Diagnostic Yield of Genetic Testing for Ocular and Oculocutaneous Albinism in a Diverse United States Pediatric Population
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronskov, K.; Ek, J.; Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet J. Rare Dis. 2007, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Bodurtha, J.N. The Wills Eye Handbook of Ocular Genetics, 1st ed.; Thieme Medical Publishers: New York, NY, USA, 2018; Volume 4, pp. 191–192. [Google Scholar]
- Tomita, Y.; Takeda, A.; Okinaga, S.; Tagami, H.; Shibahara, S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem. Biophys. Res. Commun. 1989, 164, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Rinchik, E.M.; Bultman, S.J.; Horsthemke, B.; Lee, S.T.; Strunk, K.M.; Spritz, R.A.; Avidano, K.M.; Jong, M.T.; Nicholls, R.D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature 1993, 361, 72–76. [Google Scholar] [CrossRef]
- Rosenberg, T.; Schwartz, M. X-linked ocular albinism: Prevalence and mutations—A national study. Eur. J. Hum. Genet. 1998, 6, 570–577. [Google Scholar] [CrossRef]
- Introne, W.; Boissy, R.E.; Gahl, W.A. Clinical, Molecular, and Cell Biological Aspects of Chediak–Higashi Syndrome. Mol. Genet. Metab. 1999, 68, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, H.; Yang, L.; Sun, Z.; Yuan, Z.; Li, H.; Sui, R. Molecular genetic and clinical evaluation of three Chinese families with X-linked ocular albinism. Sci. Rep. 2017, 7, 33713. [Google Scholar] [CrossRef]
- Lasseaux, E.; Plaisant, C.; Michaud, V.; Pennamen, P.; Trimouille, A.; Gaston, L.; Monferme, S.; Lacombe, D.; Rooryck, C.; Morice-Picard, F.; et al. Molecular characterization of a series of 990 index patients with albinism. Pigment Cell Melanoma Res. 2018, 31, 466–474. [Google Scholar] [CrossRef]
- Campbell, P.; Ellingford, J.M.; Parry, N.R.A.; Fletcher, T.; Ramsden, S.C.; Gale, T.; Hall, G.; Smith, K.; Kasperaviciute, D.; Thomas, E.; et al. Clinical and genetic variability in children with partial albinism. Sci. Rep. 2019, 9, 16576. [Google Scholar] [CrossRef]
- Lenassi, E.; Clayton-Smith, J.; Douzgou, S.; Ramsden, S.C.; Ingram, S.; Hall, G.; Hardcastle, C.L.; Fletcher, T.A.; Taylor, R.L.; Ellingford, J.M.; et al. Clinical utility of genetic testing in 201 preschool children with inherited eye disorders. Genet. Med. 2020, 22, 745–751. [Google Scholar] [CrossRef]
- Jackson, D.; Malka, S.; Harding, P.; Palma, J.; Dunbar, H.; Moosajee, M. Molecular diagnostic challenges for non-retinal developmental eye disorders in the United Kingdom. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 578–589. [Google Scholar] [CrossRef]
- Chan, H.W.; Schiff, E.R.; Tailor, V.K.; Malka, S.; Neveu, M.M.; Theodorou, M.; Moosajee, M. Prospective Study of the Phenotypic and Mutational Spectrum of Ocular Albinism and Oculocutaneous Albinism. Genes 2021, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Hovnik, T.; Debeljak, M.; Tekavčič Pompe, M.; Bertok, S.; Battelino, T.; Stirn Kranjc, B.; Trebušak Podkrajšek, K. Genetic Variability in Slovenian Cohort of Patients with Oculocutaneous Albinism. Acta Chim. Slov. 2021, 68, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Pennamen, P.; Tingaud-Sequeira, A.; Gazova, I.; Keighren, M.; McKie, L.; Marlin, S.; Halem, S.G.; Kaplan, J.; Delevoye, C.; Lacombe, D.; et al. Dopachrome tautomerase variants in patients with oculocutaneous albinism. Genet. Med. 2021, 23, 479–487. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Fokkema, I.; Kroon, M.; Lopez Hernandez, J.A.; Asscheman, D.; Lugtenburg, I.; Hoogenboom, J.; den Dunnen, J.T. The LOVD3 platform: Efficient genome-wide sharing of genetic variants. Eur. J. Hum. Genet. 2021, 29, 1796–1803. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Pejaver, V.; Byrne, A.B.; Feng, B.J.; Pagel, K.A.; Mooney, S.D.; Karchin, R.; O’Donnell-Luria, A.; Harrison, S.M.; Tavtigian, S.V.; Greenblatt, M.S.; et al. Calibration of computational tools for missense variant pathogenicity classification and ClinGen recommendations for PP3/BP4 criteria. Am. J. Hum. Genet. 2022, 109, 2163–2177. [Google Scholar] [CrossRef]
- Shapiro, M.B.; Senapathy, P. RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987, 15, 7155–7174. [Google Scholar] [CrossRef]
- Yeo, G.; Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved splice site detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Lin, X.; Salzberg, S.L. GeneSplicer: A new computational method for splice site prediction. Nucleic Acids Res. 2001, 29, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, K.; Kyriazopoulou Panagiotopoulou, S.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Poulter, J.A.; Al-Araimi, M.; Conte, I.; Van Genderen, M.M.; Sheridan, E.; Carr, I.M.; Parry, D.A.; Shires, M.; Carrella, S.; Bradbury, J.; et al. Recessive mutations in SLC38A8 cause foveal hypoplasia and optic nerve misrouting without albinism. Am. J. Hum. Genet. 2013, 93, 1143–1150. [Google Scholar] [CrossRef]

| Characteristic | N (%) |
|---|---|
| Age (years ± standard deviation) | 1.3 ± 2.0 |
| Sex | |
| Male | 35 (66) |
| Female | 18 (34) |
| Family history of suspected albinism | 17/48 (35) |
| Clinical diagnosis | |
| Oculocutaneous albinism | 41 (77) |
| Ocular albinism | 12 (23) |
| Ophthalmic exam findings | |
| Nystagmus | 47 (89) |
| Fundus hypopigmentation | 36 (68) |
| Foveal hypoplasia | 45 (85) |
| Iris transillumination defects | 20 (38) |
| Number of Patients with Positive Diagnostic Yield (%) | Number of Patients with Negative Diagnostic Yield (%) | p-Value | |
|---|---|---|---|
| Overall After variant reclassification | 35 (66) 37 (70) | 18 (34) 16 (30) | -- |
| Cutaneous involvement | |||
| Yes No | 31 (76) 4 (33) | 10 (24) 8 (67) | 0.007 * |
| Ocular Manifestations | |||
| Nystagmus Fundus hypopigmentation Foveal hypoplasia Iris transillumination defects | 33 (70) 25 (69) 31 (69) 16 (80) | 14 (30) 11 (31) 14 (31) 4 (20) | 0.82 |
| Race and ethnicity | |||
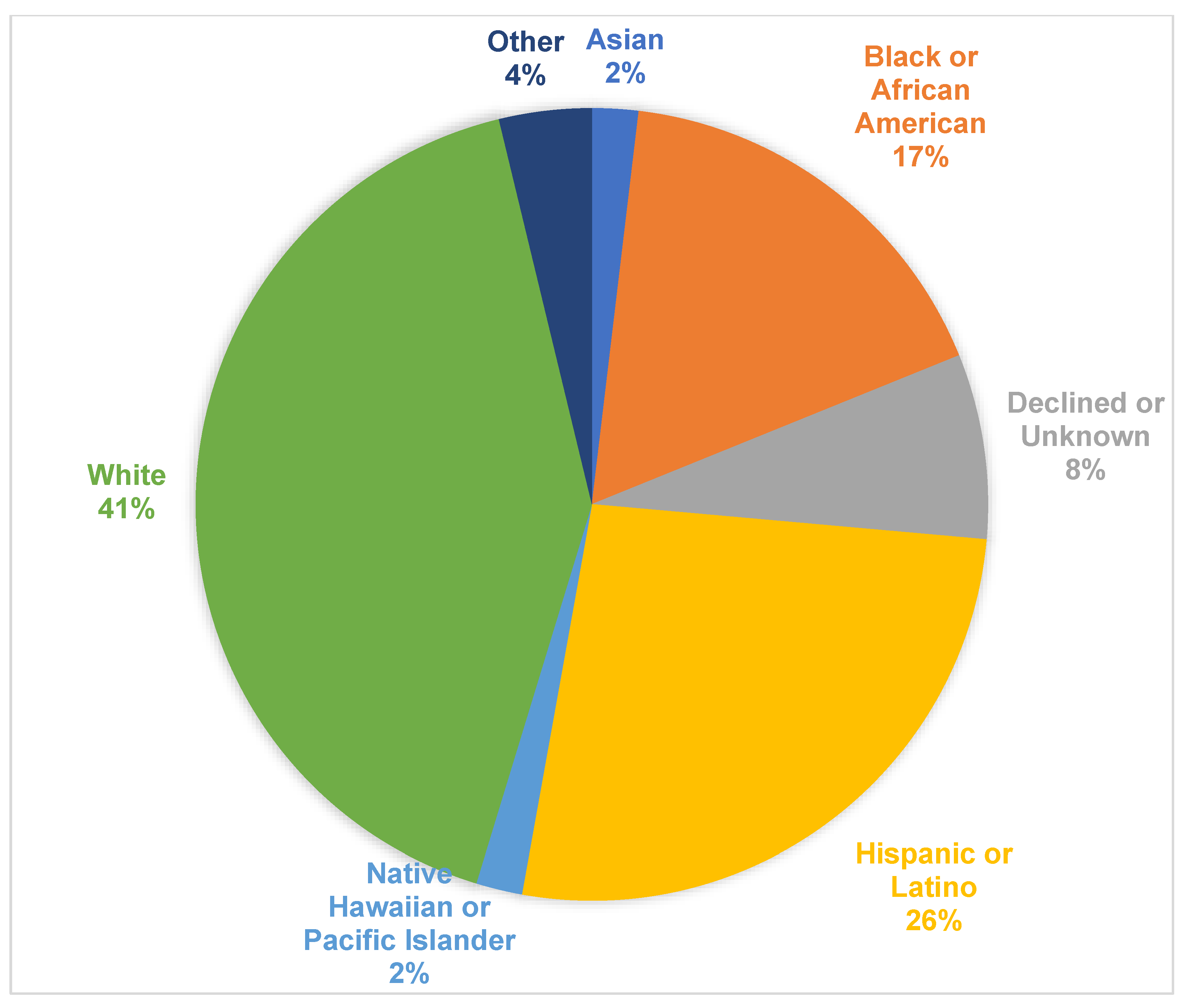
| Asian Black or African American Declined or Unknown Hispanic or Latino Native Hawaiian or Pacific Islander Other White | 0 (0) 7 (78) 3 (75) 9 (64) 1 (100) 2 (100) 13 (59) | 1 (100) 2 (22) 1 (25) 5 (36) 0 (0) 0 (0) 9 (41) | 0.59 |
| Gene | N (%) |
|---|---|
| OCA2 (%) | 15 (28) |
| TYR (%) | 11 (20) |
| HPS5 (%) | 3 (6) |
| TYRP1 (%) | 2 (4) |
| HPS1 (%) | 1 (2) |
| HPS6 (%) | 1 (2) |
| SLC45A2 (%) | 1 (2) |
| OA1/GPR143 (%) | 1 (2) |
| % Taken from total number of patients in the study (53) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.S.; Bohnsack, B.L.; Ing, A.; Drackley, A.; Castelluccio, V.; Zhang, K.X.; Ralay-Ranaivo, H.; Rossen, J.L. Diagnostic Yield of Genetic Testing for Ocular and Oculocutaneous Albinism in a Diverse United States Pediatric Population. Genes 2023, 14, 135. https://doi.org/10.3390/genes14010135
Chan KS, Bohnsack BL, Ing A, Drackley A, Castelluccio V, Zhang KX, Ralay-Ranaivo H, Rossen JL. Diagnostic Yield of Genetic Testing for Ocular and Oculocutaneous Albinism in a Diverse United States Pediatric Population. Genes. 2023; 14(1):135. https://doi.org/10.3390/genes14010135
Chicago/Turabian StyleChan, Kyle S., Brenda L. Bohnsack, Alexander Ing, Andy Drackley, Valerie Castelluccio, Kevin X. Zhang, Hanta Ralay-Ranaivo, and Jennifer L. Rossen. 2023. "Diagnostic Yield of Genetic Testing for Ocular and Oculocutaneous Albinism in a Diverse United States Pediatric Population" Genes 14, no. 1: 135. https://doi.org/10.3390/genes14010135
APA StyleChan, K. S., Bohnsack, B. L., Ing, A., Drackley, A., Castelluccio, V., Zhang, K. X., Ralay-Ranaivo, H., & Rossen, J. L. (2023). Diagnostic Yield of Genetic Testing for Ocular and Oculocutaneous Albinism in a Diverse United States Pediatric Population. Genes, 14(1), 135. https://doi.org/10.3390/genes14010135






