Further Insights on RNA Expression and Sperm Motility
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review and Database Search
2.2. Biological Sample Collection
2.3. Spermatozoa Isolation
2.4. RNA Extraction and cDNA Conversion
2.5. Genes and Primer Design
2.6. Polymerase Chain Reaction (PCR)
2.7. Definition of a List of miRNAs
2.8. Real-Time PCR (RT-PCR)
3. Results
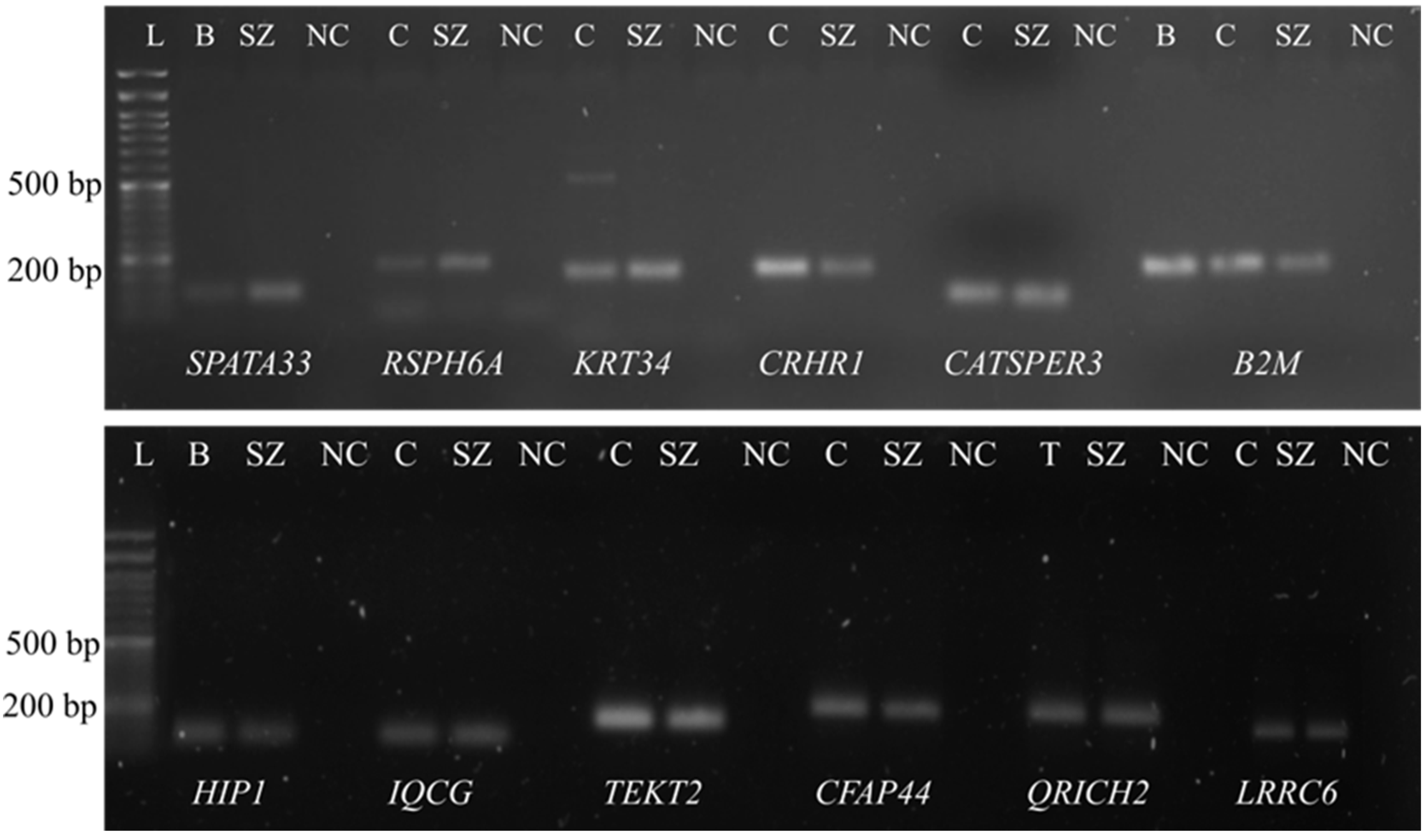
3.1. Confirmation of the Presence of mRNA in the Purified Spermatozoa
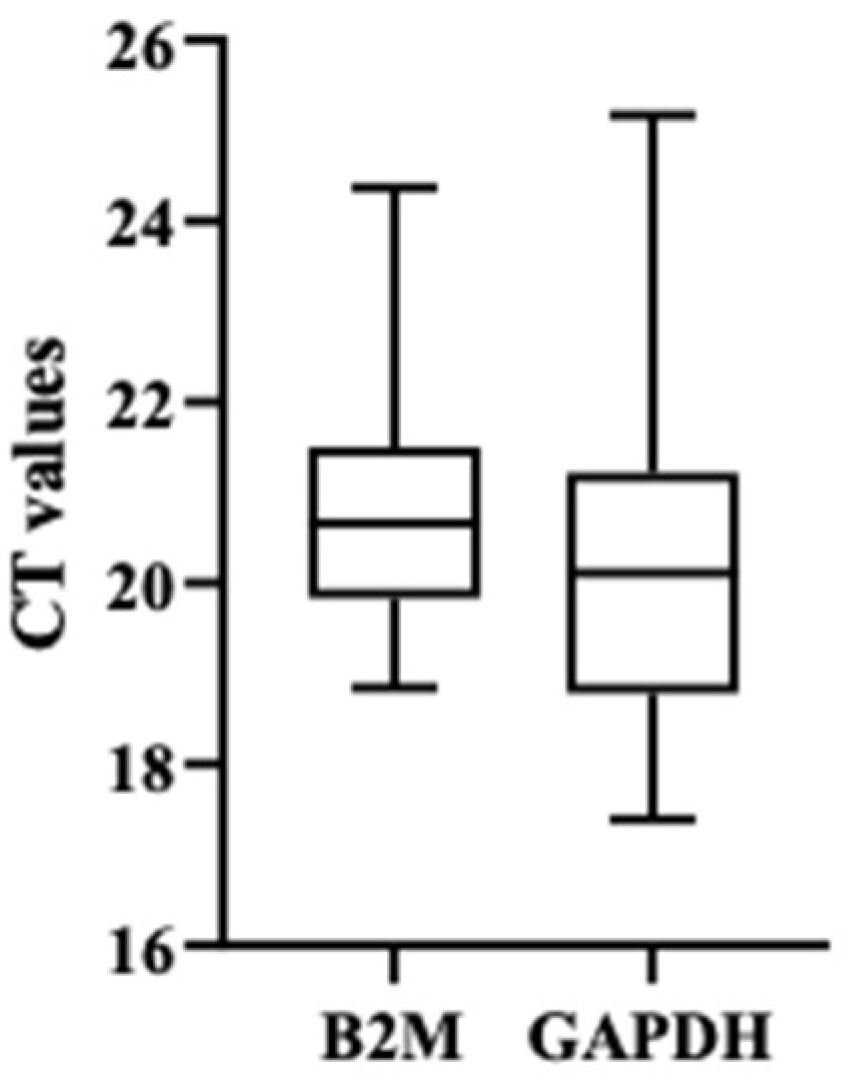
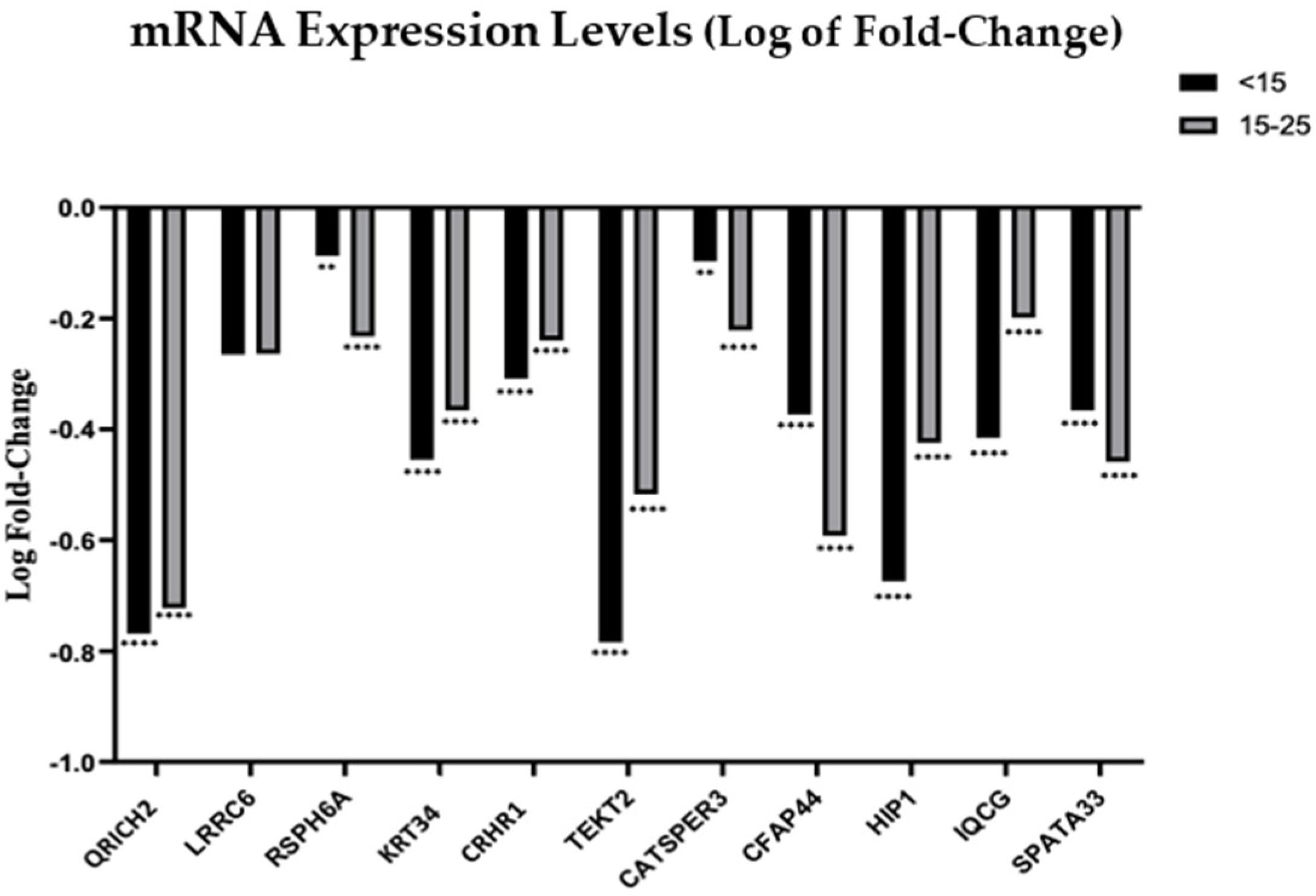
3.2. Analysis of mRNA and miRNA Expression Profiles by Quantitative RT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.Z.; Salehi, P.; Alyasin, A.; Taghiyar, S.; Deemeh, M.R. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia 2020, 52, e13463. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, W.; Cheng, Z.; Wu, S.; He, J.; Han, L.; He, Z.; Qin, W. Novel Gene Regulation in Normal and Abnormal Spermatogenesis. Cells 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silber, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef] [PubMed]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated with Rare Disorders. Front. Cell Dev. Biol. 2021, 9, 634536. [Google Scholar] [CrossRef]
- Pereira, R.; Sa, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Mortimer, D. The functional anatomy of the human spermatozoon: Relating ultrastructure and function. Mol. Hum. Reprod. 2018, 24, 567–592. [Google Scholar] [CrossRef]
- Chemes, H.E.; Alvarez Sedo, C. Tales of the tail and sperm head aches: Changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J. Androl. 2012, 14, 14–23. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rawe, V.Y. The making of abnormal spermatozoa: Cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010, 341, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pessot, C.A.; Brito, M.; Figueroa, J.; Concha, I.I.; Yañez, A.; Burzio, L.O. Presence of RNA in the sperm nucleus. Biochem. Biophys. Res. Commun. 1989, 158, 272–278. [Google Scholar] [CrossRef]
- Dadoune, J.P.; Pawlak, A.; Alfonsi, M.F.; Siffroi, J.P. Identification of transcripts by macroarrays, RT–PCR and in situ hybridization in human ejaculate spermatozoa. Mol. Hum. Reprod. 2005, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, T. Human spermatozoal RNAs. Fertil. Steril. 2012, 97, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.; Shah, C.; Sachdeva, G.; Gadkar, S.; Bhartiya, D.; Puri, C. Ontogeny and cellular localization of SRY transcripts in the human testes and its detection in spermatozoa. Reproduction 2005, 130, 603–613. [Google Scholar] [CrossRef]
- Krawetz, S.A. Paternal contribution: New insights and future challenges. Nat. Rev. Genet. 2005, 6, 633–642. [Google Scholar] [CrossRef]
- Jodar, M. Sperm and seminal plasma RNAs: What roles do they play beyond fertilization? Reproduction 2019, 158, R113–R123. [Google Scholar] [CrossRef]
- Bansal, S.K.; Gupta, N.; Sankhwar, S.N.; Rajender, S. Differential Genes Expression between Fertile and Infertile Spermatozoa Revealed by Transcriptome Analysis. PLoS ONE 2015, 10, e0127007. [Google Scholar] [CrossRef]
- Walker, W.H. Regulation of mammalian spermatogenesis by miRNAs. Semin. Cell Dev. Biol. 2022, 121, 24–31. [Google Scholar] [CrossRef]
- Barbu, M.G.; Thompson, D.C.; Suciu, N.; Voinea, S.C.; Cretoiu, D.; Predescu, D.V. The Roles of MiRNAs in Male Infertility. Int. J. Mol. Sci. 2021, 22, 2910. [Google Scholar] [CrossRef]
- Bartel, D.P. MiRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Tomara, P.; Louka, I.; Maziotis, E.; Pantou, A.; Nitsos, N.; Vaxevanoglou, T.; Kokkali, G.; Agarwal, A.; et al. Investigating the Role of the miRNA-34/449 Family in Male Infertility: A Critical Analysis and Review of the Literature. Front. Endocrinol. 2021, 12, 709943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jia, Q.; Guo, X.; Li, K.; Chen, W.; Shen, Q.; Xu, C.; Fu, Y. miRNA-34 family: From mechanism to potential applications. Int. J. Biochem. Cell Biol. 2022, 144, 106168. [Google Scholar] [CrossRef] [PubMed]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorram Khorshid, H.R. MiRNA profiling in spermatozoa of men with unexplained asthenozoospermia. Andrologia 2019, 51, e13284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Zhou, Q.-Z.; Yang, J.-K.; Lyu, X.-M.; Bian, J.; Guo, W.-B.; Chen, Z.-J.; Xia, M.; Xia, H.; Qi, T.; et al. MiRNA-27a-mediated repression of cysteine-rich secretory protein 2 translation in asthenoteratozoospermic patients. Asian J. Androl. 2017, 19, 591–595. [Google Scholar] [CrossRef]
- Pereira, R.; Oliveira, M.E.; Santos, R.; Oliveira, E.; Barbosa, T.; Santos, T.; Gonçalves, P.; Ferraz, L.; Pinto, S.; Barros, A.; et al. Characterization of CCDC103 expression profiles: Further insights in primary ciliary dyskinesia and in human reproduction. J. Assist. Reprod. Genet. 2019, 36, 1683–1700. [Google Scholar] [CrossRef]
- Busk, P.K. A tool for design of primers for miRNA-specific quantitative RT-qPCR. BMC Bioinform. 2014, 15, 29–38. [Google Scholar] [CrossRef]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zou, Q.; Zheng, A.; Li, H.; Yang, S.; Xiang, J. Patient with CATSPER3 mutations-related failure of sperm acrosome reaction with successful pregnancy outcome from intracytoplasmic sperm injection (ICSI). Mol. Genet. Genom. Med 2021, 9, e1579. [Google Scholar] [CrossRef]
- Li, H.-G.; Ding, X.-F.; Liao, A.-H.; Kong, X.-B.; Xiong, C.-L. Expression of CatSper family transcripts in the mouse testis during post-natal development and human ejaculated spermatozoa: Relationship to sperm motility. Mol. Hum. Reprod. 2007, 13, 299–306. [Google Scholar] [CrossRef]
- Carkci, S.; Etem, E.O.; Ozaydin, S.; Karakeci, A.; Tektemur, A.; Ozan, T.; Orhan, I. Ion channel gene expressions in infertile men: A case-control study. Int. J. Reprod. Biomed. 2017, 15, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, X.; Li, W.; Yang, X.; Li, Z.; Liu, W.; Li, C.; Zhu, Z.; Wang, L.; Wang, J.; et al. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am. J. Hum. Genet. 2017, 100, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Coutton, C.; Vargas, A.S.; Amiri-Yekta, A.; Kherraf, Z.-E.; Ben Mustapha, S.F.; Le Tanno, P.; Wambergue-Legrand, C.; Karaouzène, T.; Martinez, G.; Crouzy, S.; et al. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018, 9, 686. [Google Scholar] [CrossRef]
- Kamel, A.; Saberiyan, M.; Mirfakhraie, R.; Teimori, H. Reduced expression of CFAP44 and CFAP44-AS1 may affect sperm motility and morphology. Andrologia 2022, 54, e14447. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Miyata, H.; Shimada, K.; Morohoshi, A.; Nozawa, K.; Matsumura, T.; Xu, Z.; Pratiwi, P.; Ikawa, M. RSPH6A is required for sperm flagellum formation and male fertility in mice. J. Cell Sci. 2018, 131, jcs221648. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Yang, P. The radial spokes and central apparatus: Mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskelet. 2004, 57, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Grossman-Haham, I.; Coudray, N.; Yu, Z.; Wang, F.; Zhang, N.; Bhabha, G.; Vale, R.D. Structure of the radial spoke head and insights into its role in mechanoregulation of ciliary beating. Nat. Struct. Mol. Biol. 2021, 28, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yi, M.; Sheng, Y.; Cheng, H.; Zhou, R. A Novel Testis-Enriched Gene Spata33 Is Expressed during Spermatogenesis. PLoS ONE 2013, 8, e67882. [Google Scholar] [CrossRef]
- Miyata, H.; Oura, S.; Morohoshi, A.; Shimada, K.; Mashiko, D.; Oyama, Y.; Kaneda, Y.; Matsumura, T.; Abbasi, F.; Ikawa, M. SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2106673118. [Google Scholar] [CrossRef]
- Aprea, I.; Raidt, J.; Höben, I.M.; Loges, N.T.; Nöthe-Menchen, T.; Pennekamp, P.; Olbrich, H.; Kaiser, T.; Biebach, L.; Tüttelmann, F.; et al. Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility. PLoS Genet. 2021, 17, e1009306. [Google Scholar] [CrossRef]
- Horani, A.; Ferkol, T.W.; Shoseyov, D.; Wasserman, M.G.; Oren, Y.S.; Kerem, B.; Amirav, I.; Cohen-Cymberknoh, M.; Dutcher, S.K.; Brody, S.L.; et al. LRRC6 mutation causes primary ciliary dyskinesia with dynein arm defects. PLoS ONE 2013, 8, e59436. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, E.W.; Grammatopoulos, D.K. The Molecular Mechanisms Underlying the Regulation of the Biological Activity of Corticotropin-Releasing Hormone Receptors: Implications for Physiology and Pathophysiology. Endocr. Rev. 2006, 27, 260–286. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Barbosa, T.; Alves, A.; Santos, R.; Oliveira, J.; Sousa, M. Unveiling the genetic etiology of primary ciliary dyskinesia: When standard genetic approach is not enough. Adv. Med. Sci. 2020, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Legendre-Guillemin, V.; Gan, L.; Chopra, V.; Kwok, A.; McPherson, P.S.; Hayden, M.R. HIP1 Functions in Clathrin-mediated Endocytosis through Binding to Clathrin and Adaptor Protein 2. J. Biol. Chem. 2001, 276, 39271–39276. [Google Scholar] [CrossRef] [PubMed]
- Khatchadourian, K.; Smith, C.E.; Metzler, M.; Gregory, M.; Hayden, M.R.; Cyr, D.G.; Hermo, L. Structural abnormalities in spermatids together with reduced sperm counts and motility underlie the reproductive defect in HIP1−/− mice. Mol. Reprod. Dev. 2007, 74, 341–359. [Google Scholar] [CrossRef]
- Chen, L.-T.; Liang, W.-X.; Chen, S.; Li, R.-K.; Tan, J.-L.; Xu, P.-F.; Luo, L.-F.; Wang, L.; Yu, S.-H.; Meng, G.; et al. Functional and molecular features of the calmodulin-interacting protein IQCG required for haematopoiesis in zebrafish. Nat. Commun. 2014, 5, 3811. [Google Scholar] [CrossRef]
- Harris, T.P.; Schimenti, K.J.; Munroe, R.J.; Schimenti, J.C. IQ Motif-Containing G (Iqcg) Is Required for Mouse Spermiogenesis. G3 Genes Genomes Genet. 2014, 4, 367–372. [Google Scholar] [CrossRef]
- Li, R.-K.; Tan, J.-L.; Chen, L.-T.; Feng, J.-S.; Liang, W.-X.; Guo, X.-J.; Liu, P.; Chen, Z.; Sha, J.-H.; Wang, Y.-F.; et al. Iqcg Is Essential for Sperm Flagellum Formation in Mice. PLoS ONE 2014, 9, e98053. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, F.; Li, F.; Jiang, X.; Yang, Y.; Li, X.; Li, W.; Wang, X.; Cheng, J.; Liu, M.; et al. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat. Commun. 2019, 10, 433. [Google Scholar] [CrossRef]
- Zhang, G.; Li, D.; Tu, C.; Meng, L.; Tan, Y.; Ji, Z.; Cheng, J.; Lu, G.; Lin, G.; Zhang, H.; et al. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum. Mol. Genet. 2021, 31, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Kherraf, Z.-E.; Cazin, C.; Coutton, C.; Amiri-Yekta, A.; Martinez, G.; Boguenet, M.; Fourati Ben Mustapha, S.; Kharouf, M.; Gourabi, H.; Hosseini, S.H.; et al. Whole exome sequencing of men with multiple morphological abnormalities of the sperm flagella reveals novel homozygous QRICH2 mutations. Clin. Genet. 2019, 96, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, M.; Janett, F.; Mapel, X.M.; Kadri, N.K.; Fang, Z.-H.; Schwarzenbacher, H.; Seefried, F.R.; Spengeler, M.; Witschi, U.; Pausch, H. A 1-bp deletion in bovine QRICH2 causes low sperm count and immotile sperm with multiple morphological abnormalities. Genet. Sel. Evol. 2022, 54, 18. [Google Scholar] [CrossRef] [PubMed]
- Linck, R. Tektins and Microtubules. Adv. Mol. Cell Biol. 1990, 3, 35–63. [Google Scholar] [CrossRef]
- Setter, P.W.; Malvey-Dorn, E.; Steffen, W.; Stephens, R.E.; Linck, R.W. Tektin interactions and a model for molecular functions. Exp. Cell Res. 2006, 312, 2880–2896. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Iguchi, N.; Toyama, Y.; Kitamura, K.; Takahashi, T.; Kaseda, K.; Maekawa, M.; Nishimune, Y. Mice Deficient in the Axonemal Protein Tektin-t Exhibit Male Infertility and Immotile-Cilium Syndrome Due to Impaired Inner Arm Dynein Function. Mol. Cell. Biol. 2004, 24, 7958–7964. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, C.; Pinzani, P.; Bianchi, S.; Paglierani, M.; Distante, V.; Pazzagli, M.; Bustin, S.A.; Orlando, C. Quantitative real-time reverse transcription polymerase chain reaction: Normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal. Biochem. 2002, 309, 293–300. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Lee, P.D.; Sladek, R.; Greenwood, C.M.T.; Hudson, T.J. Control Genes and Variability: Absence of Ubiquitous Reference Transcripts in Diverse Mammalian Expression Studies. Genome Res. 2002, 12, 292–297. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Zeng, C.; He, L.; Peng, W.; Ding, L.; Tang, K.; Fang, D.; Zhang, Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology 2014, 68, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MiRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Aliakbari, F.; Eshghifar, N.; Mirfakhraie, R.; Pourghorban, P.; Azizi, F. Coding and Non-Coding RNAs, as Male Fertility and Infertility Biomarkers. Int. J. Fertil. Steril. 2021, 15, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, R.; Mofidi Chelan, E. Using miRNAs as molecular biomarkers for the evaluation of male infertility. Andrologia 2022, 54, e14298. [Google Scholar] [CrossRef]
- de Mateo, S.; Sassone-Corsi, P. Regulation of spermatogenesis by small non-coding RNAs: Role of the germ granule. Semin. Cell Dev. Biol. 2014, 29, 84–92. [Google Scholar] [CrossRef]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for miRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef]
- Corral-Vazquez, C.; Blanco, J.; Salas-Huetos, A.; Vidal, F.; Anton, E. Normalization matters: Tracking the best strategy for sperm miRNA quantification. Mol. Hum. Reprod. 2017, 23, 45–53. [Google Scholar] [CrossRef][Green Version]
- Guo, D.; Barry, L.; Lin, S.S.H.; Huang, V.; Li, L.-C. RNAa in action: From the exception to the norm. RNA Biol. 2014, 11, 1221–1225. [Google Scholar] [CrossRef][Green Version]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MiRNA-10a Binds the 5′UTR of Ribosomal Protein mRNAs and Enhances Their Translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MiRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.; Viana, P.; Barros, A.; Sá, R.; Sousa, M.; Pereira, R. Further Insights on RNA Expression and Sperm Motility. Genes 2022, 13, 1291. https://doi.org/10.3390/genes13071291
Silva C, Viana P, Barros A, Sá R, Sousa M, Pereira R. Further Insights on RNA Expression and Sperm Motility. Genes. 2022; 13(7):1291. https://doi.org/10.3390/genes13071291
Chicago/Turabian StyleSilva, Carolina, Paulo Viana, Alberto Barros, Rosália Sá, Mário Sousa, and Rute Pereira. 2022. "Further Insights on RNA Expression and Sperm Motility" Genes 13, no. 7: 1291. https://doi.org/10.3390/genes13071291
APA StyleSilva, C., Viana, P., Barros, A., Sá, R., Sousa, M., & Pereira, R. (2022). Further Insights on RNA Expression and Sperm Motility. Genes, 13(7), 1291. https://doi.org/10.3390/genes13071291






