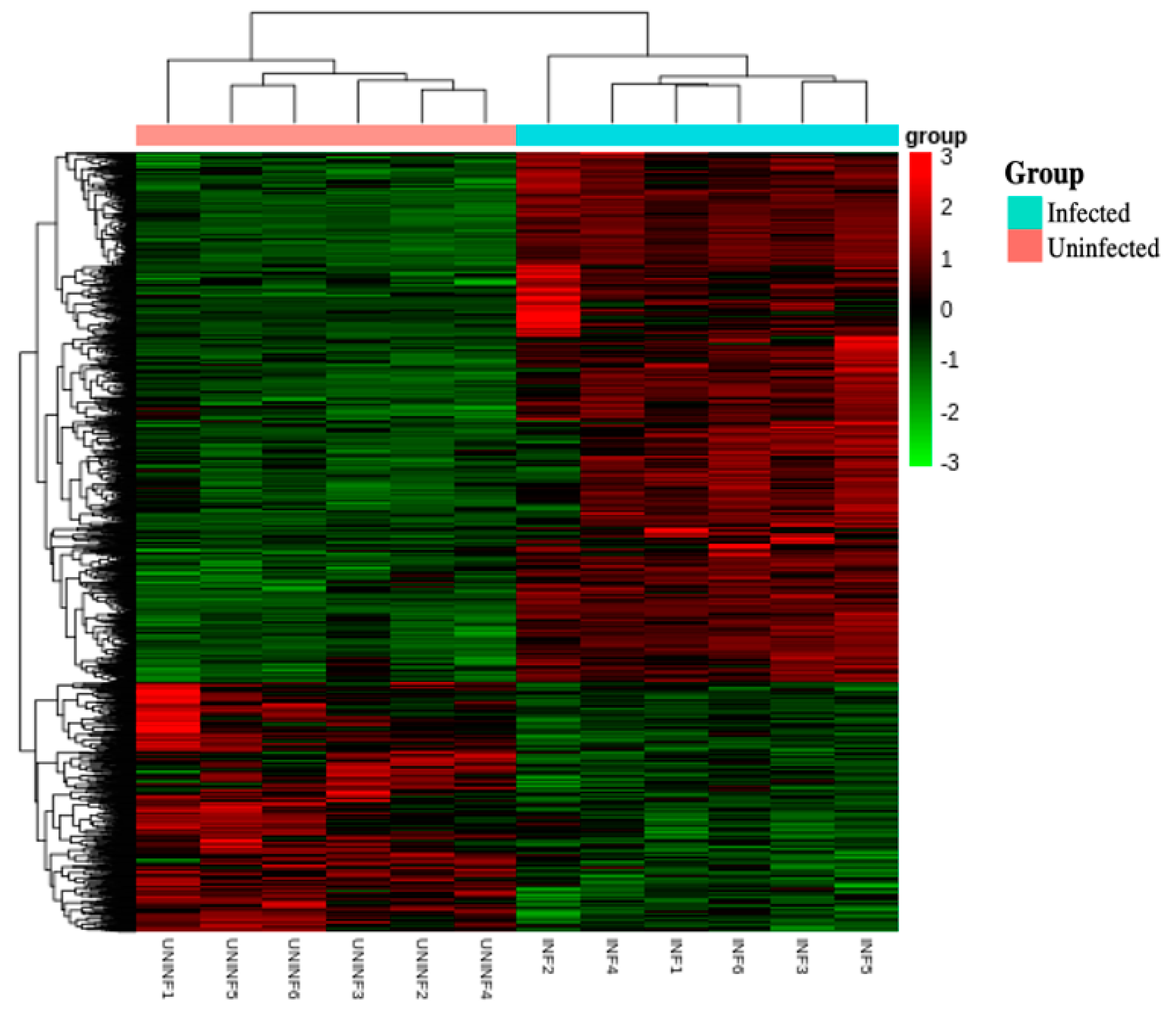
3.2.3. Combined Heat Map of Differentially Expressed Genes between Infected and Uninfected Fire Ant Workers
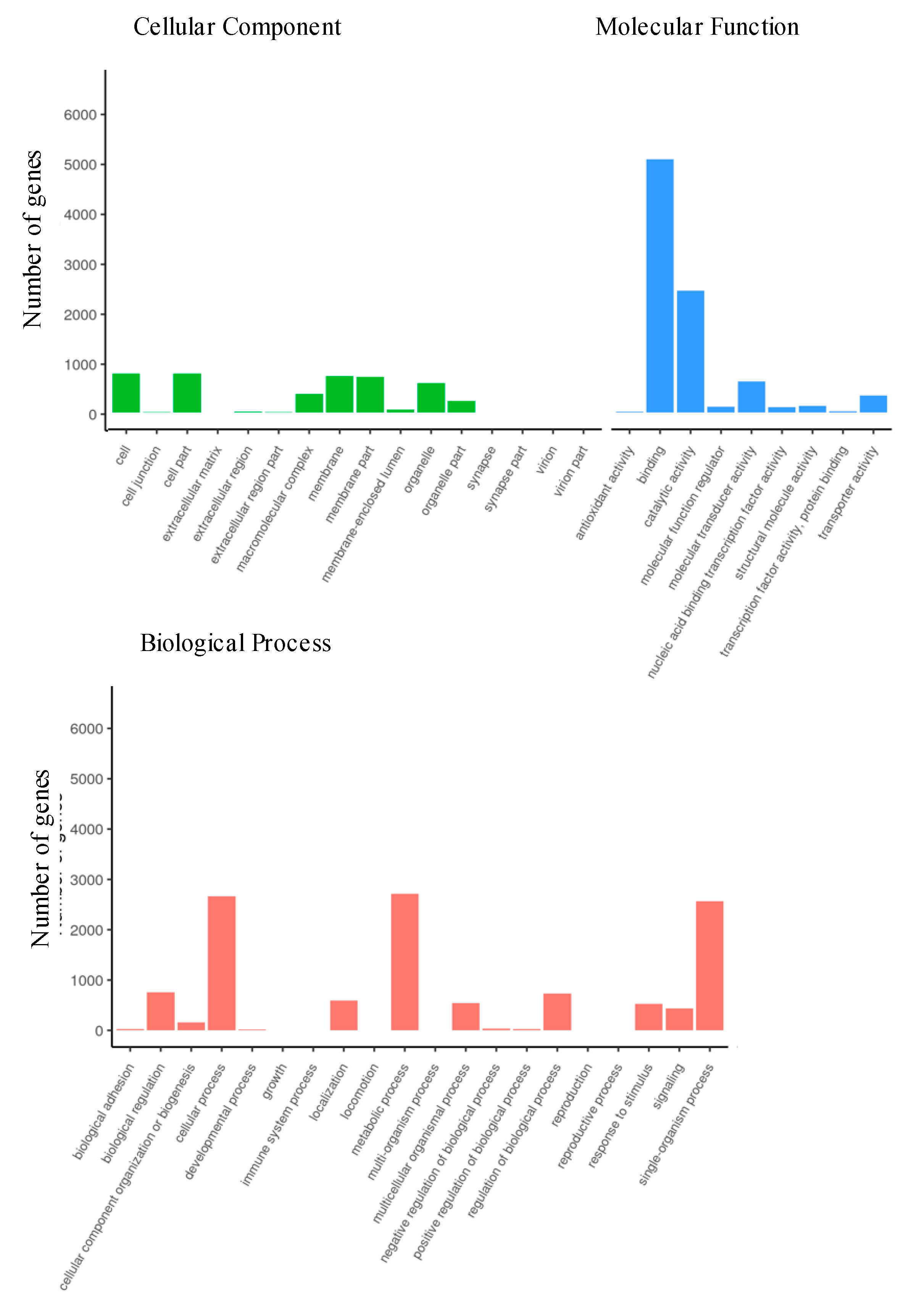
3.2.4. Gene Ontology of Differentially Expressed Genes
3.2.5. KEGG Enrichment Analysis of Differentially Expressed Genes
3.2.6. Glycolysis Pathway
4. Discussion
4.1. Janus Kinase (JNK)
4.2. Allatostatin
4.3. Antimicrobial Peptides (AMPs)
4.4. Cytochrome p450
4.5. Toll and Immune Deficiency (Imd) Pathways
4.6. Apoptosis
4.7. Glycolysis
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, A.N. Diversity, Biogeography and Community Ecology of Ants: Introduction to the Special Issue. Diversity 2021, 13, 625. [Google Scholar] [CrossRef]
- Morrison, L.W.; Porter, S.D.; Daniels, E.; Korzukhin, M.D. Potential global range expansion of the invasive fire ant, Solenopsis invicta. Biol. Invasions 2004, 6, 183–191. [Google Scholar] [CrossRef]
- Ascunce, M.S.; Yang, C.-C.; Oakey, J.; Calcaterra, L.; Wu, W.-J.; Shih, C.-J.; Goudet, J.; Ross, K.G.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef]
- Calcaterra, L.A.; Livore, J.; Delgado, A.; Briano, J.A. Ecological dominance of the red imported fire ant, Solenopsis invicta, in its native range. Oecologia 2008, 156, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Drees, B.M.; Calixto, A.A.; Nester, P.R. Integrated pest management concepts for red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae). Insect Sci. 2013, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Reed, J.; Brown, R. Timing bait applications for control of imported fire ants (Hymenoptera: Formicidae) in Mississippi: Efficacy and effects on non-target ants. Int. J. Pest Manag. 2005, 51, 121–130. [Google Scholar] [CrossRef]
- Williams, D.F. Control of the introduced pest Solenopsis invicta in the United States. In Exotic Ants; CRC Press: Boca Raton, FL, USA, 2021; pp. 282–292. [Google Scholar]
- Williams, D.F.; Collins, H.L.; Oi, D.H. The red imported fire ant (Hymenoptera: Formicidae): An historical perspective of treatment programs and the development of chemical baits for control. Am. Entomol. 2001, 47, 146–159. [Google Scholar] [CrossRef]
- Porter, S.D.; Valles, S.M.; Oi, D.H. Host specificity and colony impacts of the fire ant pathogen, Solenopsis invicta virus 3. J. Invertebr. Pathol. 2013, 114, 1–6. [Google Scholar] [CrossRef]
- Holmes, V.R.; Johnston, J.S. The Innate Immune Response of Eusocial Hymenopterans to Viral Pathogen Challenge. Ann. Entomol. Soc. Am. 2022, 115, 141–147. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D.; Firth, A.E. Solenopsis invicta virus 3: Pathogenesis and stage specificity in red imported fire ants. Virology 2014, 460, 66–71. [Google Scholar] [CrossRef]
- Al, H.e. Prevalence and Distribution of the Fire Ant Pathogen Solenopsis invicta Virus-3 in the Brazos Valley of Texas; Texas A & M University, Department of Entomology: College Station, TX, USA, 2022; manuscript in preparation; to be submitted. [Google Scholar]
- Wilson-Rich, N.; Dres, S.T.; Starks, P.T. The ontogeny of immunity: Development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 2008, 54, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015, 16, 83. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and dsRNA-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Brahma, A.; Leon, R.G.; Hernandez, G.L.; Wurm, Y. Larger, more connected societies of ants have a higher prevalence of viruses. Mol. Ecol. 2022, 31, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.P. Examining the Prevalence of Solenopsis Invicta Virus 3 (SINV-3) in Solenopsis invicta (Hymenoptera: Formicidae) Alates Collected in North Florida; Florida Agricultural and Mechanical University: Tallahassee, FL, USA, 2019. [Google Scholar]
- Valles, S.M.; Oi, D.H.; Weeks, R.D.; Addesso, K.M.; Oliver, J.B. Field evaluation of Solenopsis invicta virus 3 against its host Solenopsis invicta. J. Invertebr. Pathol. 2022, 191, 107767. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Advancement on techniques for the separation and maintenance of the red imported fire ant colonies. Insect Sci. 2007, 14, 1–4. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D. Identification of polygyne and monogyne fire ant colonies (Solenopsis invicta) by multiplex PCR of Gp-9 alleles. Insectes Sociaux 2003, 50, 199–200. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb. prot5439. [Google Scholar] [CrossRef]
- Valles, S.M.; Oi, D.H. Successful transmission of Solenopsis invicta virus 3 to field colonies of Solenopsis invicta (Hymenoptera: Formicidae). Fla. Entomol. 2014, 97, 1244–1246. [Google Scholar] [CrossRef]
- Dussutour, A.; Simpson, S.J. Communal nutrition in ants. Curr. Biol. 2009, 19, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Li, J.; Luo, L.; Kang, L.; Cui, F. The c-Jun N-terminal kinase pathway of a vector insect is activated by virus capsid protein and promotes viral replication. eLife 2017, 6, e26591. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Bachtel, N.D.; Hovsepian, G.A.; Nixon, D.F.; Eleftherianos, I. Allatostatin C modulates nociception and immunity in Drosophila. Sci. Rep. 2018, 8, 7501. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, H.; Lin, Z.-G.; Niu, Q.-S.; Wang, Z.; Gao, F.-C.; Ji, T. Carbendazim exposure during the larval stage suppresses major royal jelly protein expression in nurse bees (Apis mellifera). Chemosphere 2021, 266, 129011. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, B.-Y.; Kim, J.-M.; Choi, Y.-S.; Lee, M.-Y.; Lee, K.-S.; Jin, B.-R. Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion. Insects 2022, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Wen, Z. Cytochromes P450 of insects: The tip of the iceberg. Pest Manag. Sci. 2001, 57, 958–967. [Google Scholar] [CrossRef]
- Al, K.e. Quit Bugging Me: The Presence of Phorid Fly Parasitoids Affects Expression of Hymenoptaecin and Defensin-2 in Foraging Fire Ant Workers; Texas A & M University: College Station, TX, USA, 2022; manuscript in preparation; to be submitted. [Google Scholar]
- Strasser, P. Warburg Effect in Lymph Gland of Drosophila melanogaster upon Parasitoid Wasp Infection. Bachelor’s Thesis, University of South Bohemia České Budějovice, České Budějovice, Czech Repulic, 2016. [Google Scholar]
- Wang, L.; Su, M.; Zhao, X.; Hong, J.; Yu, X.; Xu, B.; Sheng, L.; Liu, D.; Shen, W.; Li, B. Nanoparticulate TiO2 protection of midgut damage in the silkworm (Bombyx mori) following phoxim exposure. Arch. Environ. Contam. Toxicol. 2015, 68, 534–542. [Google Scholar] [CrossRef]
- Erler, S.; Popp, M.; Lattorff, H.M.G. Dynamics of immune system gene expression upon bacterial challenge and wounding in a social insect (Bombus terrestris). PLoS ONE 2011, 6, e18126. [Google Scholar] [CrossRef]
- Choe, K.-M.; Werner, T.; Stöven, S.; Hultmark, D.; Anderson, K.V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002, 296, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002, 416, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Zaidman-Rémy, A.; Hervé, M.; Poidevin, M.; Pili-Floury, S.; Kim, M.-S.; Blanot, D.; Oh, B.-H.; Ueda, R.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 2006, 24, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila imd signaling pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- Evans, J.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Baty, J.W.; Bulgarella, M.; Dobelmann, J.; Felden, A.; Lester, P.J. Viruses and their effects in ants (Hymenoptera: Formicidae). Myrmecol. News 2020, 30, 213–228. [Google Scholar]
- Lester, P.J.; Buick, K.H.; Baty, J.W.; Felden, A.; Haywood, J. Different bacterial and viral pathogens trigger distinct immune responses in a globally invasive ant. Sci. Rep. 2019, 9, 5780. [Google Scholar] [CrossRef]
- Rosales, C.; Vonnie, S. Cellular and molecular mechanisms of insect immunity. In Insect Physiology and Ecology; IntechOpen: London, UK, 2017; pp. 179–212. [Google Scholar]
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.; Bull, J.C. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Yang, X.; Nino, E.L.; Yi, S.; Grozinger, C. Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog. 2015, 11, e1004713. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.E.; Clem, R.J. Insect defenses against virus infection: The role of apoptosis. Int. Rev. Immunol. 2003, 22, 401–424. [Google Scholar] [CrossRef]
- Nainu, F.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y. Protection of insects against viral infection by apoptosis-dependent phagocytosis. J. Immunol. 2015, 195, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Settles, E.W.; Friesen, P.D. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. J. Virol. 2008, 82, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Bertin, J.; Mendrysa, S.M.; LaCount, D.J.; Gaur, S.; Krebs, J.F.; Armstrong, R.C.; Tomaselli, K.J.; Friesen, P.D. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J. Virol. 1996, 70, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Vander Meer, R.K.; Coy, M.; Scharf, M.E. Phenotypic impacts of PBAN RNA interference in an ant, Solenopsis invicta, and a moth, Helicoverpa zea. J. Insect Physiol. 2012, 58, 1159–1165. [Google Scholar] [CrossRef]




| Sample Identity | Total Reads | Total Reads Mapped to S. invicta Genome |
|---|---|---|
| Infected colony #1 | 41059816 | 36869843 (89.8%) |
| Infected colony #2 | 63672854 | 35140919 (55.19%) |
| Infected colony #3 | 43475766 | 37892796 (87.16%) |
| Infected colony #4 | 41430720 | 37170634 (89.72%) |
| Infected colony #5 | 50718212 | 45360352 (89.44%) |
| Infected colony #6 | 52744884 | 42313142 (80.22%) |
| Uninfected colony #1 | 43370224 | 36954917 (85.21%) |
| Uninfected colony #2 | 52867232 | 46842630 (88.6%) |
| Uninfected colony #3 | 50842784 | 42388449 (83.37%) |
| Uninfected colony #4 | 59334108 | 38106930 (64.22%) |
| Uninfected colony #5 | 52962938 | 44766349 (84.52%) |
| Uninfected colony #6 | 37330324 | 32719501 (87.65%) |
| Upregulated Components of the Glycolysis Pathway | ||
|---|---|---|
| Figure Identification Number | Gene ID | Pathway Component Identification |
| 5.4.2.2 | 105199081 | phosphoglucomutase-2 |
| 2.7.1.1 | 105202325 | hexokinase-2 |
| 2.7.1.147 | 105205417 | ADP-dependent glucokinase |
| 5.1.3.3 | 105199419 | aldose 1-epimerase isoform X1 |
| 5.1.3.15 | 105207919 | glucose-6-phosphate 1-epimerase |
| 5.3.1.9 | 105206436 | glucose-6-phosphate isomerase |
| 3.1.3.11 | 105198274 | fructose-1,6-bisphosphatase 1 |
| 2.7.1.11 | 105204005 | ATP-dependent 6-phosphofructokinase isoform X2 |
| 4.1.2.13 | 105198376 | fructose-bisphosphate aldolase isoform X1 |
| 5.3.1.1 | 105192814 | triosephosphate isomerase |
| 1.2.1.12 | 105196341 | glyceraldehyde-3-phosphate dehydrogenase 2-like |
| 2.7.2.3 | 105198397 | phosphoglycerate kinase |
| 5.4.2.11 | 105193833 | phosphoglycerate mutase 2 |
| 3.1.3.80 | 105194144 | multiple inositol polyphosphate phosphatase 1 isoform X1 |
| 4.2.1.11 | 105197049 | enolase |
| 4.1.1.32 | 105192993 | phosphoenolpyruvate carboxykinase |
| 2.7.1.40 | 105201593 | pyruvate kinase isoform X1 |
| 2.3.1.12 | 105197159 | dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex, mitochondrial isoform X1 |
| 1.2.4.1 | 105198508 | pyruvate dehydrogenase E1 component subunit beta, mitochondrial-like |
| 1.1.1.27 | 105201857 | L-lactate dehydrogenase B chain |
| 6.2.1.1 | 105199849 | acetyl-coenzyme A synthetase |
| 1.8.1.4 | 105198927 | dihydrolipoyl dehydrogenase, mitochondrial |
| 1.2.1.3 | 105192904 | retinal dehydrogenase 1 |
| 1.1.1.1 | 105194743 | alcohol dehydrogenase class-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, V.R.; Johnston, J.S. Differential Gene Expression of Innate Immune Response Genes Consequent to Solenopsis invicta Virus-3 Infection. Genes 2023, 14, 188. https://doi.org/10.3390/genes14010188
Holmes VR, Johnston JS. Differential Gene Expression of Innate Immune Response Genes Consequent to Solenopsis invicta Virus-3 Infection. Genes. 2023; 14(1):188. https://doi.org/10.3390/genes14010188
Chicago/Turabian StyleHolmes, V. Renee, and J. Spencer Johnston. 2023. "Differential Gene Expression of Innate Immune Response Genes Consequent to Solenopsis invicta Virus-3 Infection" Genes 14, no. 1: 188. https://doi.org/10.3390/genes14010188
APA StyleHolmes, V. R., & Johnston, J. S. (2023). Differential Gene Expression of Innate Immune Response Genes Consequent to Solenopsis invicta Virus-3 Infection. Genes, 14(1), 188. https://doi.org/10.3390/genes14010188






