ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity
Abstract
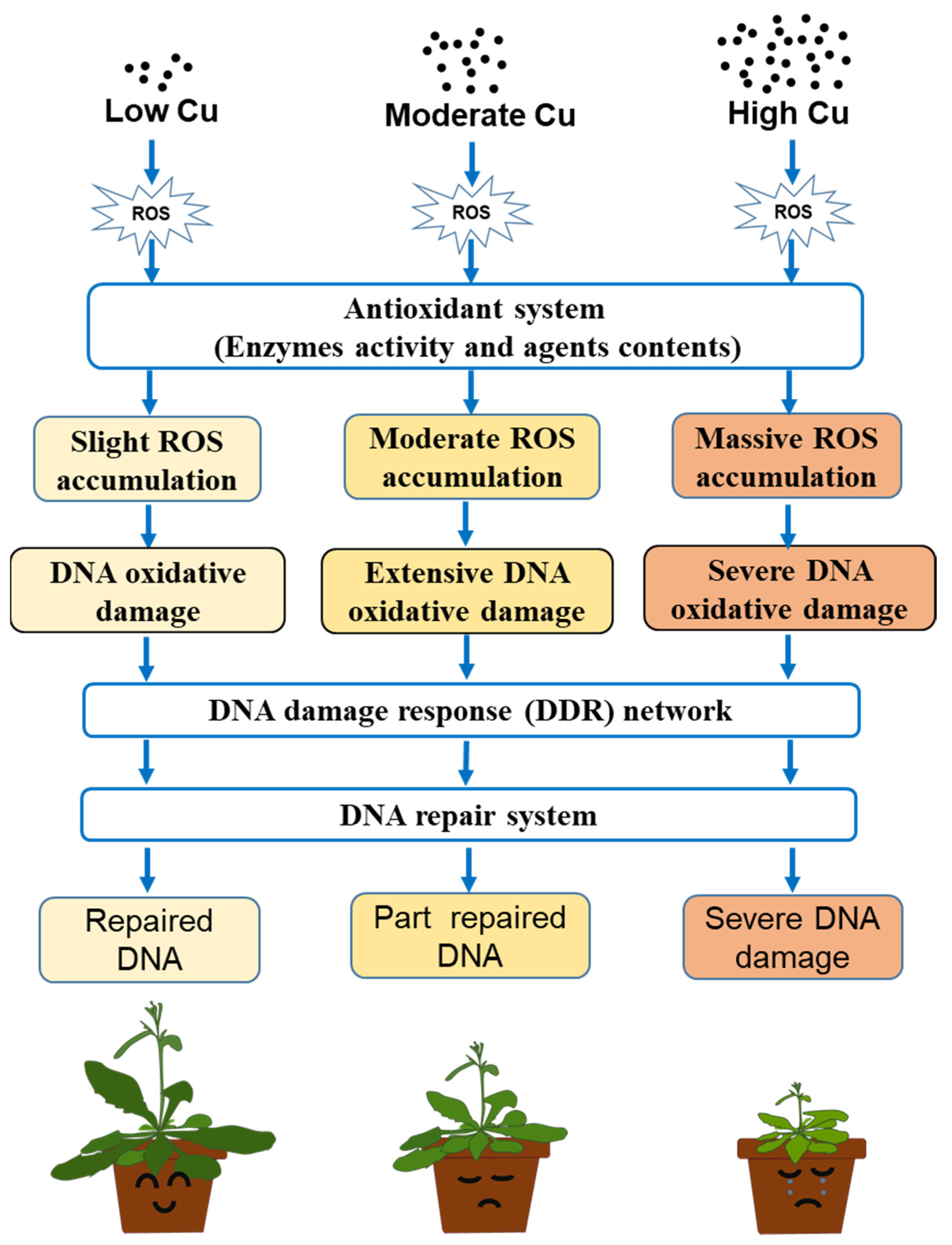
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Cu Treatment
2.2. Determination of Physiological Indices
2.3. Histochemical Localization Analysis
2.4. RNA Isolation and qRT-PCR Analysis
2.5. Statistical Analysis
3. Results and Discussions
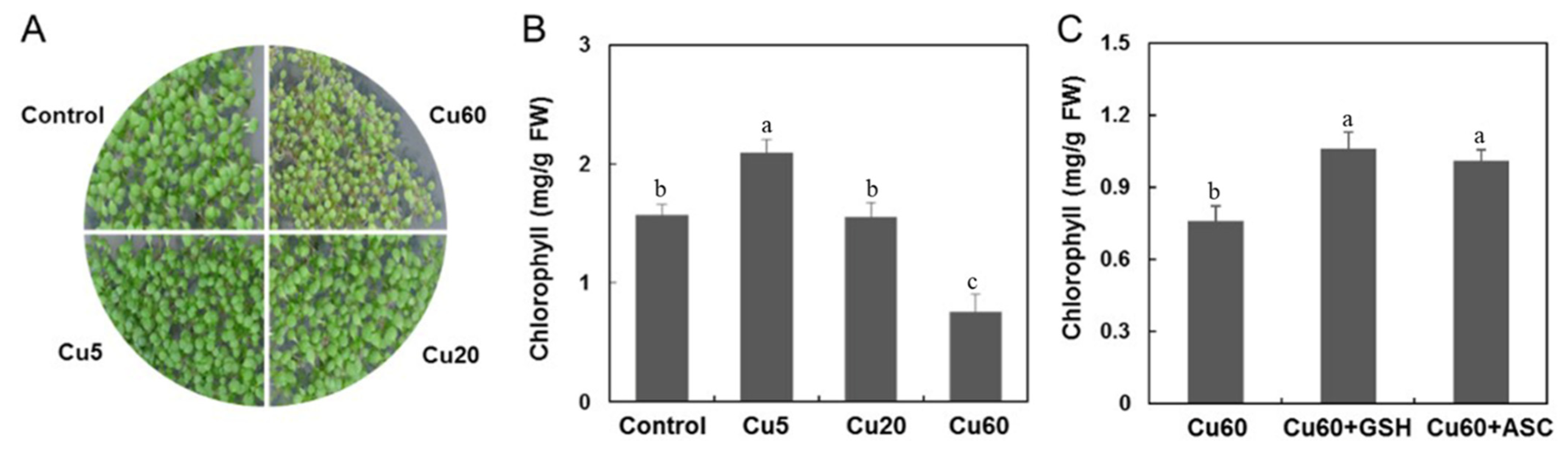
3.1. Reproductive Performance of Arabidopsis upon Cu Treatment
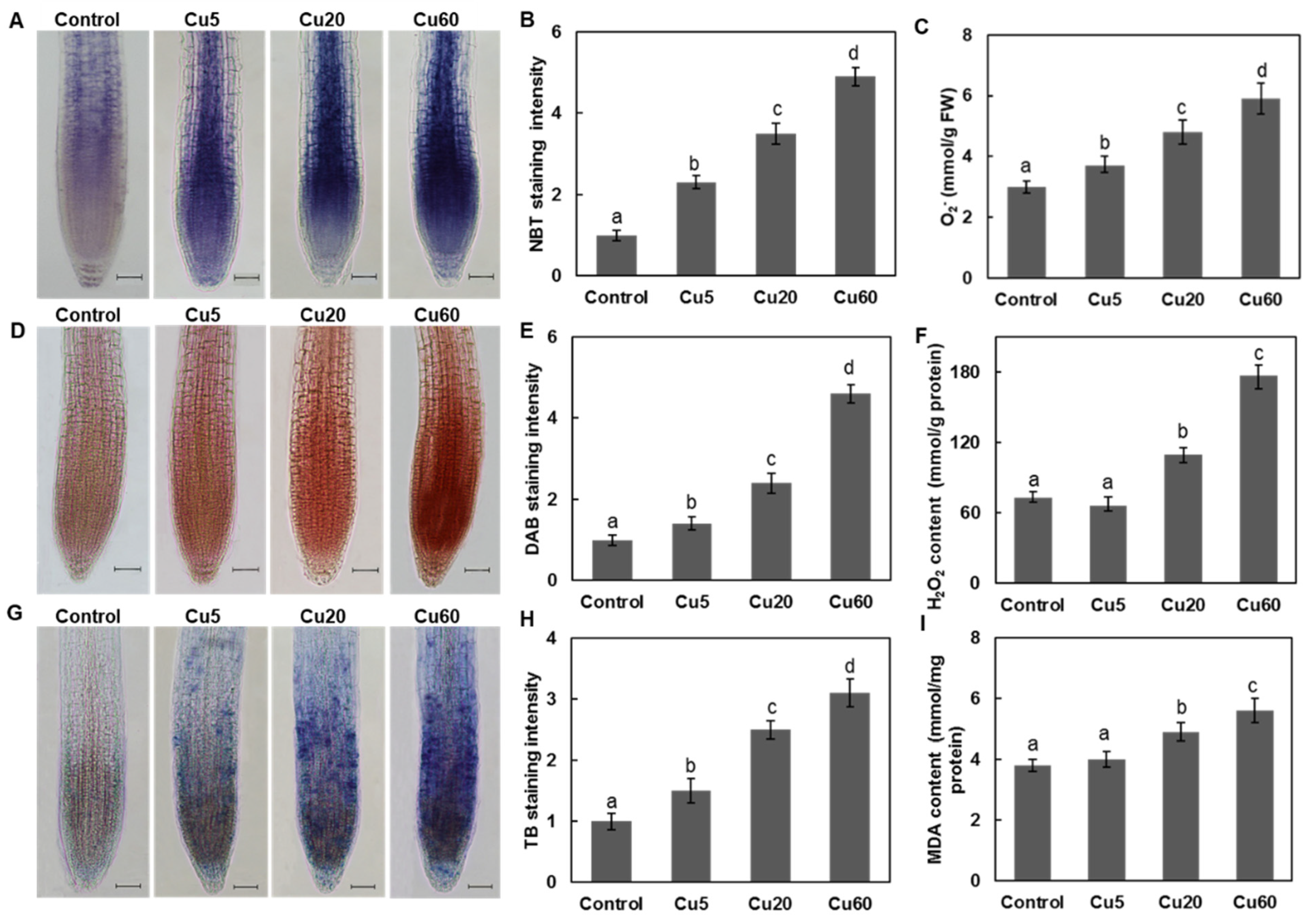
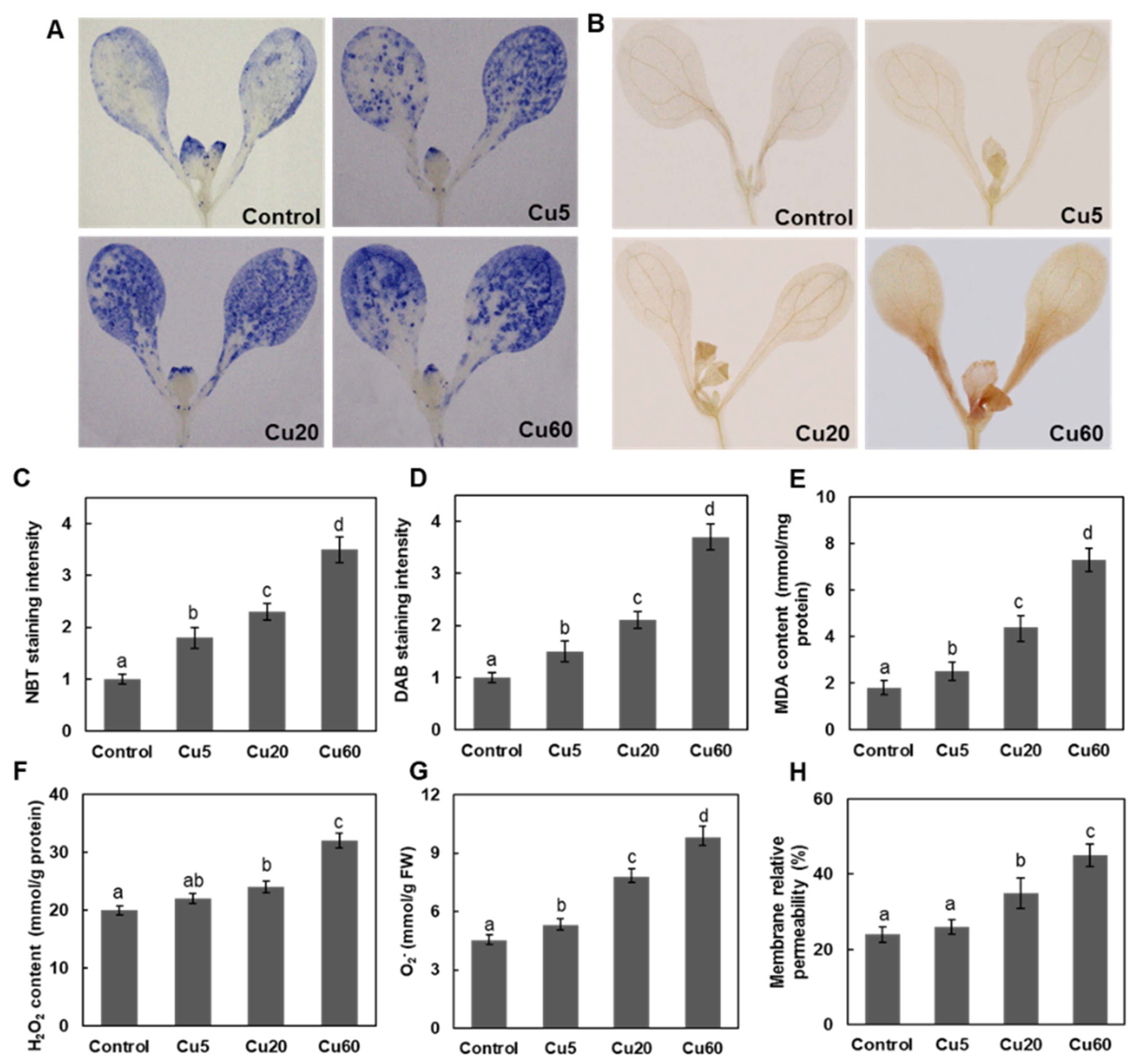
3.2. Dose-Dependent Accumulation of ROS Induced by Cu in Arabidopsis Seedlings
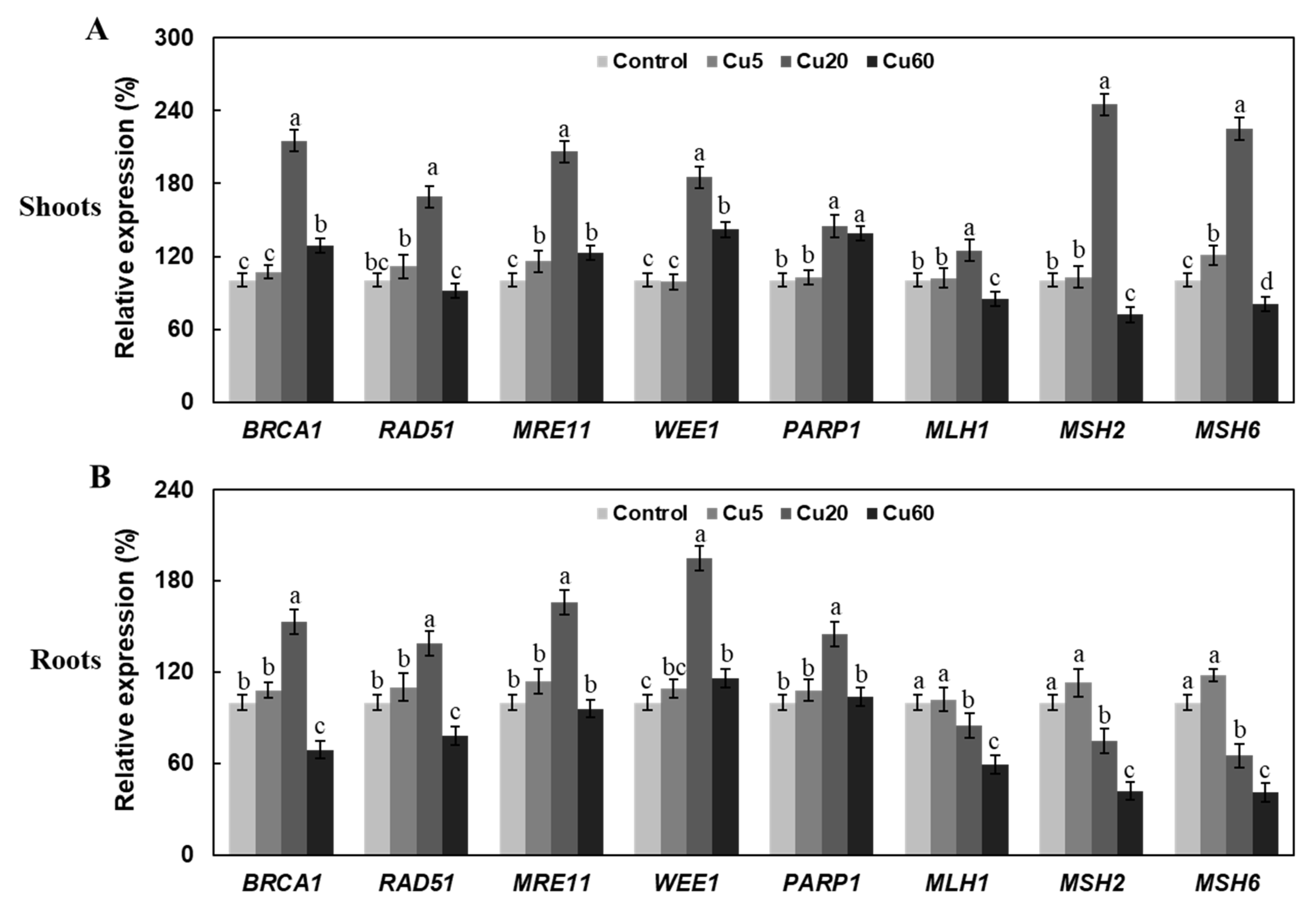
3.3. Cu Induces Oxidative DNA Damage Response in Arabidopsis Seedlings
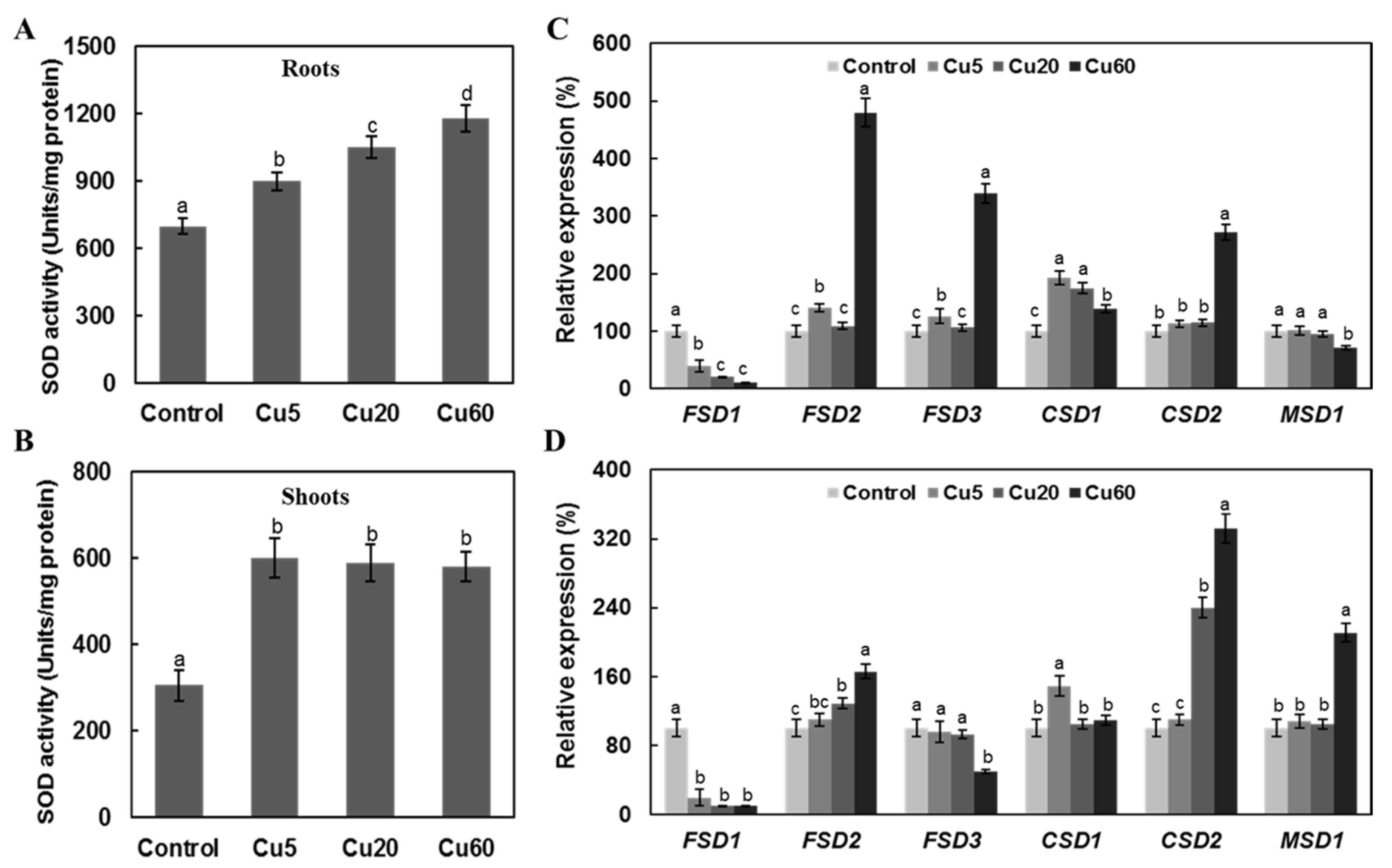
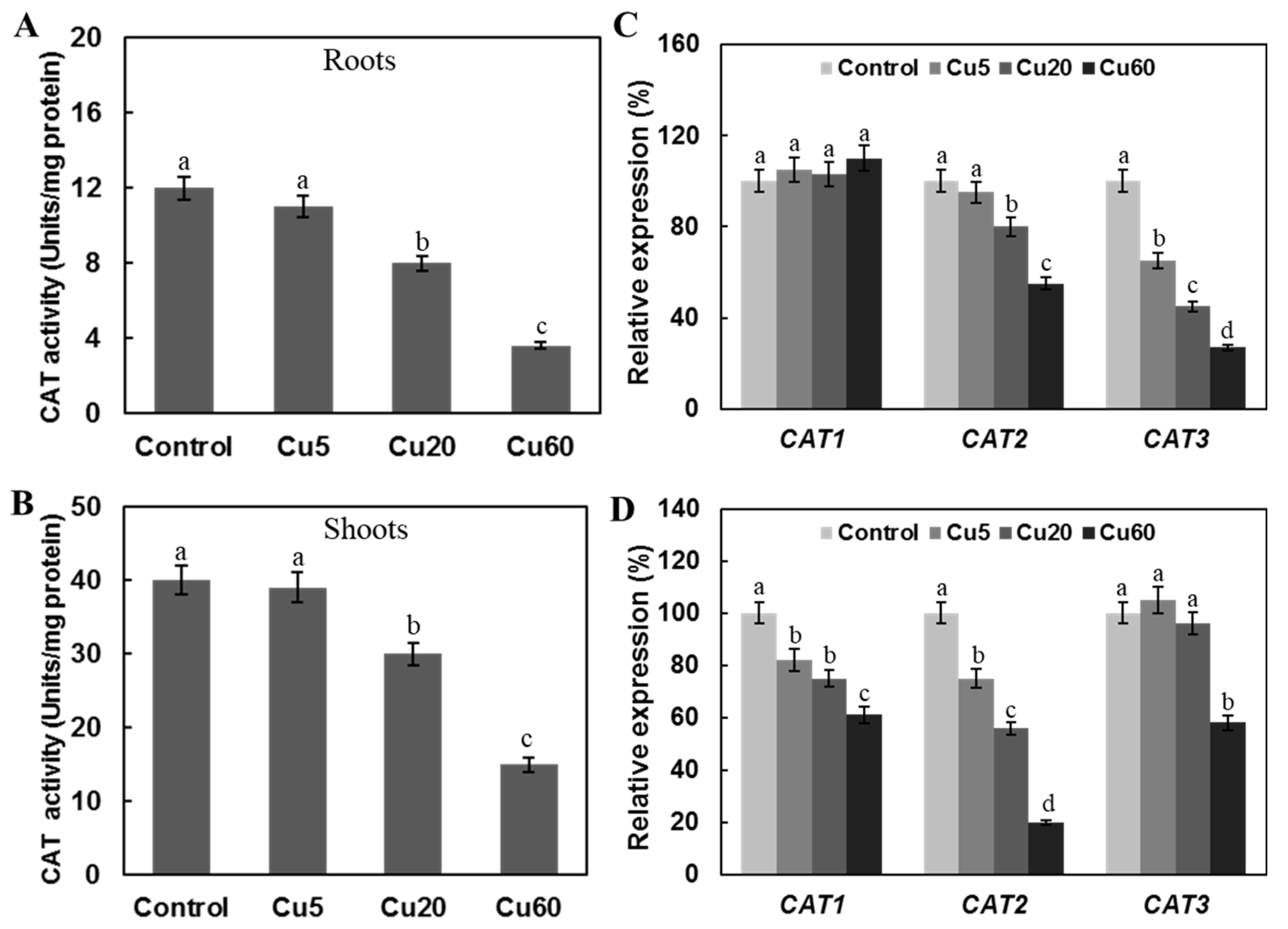
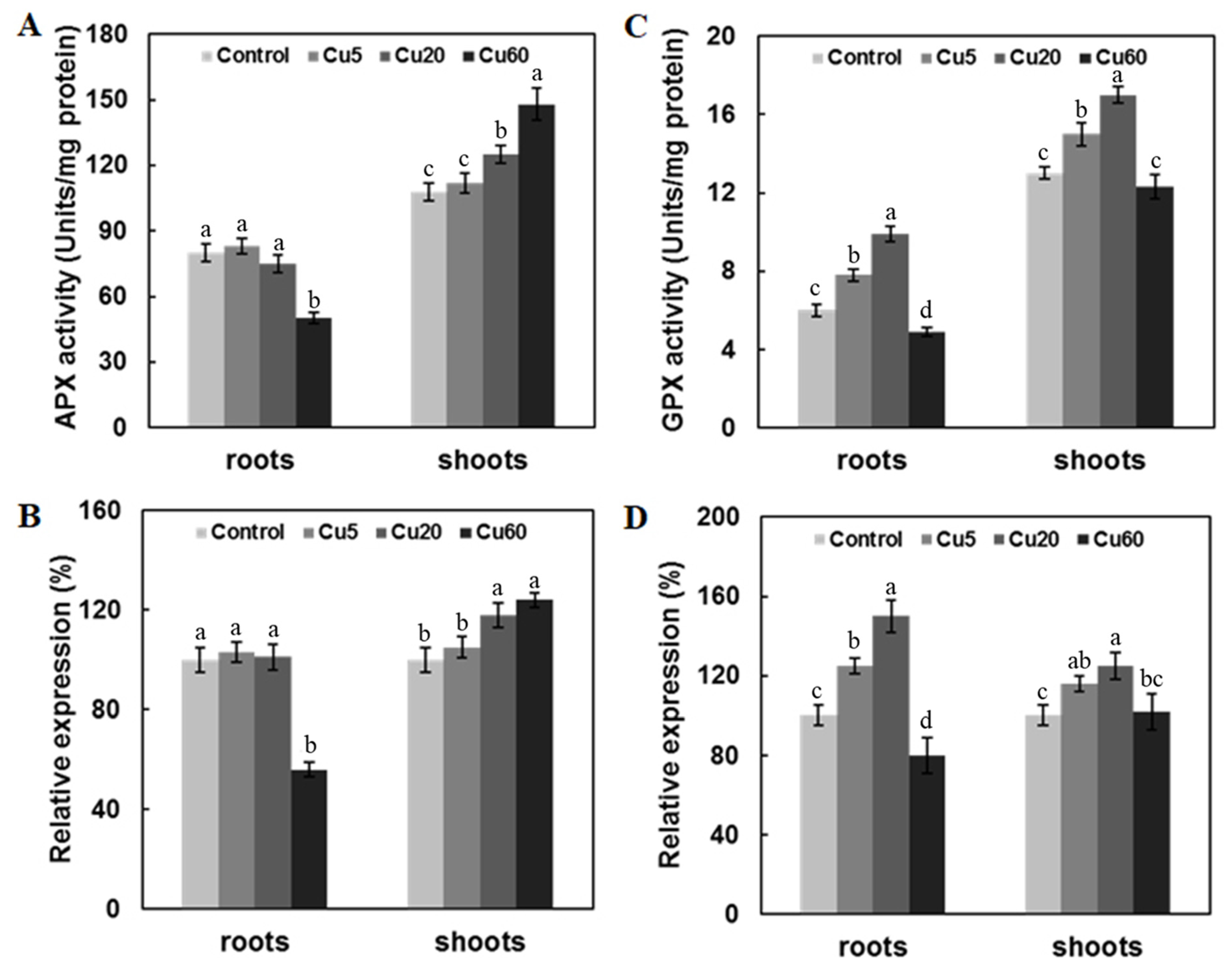
3.4. Cu Regulates Antioxidative Enzyme Activity in Arabidopsis Seedlings
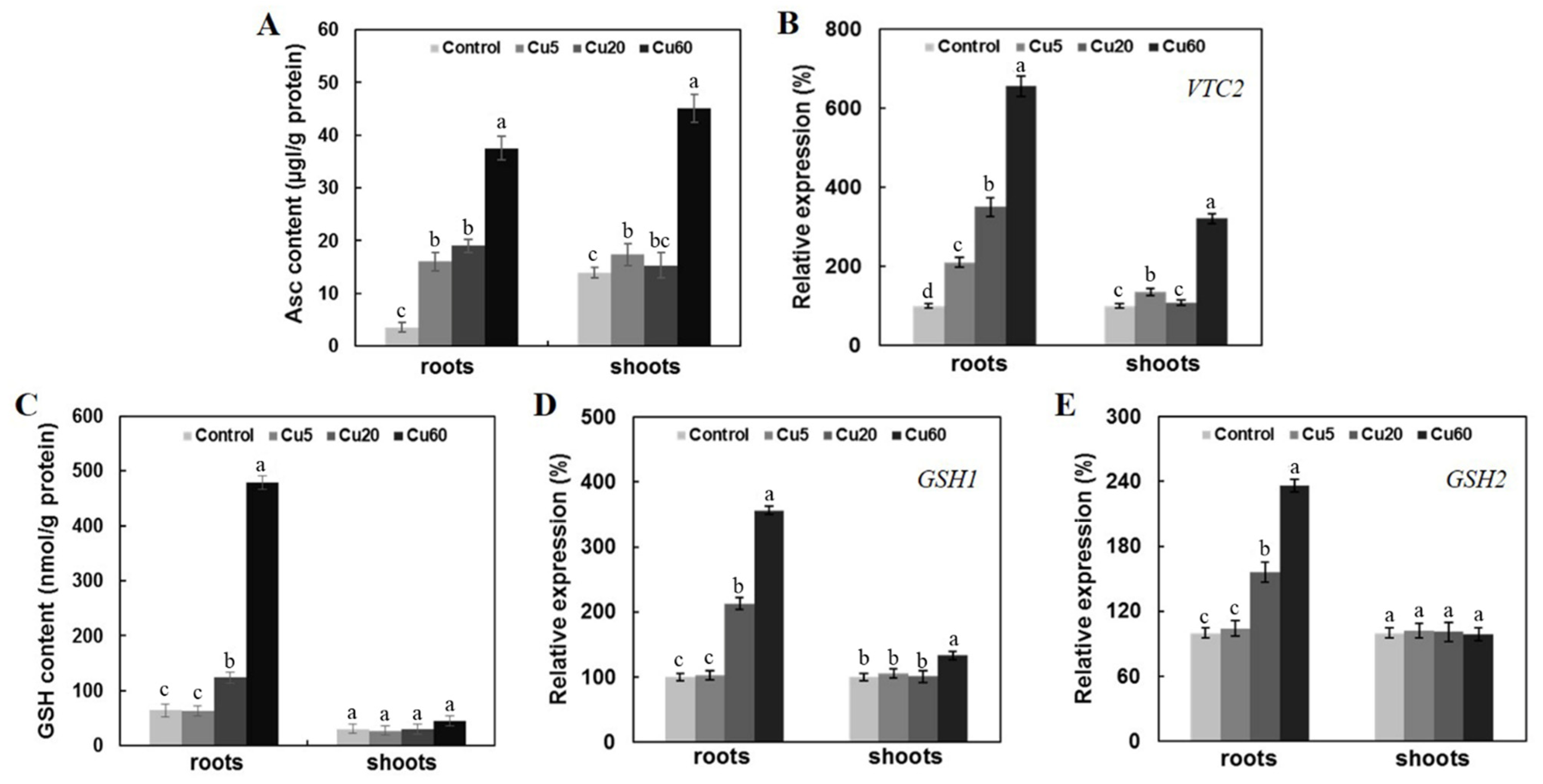
3.5. Cu-Induced Changes of AsA and GSH Contents in Arabidopsis Seedlings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Reis, R.; Hendrix, S.; Mourato, M.P.; Martins, L.L.; Vangronsveld, J.; Cuypers, A. Efficient regulation of copper homeostasis underlies accession-specific sensitivities to excess copper and cadmium in roots of Arabidopsis thaliana. J. Plant Physiol. 2021, 261, 153434. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, L.; Dai, T.; Zhou, J.; Li, Z. Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol. Environ. Saf. 2019, 171, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, L.; Yang, S.; Wang, C.; Zhang, T.; Wang, J. Dose-dependent sensitivity of Arabidopsis thaliana seedling root to copper is regulated by auxin homeostasis. Environ. Exp. Bot. 2017, 139, 23–30. [Google Scholar] [CrossRef]
- Zehra, A.; Choudhary, S.; Wani, K.I.; Naeem, M.; Aftab, T. Exogenous abscisic acid mediates ROS homeostasis and maintains glandular trichome to enhance artemisinin biosynthesis in Artemisia annua under copper toxicity. Plant Physiol. Bioch. 2020, 156, 125–134. [Google Scholar] [CrossRef]
- Pető, A.; Lehotai, N.; Feigl, G.; Tugyi, N.; Ördög, A.; Gémes, K.; Tari, I.; Erdei, L.; Kolbert, Z. Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep. 2013, 32, 1913–1923. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wójcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.; Xia, X.; Wang, M.; Yin, X.; Zhang, B.; Zhang, X.; Cui, Y. Deciphering the physiological and molecular mechanisms for copper tolerance in autotetraploid Arabidopsis. Plant Cell Rep. 2017, 36, 1585–1597. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase—Representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. R. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.; Prasad, M. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Teisseire, H.; Vernet, G. Ascorbate and glutathione contents in duckweed, Lemna minor, as biomarkers of the stress generated by copper, folpet and diuron. Biomarkers 2000, 5, 263–273. [Google Scholar] [CrossRef]
- Hu, Z.; Cools, T.; De Veylder, L. Mechanisms used by plants to cope with DNA damage. Ann. Rev. Plant Biol. 2016, 29, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.U.; Huang, Y.; Benhamed, M.; Raynaud, C. The plant DNA damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Pedroza-Garcia, J.A.; Xiang, Y.; De Veylder, L. Cell cycle checkpoint control in response to DNA damage by environmental stresses. Plant J. 2022, 109, 490–507. [Google Scholar] [CrossRef]
- Wang, Q.; La, Y.; Xia, H.; Zhou, S.; Zhai, Z.; La, H. Roles of MEM1 in safeguarding Arabidopsis genome against DNA damage, inhibiting ATM/SOG1-mediated DNA damage response, and antagonizing global DNA hypermethylation. J. Integr. Plant Biol. 2022, 64, 87–104. [Google Scholar] [CrossRef]
- Cools, T.; Iantcheva, A.; Weimer, A.K.; Boens, S.; Takahashi, N.; Maes, S.; Van den Daele, H.; Van Isterdael, G.; Schnittger, A.; De Veylder, L. The Arabidopsis thaliana checkpoint kinase WEE1 protects against premature vascular differentiation during replication stress. Plant Cell. 2011, 23, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Amiard, S.; Charbonnel, C.; Allain, E.; Depeiges, A.; White, C.I.; Gallego, M.E. Distinct roles of the ATR kinase and the Mre11-Rad50-Nbs1 complex in the maintenance of chromosomal stability in Arabidopsis. Plant Cell. 2010, 22, 3020–3033. [Google Scholar] [CrossRef]
- Shen, M.; Nie, Y.; Chen, Y.; Zhang, X.; Zhao, J. OsMre11 is required for mitosis during rice growth and development. Int. J. Mol. Sci. 2021, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Black, B.E. Rapid detection and signaling of DNA damage by PARP-1. Trends Biochem. Sci. 2021, 46, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nan, N.; Shi, L.; Li, N.; Huang, S.; Zhang, A.; Liu, Y.; Guo, P.; Liu, B.; Xu, Z.Y. Arabidopsis BRCA1 represses RRTF1-mediated ROS production and ROS-responsive gene expression under dehydration stress. New Phytol. 2020, 228, 1591–1610. [Google Scholar] [CrossRef]
- Maksymiec, W.; Krupa, Z. The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ. Exp. Bot. 2006, 57, 187–194. [Google Scholar] [CrossRef]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef]
- Wang, J.; Moeen-Ud-Din, M.; Yang, S. Dose-dependent responses of Arabidopsis thaliana to zinc are mediated by auxin homeostasis and transport. Environ. Exp. Bot. 2021, 189, 104554. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Wang, J.; Tian, L.; Li, F.; Liu, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Huang, Y.; et al. C60 fullerols enhance copper toxicity and alter the leaf metabolite and protein profile in Cucumber. Environ. Sci. Technol. 2019, 53, 2171–2180. [Google Scholar] [CrossRef]
- Grellet, C.F.; Bournonville, J.; Díaz-Ricci, C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Guan, Z.Q.; Chai, T.Y.; Zhang, Y.X.; Xu, J.; Wei, W. Enhancement of Cd tolerance in transgenic tobacco plants overexpressing a Cd-induced catalase cDNA. Chemosphere 2009, 76, 623–630. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, W.; Bao, L.; Wang, Y.; Sodmergen, K.C.; Han, Z.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.J.; Lo, J.C.; Yeh, K.C. Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol. Bioch. 2012, 159, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Sancenon, V.; Puig, S.; Mateu-Andres, I.; Dorcey, E.; Thiele, D.J.; Penarrubia, L. The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J. Biol. Chem. 2004, 279, 15348. [Google Scholar] [CrossRef]
- Cuypers, A.; Karen, S.; Jos, R.; Kelly, O.; Els, K.; Tony, R.; Nele, H.; Nathalie, V.; Yves, G.; Jan, C.; et al. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant. Physiol. 2011, 168, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Drazkiewicz, M.; Skórzyńska-Polit, E.; Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. BioMetals 2004, 17, 379–387. [Google Scholar] [CrossRef]
- Choudhary, M.; Jetley, U.K.; Khan, M.A.; Zutshi, S.; Fatma, T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotox. Environ. Saf. 2007, 66, 204–209. [Google Scholar] [CrossRef]
- Rodrigo-Moreno, A.; Andrés-Colás, N.; Poschenrieder, C.; Gunsé, B.; Penarrubia, L.; Shabala, S. Calcium- and potassium-permeable plasma membrane transporters are activated by copper in Arabidopsis root tips: Linking copper transport with cytosolic hydroxyl radical production. Plant Cell Environ. 2013, 36, 844–855. [Google Scholar] [CrossRef]
- De Schutter, K.; Joubès, J.; Cools, T.; Verkest, A.; Corellou, F.; Babiychuk, E.; Van Der Schueren, E.; Beeckman, T.; Kushnir, S.; Inze, D.; et al. Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA Integrity checkpoint. Plant Cell. 2007, 19, 211–225. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Zhuang, D.; Zhu, H.; Du, Y.; Cheng, Z.; Cui, W.; Rogers, H.J.; Zhang, Q.; Jia, C.; et al. Roles of MSH2 and MSH6 in cadmium-induced G2/M checkpoint arrest in Arabidopsis roots. Chemosphere 2018, 201, 586–594. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Smeets, K.; Opdenakker, K.; Remans, T.; Sanden, S.V.; Belleghem, F.V.; Semane, B.; Horemans, N.; Guisez, Y.; Vangronsveld, J.; Cuypers, A. Oxidative stress-related responses at transcriptional and enzymatic levels after exposure to Cd or Cu in a multipollution context. J. Plant. Physiol. 2009, 166, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Demirevska-Kepova, K.; Simova-Stoilova, L.; Stoyanova, Z.; Hölzer, R.; Feller, U. Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 2004, 52, 253–266. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Horemans, N.; Wannijn, J.; Nauts, R.; Hees, M.V.; Vandenhove, H. Primary stress responses in Arabidopsis thaliana exposed to gamma radiation. J. Environ. Radioact. 2014, 129, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, S.; Leisner, S. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. J. Plant. Physiol. 2011, 168, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, S.E. Contribution of plastocyanin isoforms to photosynthesis and copper homeostasis in Arabidopsis thaliana grown at different copper regimes. Planta 2009, 229, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid. Med. Cell. Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, Y.; Zhang, X.; Xie, G.; Qin, M. Copper stress-induced changes in biomass accumulation, antioxidant activity and flavonoid contents in Belamcanda chinensis calli. Plant Cell Tiss Organ Cult. 2020, 142, 299–311. [Google Scholar] [CrossRef]
- Altaf, M.M.; Diao, X.P.; Altaf, M.A.; ur Rehman, A.; Shakoor, A.; Khan, L.U.; Jan, B.L.; Ahmad, P. Silicon-mediated metabolic upregulation of ascorbate glutathione (AsA-GSH) and glyoxalase reduces the toxic effects of vanadium in rice. J. Hazard. Mater. 2022, 436, 129145. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Xiang, C.; Oliver, D.J. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998, 10, 1539–1550. [Google Scholar] [CrossRef]
- Aguirre, G.; Pilon, M. Copper delivery to chloroplast proteins and its regulation. Front. Plant Sci. 2016, 6, 1250. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, E.A.; Klaus, A.A.; Kartashov, A.V.; Kusnetsov, V.V. Specificity of Cd, Cu, and Fe effects on barley growth, metal contents in leaves and chloroplasts, and activities of photosystem I and photosystem II. Plant Physiol. Bioch. 2020, 147, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol. 1985, 113, 548–555. [Google Scholar]
- Kim, Y.O.; Bae, H.J.; Cho, E.; Kang, H. Exogenous glutathione enhances mercury tolerance by inhibiting mercury entry into plant cells. Front. Plant Sci. 2017, 8, 683. [Google Scholar] [CrossRef]
- Ramírez, L.; Bartoli, C.G.; Lamattina, L. Glutathione and ascorbic acid protect Arabidopsis plants against detrimental effects of iron deficiency. J. Exp. Bot. 2013, 64, 3169–3178. [Google Scholar] [CrossRef]
| CuSO4 (µM) | Control | Cu5 | Cu20 | Cu60 |
|---|---|---|---|---|
| Stem height (cm) | 39.0 ± 1.2 a | 39.1 ± 1.8 a | 39.1 ± 1.9 a | 38.6 ± 2.1 a |
| Bolting time (d) | 31.0 ± 2.0 b | 28.0 ± 3.0 c | 33.0 ± 3.0 b | 37.0 ± 2.0 a |
| Silique lengths (cm) | 1.2 ± 0.3 a | 1.3 ± 0.2 a | 1.1 ± 0.3 ab | 1.0 ± 0.1 b |
| Thousand seed weight (mg DW) | 18.9 ± 0.9 b | 21.9 ± 0.5 a | 19.9 ± 1.1 ab | 18.6 ± 0.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Moeen-ud-din, M.; Yin, R.; Yang, S. ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity. Genes 2023, 14, 11. https://doi.org/10.3390/genes14010011
Wang J, Moeen-ud-din M, Yin R, Yang S. ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity. Genes. 2023; 14(1):11. https://doi.org/10.3390/genes14010011
Chicago/Turabian StyleWang, Jiehua, Muhammad Moeen-ud-din, Rong Yin, and Shaohui Yang. 2023. "ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity" Genes 14, no. 1: 11. https://doi.org/10.3390/genes14010011
APA StyleWang, J., Moeen-ud-din, M., Yin, R., & Yang, S. (2023). ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity. Genes, 14(1), 11. https://doi.org/10.3390/genes14010011






