Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Cells
2.2. Prediction of miRNA Targeting DUSP1
2.3. Synthesis of Primers
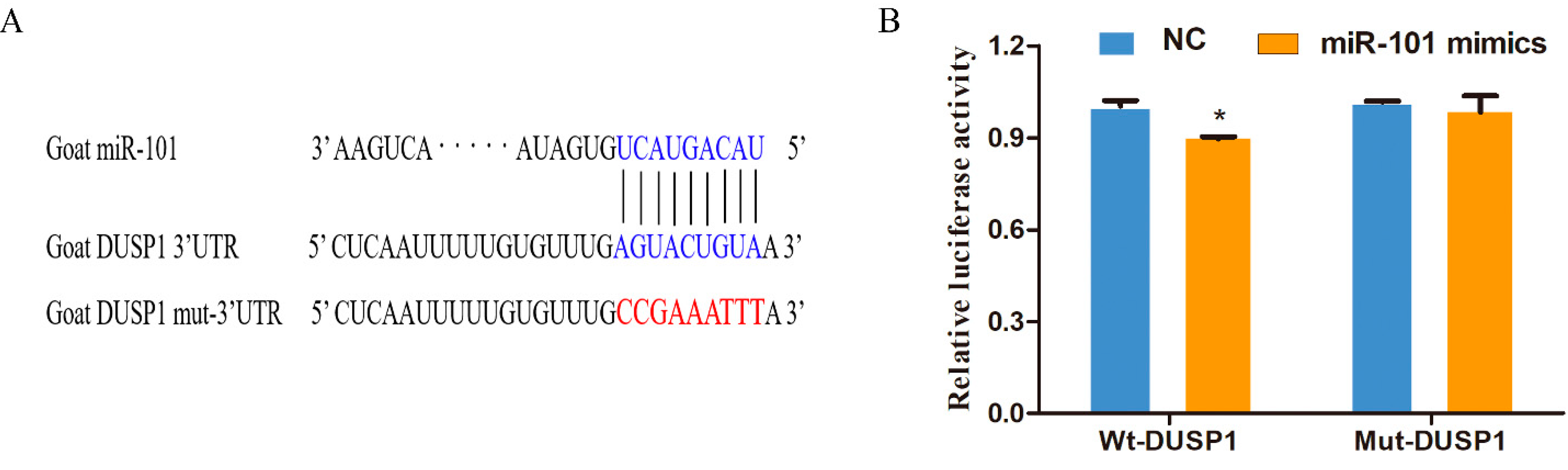
2.4. Double Luciferase Reporter Assay
2.5. Construction and Transfection of Plasmid
2.6. Quantitative Real-Time PCR
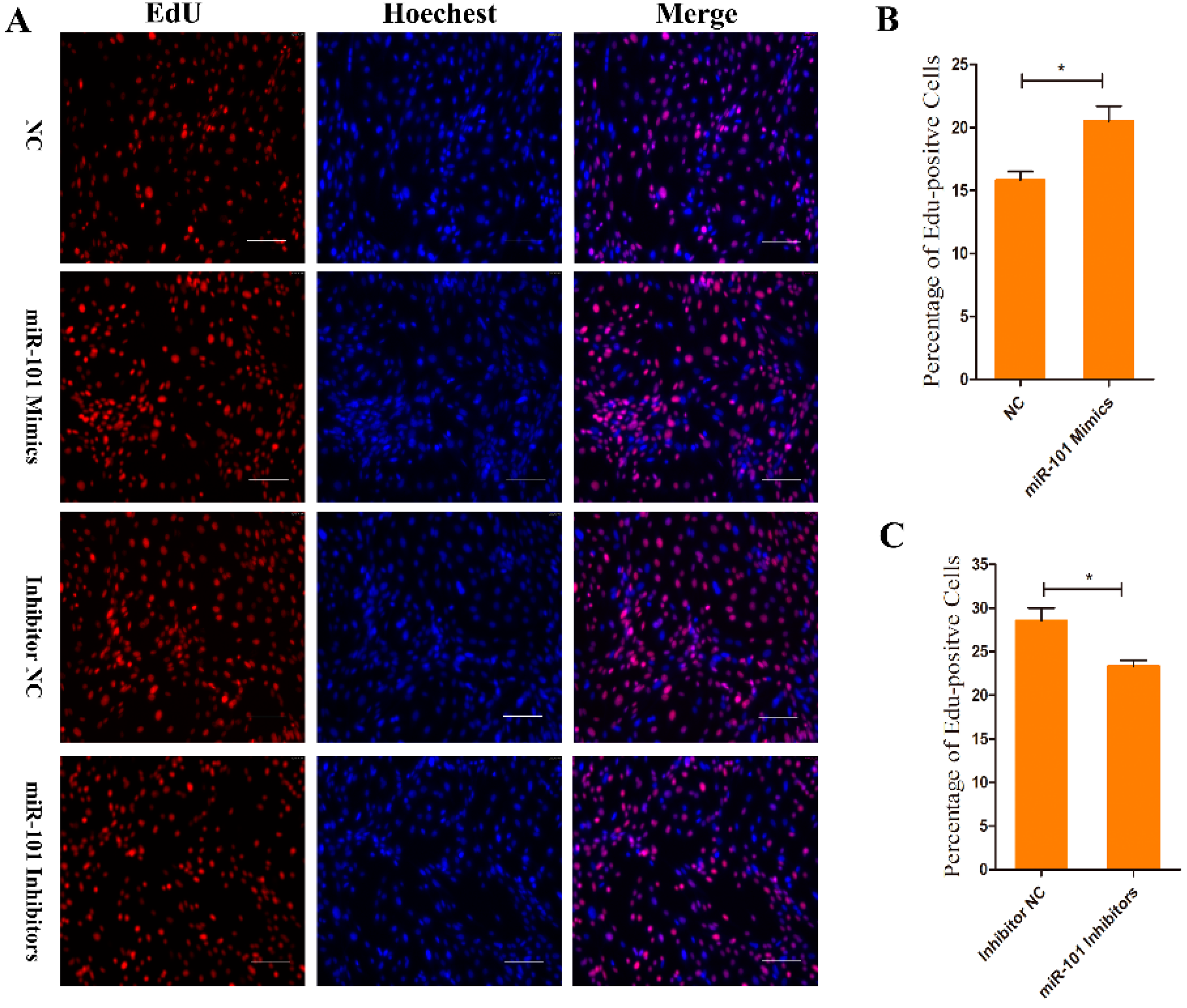
2.7. EdU Incorporation Assay
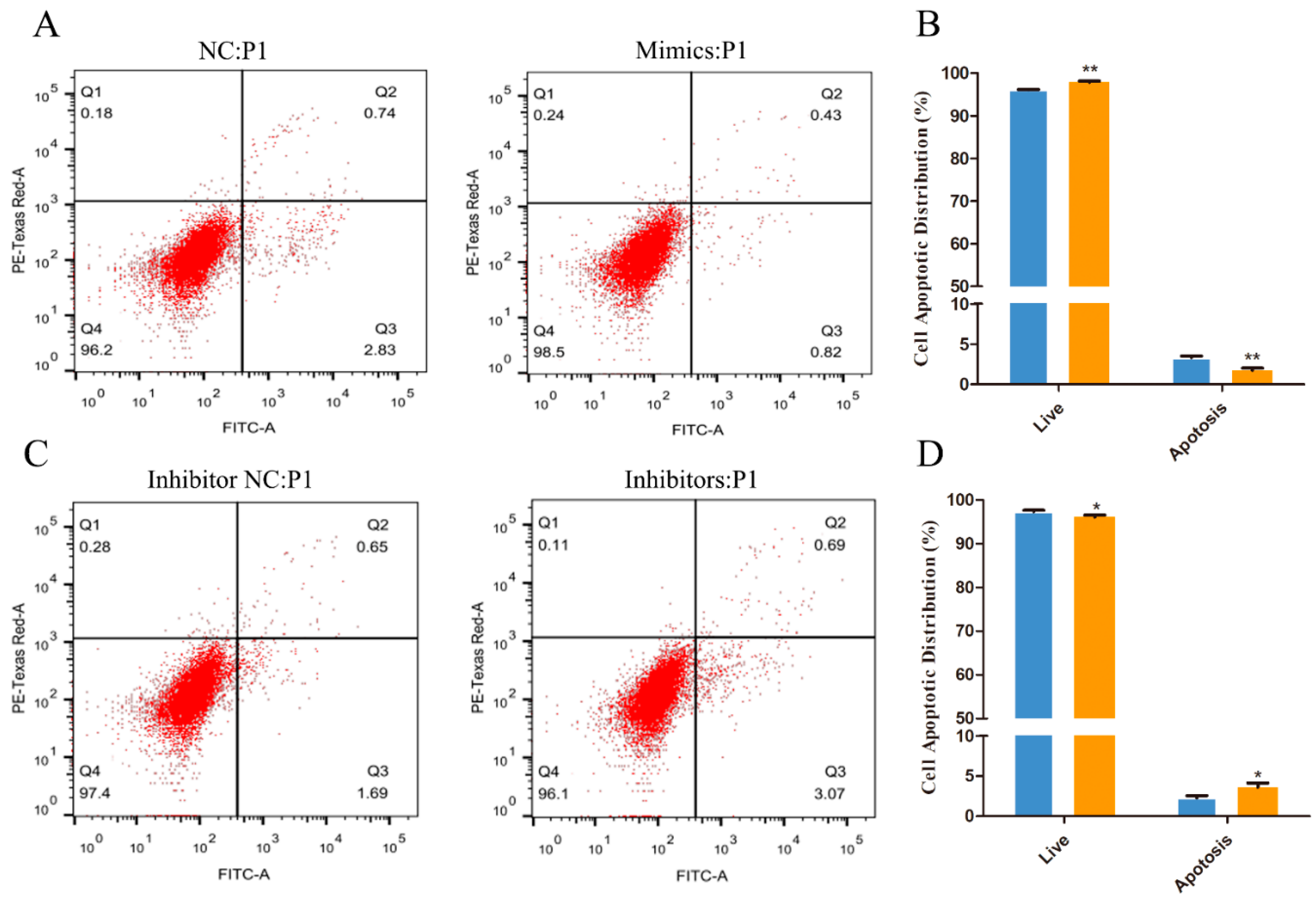
2.8. Apoptosis Assay
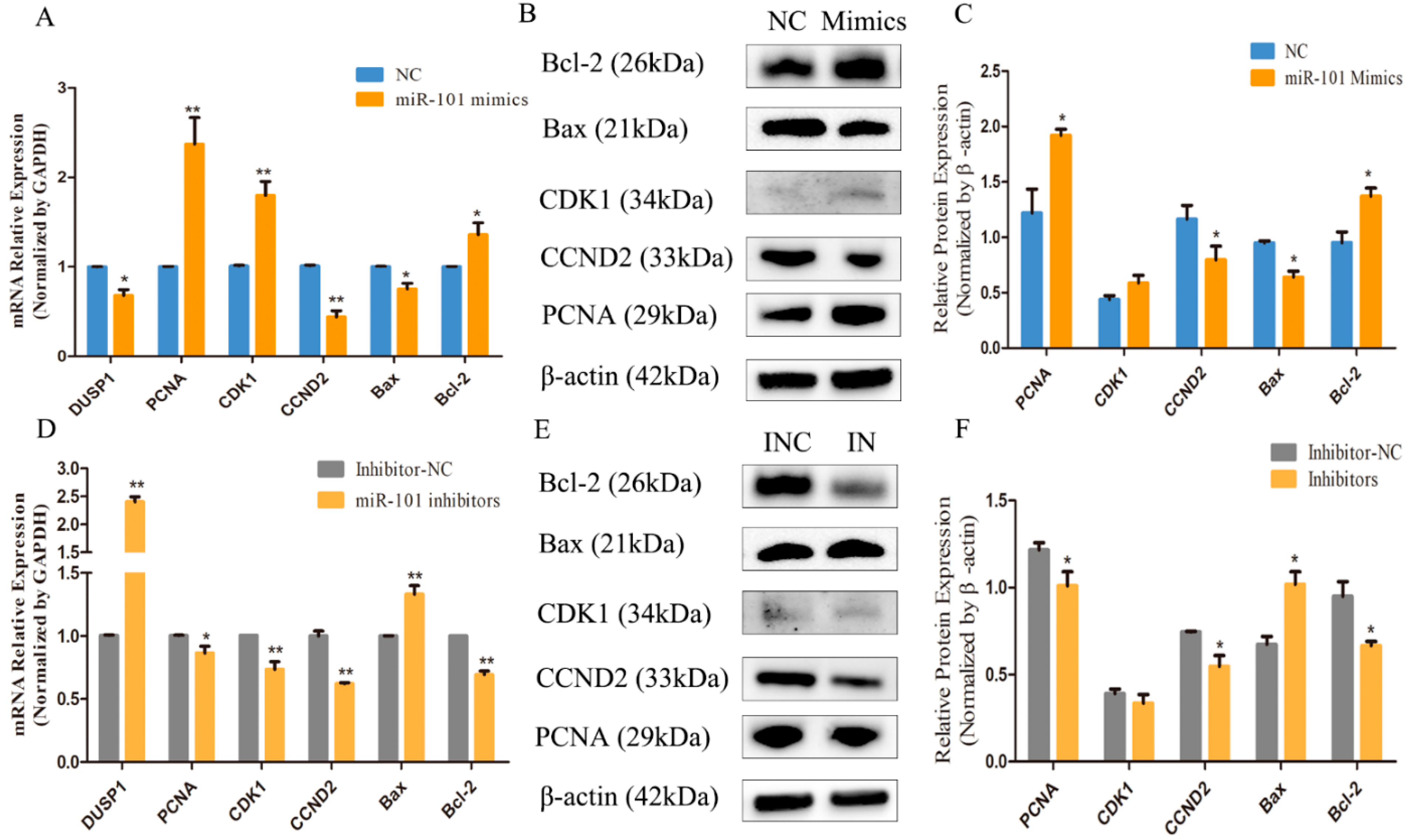
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. DUSP1 Is a Direct Target of miR-101
3.2. Effect of miR-101 on the Proliferation of HFSCs
3.3. Effect of miR-101 on the Apoptosis of HFSCs
3.4. Effect of miR-101 on the Expression Levels of Proliferation and Apoptosis-Related Genes and Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- National livestock and Poultry Genetic Resources Committee. Chinese Livestock and Poultry Genetic Resources; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Li, Y.; Huang, Y. The production of wool goat and writing brush in our country. China Herbiv. 2005, 25, 44–46. [Google Scholar]
- Gat, U.; DasGupta, L.; Fuchs, E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 1998, 95, 605–614. [Google Scholar] [CrossRef]
- Theodosiou, A.; Ashworth, A. MAP kinase phosphatases. Genome Biol. 2002, 3, reviews3009.1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, J.; Ling, Z.Q.; Ge, M.H. Expression of DUSP1 in salivary adenoid cystic carcinoma and its clinicopathological significance. Zhejiang Med. J. 2016, 38, 379–382. [Google Scholar]
- Celaya, A.M.; Sánchez-Pérez, I.; Bermúdez-Muñoz, J.M.; Rodríguez-de la Rosa, L.; Pintado-Berninches, L.; Perona, R.; Murillo-Cuesta, S.; Varela-Nieto, I. Deficit of mitogen-activated protein kinase phosphatase 1 (DUSP1) accelerates progressive hearing loss. eLife 2019, 8, e39159. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, G.; Li, Y. A Screen for Key Genes and Pathways Involved in High-Quality Brush Hair in the Yangtze River Delta White Goat. PLoS ONE 2017, 12, e0169820. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Zhang, J.; Ji, D.; Zhang, G.; Yang, B. Identification of genes influencing formation of the Type III Brush Hair in Yangtze River Delta white goats by differential display of mRNA. Gene 2013, 526, 205–209. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Yi, R.; O’Caroll, D.; Pasolli, H.A.; Zhang, Z.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef]
- Pal, A.S.; Kasinski, A.L. Animal Models to Study MicroRNA Function. Adv. Cancer Res. 2017, 135, 53–118. [Google Scholar] [PubMed]
- Zhao, B.; Chen, Y.; Yang, N.; Chen, Q.; Bao, Z.; Liu, M.; Hu, S.; Li, J.; Wu, X. miR-218-5p regulates skin and hair follicle development through Wnt/β-catenin signaling pathway by targeting SFRP2. J. Cell Physiol. 2019, 234, 20329–20341. [Google Scholar] [CrossRef]
- Ma, T.; Li, J.; Jiang, Q.; Wu, H.; Jiang, H.; Zhang, Q. Differential expression of miR-let7a in hair follicle cycle of Liaoning cashmere goats and identification of its targets. Funct. Integr. Genom. 2018, 18, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Du, K.-T.; Deng, J.-Q.; He, X.-G.; Liu, Z.-p.; Peng, C.; Zhang, M.-S. MiR-214 Regulates the Human Hair Follicle Stem Cell Proliferation and Differentiation by Targeting EZH2 and Wnt/β-Catenin Signaling Way In Vitro. Tissue Eng. Regen. Med. 2018, 15, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, Y.; He, X.; Wang, J.; Cai, W.; Jia, Y.; Xiao, D.; Zhang, J.; Zhao, M.; et al. Overexpression of miR-101 suppresses collagen synthesis by targeting EZH2 in hypertrophic scar fibroblasts. Burn. Trauma 2021, 9, tkab038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-g.; Guo, J.-F.; Liu, D.-L.; Liu, Q.; Wang, J.-J. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J. Thorac. Oncol. 2011, 6, 671–678. [Google Scholar] [CrossRef]
- Friedman, J.M.; Liang, G.; Liu, C.-C.; Wolff, E.M.; Tsai, Y.C.; Ye, W.; Zhou, X.; Jones, P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009, 69, 2623–2629. [Google Scholar] [CrossRef]
- Vella, S.; Pomella, S.; Leoncini, P.P.; Colletti, M.; Conti, B.; Marquez, V.E.; Strillacci, A.; Roma, J.; Gallego, S.; Milano, G.M.; et al. MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin. Epigenetics 2015, 7, 82. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Mi, N.; Zhou, L. miR-101-3p and miR-199b-5p promote cell apoptosis in oral cancer by targeting BICC1. Mol. Cell Probes 2020, 52, 101567. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, J.; Li, Y.; Ji, D.; Zhang, H.; Yin, X.; Wang, J.; Niu, H. Hair follicle stem cells isolated from newborn Yangtze River Delta White Goats. Gene 2019, 698, 19–26. [Google Scholar] [CrossRef]
- Ouji, Y.; Yoshikawa, M.; Nishiofuku, M.; Ouji-Sageshima, N.; Kubo, A.; Ishizaka, S. Effects of Wnt-10b on proliferation and differentiation of adult murine skin-derived CD34 and CD49f double-positive cells. J. Biosci. Bioeng. 2010, 110, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Arellano, M.; Moreno, S. Regulation of CDK/cyclin complexes during the cell cycle. Int. J. Biochem. Cell Biol. 1997, 29, 559–573. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, Y.; Li, L.; Zhang, G. MiR-101 inhibits cell growth and tumorigenesis of Helicobacter pylori related gastric cancer by repression of SOCS2. Cancer Biol. Ther. 2015, 16, 160–169. [Google Scholar] [CrossRef]
- Kraus, S.; Naor, Z.; Seger, R. Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor. Arch. Med. Res. 2001, 32, 499–509. [Google Scholar] [CrossRef]
- Keyse, S.M. Protein phosphatases and the regulation of MAP kinase activity. Semin. Cell Dev. Biol. 1998, 9, 143–152. [Google Scholar] [CrossRef]
- Brondello, J.M.; Brunet, A.; Pouyssegur, J.; McKenzie, F.R. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem. 1997, 272, 1368–1376. [Google Scholar] [CrossRef]
- Kondoh, K.; Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta 2007, 1773, 1227–1237. [Google Scholar] [CrossRef]
- Farooq, A.; Zhou, M.M. Structure and regulation of MAPK phosphatases. Cell Signal 2004, 16, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Purwana, I.N.; Kanasaki, H.; Mijiddorj, T.; Oride, A.; Miyazaki, K. Induction of dual-specificity phosphatase 1 (DUSP1) by pulsatile gonadotropin-releasing hormone stimulation: Role for gonadotropin subunit expression in mouse pituitary LbetaT2 cells. Biol. Reprod. 2011, 84, 996–1004. [Google Scholar] [CrossRef]
- Yang, J.; Sun, L.; Han, J.; Zheng, W.; Peng, W. DUSP1/MKP-1 regulates proliferation and apoptosis in keratinocytes through the ERK/Elk-1/Egr-1 signaling pathway. Life Sci. 2019, 223, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Yang, B.; Li, Y.; Cai, M.; Zhang, W.; Cheng, G.; Guo, H. Transcriptomic inspection revealed a possible pathway regulating the formation of the high-quality brush hair in Chinese Haimen goat (Capra hircus). R. Soc. Open Sci. 2018, 5, 170907. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Feng, Y.; Zhang, L.; Hu, H.; Wang, Q.; Chu, C.; Qu, J.; Wang, Y.; Li, Y. Silencing MAP3K1 expression inhibits the proliferation of goat hair follicle stem cells. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 428–437. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Chu, Z.; Dang, Y.; Zhu, J.; Su, X. MicroRNA-101 in the ventrolateral orbital cortex (VLO) modulates depressive-like behaviors in rats and targets dual-specificity phosphatase 1 (DUSP1). Brain Res. 2017, 1669, 55–62. [Google Scholar] [CrossRef]
- Xin, Y.; Tang, L.; Chen, J.; Chen, D.; Wen, W.; Han, F. Inhibition of miR-101-3p protects against sepsis-induced myocardial injury by inhibiting MAPK and NF-κB pathway activation via the upregulation of DUSP1. Int. J. Mol. Med. 2021, 47, 20. [Google Scholar] [CrossRef]
- Wei, X.; Tang, C.; Lu, X.; Liu, R.; Zhou, M.; He, D.; Zheng, D.; Sun, C.; Wu, Z. MiR-101 targets DUSP1 to regulate the TGF-β secretion in sorafenib inhibits macrophage-induced growth of hepatocarcinoma. Oncotarget 2015, 6, 18389–18405. [Google Scholar] [CrossRef]
- Ye, Y.; Bao, C.; Fan, W. Overexpression of miR-101 May Target DUSP1 to Promote the Cartilage Degradation in Rheumatoid Arthritis. J. Comput. Biol. 2019, 26, 1067–1079. [Google Scholar] [CrossRef]
| Genes | Gene ID | Primer Sequence (5′–3′) |
|---|---|---|
| GAPDH | 100860872 | F: AGGTCGGAGTGAACGGATTC |
| R: CCAGCATCACCCCACTTGAT | ||
| DUSP1 | 539175 | F: CCACCACCACCGTCTTCAACTTC |
| R: GCTGGGAGAGGTCGTGATAGGG | ||
| PCNA | 102172276 | F: ATCAGCTCAAGTGGCGTGAA |
| R: TGCCAAGGTGTCCGCATTAT | ||
| CDK1 | 10086361 | F: AGATTTTGGCCTTGCCAGAG |
| R: AGCTGACCCCAGCAATACTT | ||
| CCND2 | 102180657 | F: GGGCAAGTTGAAATGGAA |
| R: TCATCGACGGCGGGTAC | ||
| Bax | 100846984 | F: GGGCAAGTTGAAATGGAA |
| R: TCATCGACGGCGGGTAC | ||
| Bcl-2 | 100861254 | F:ATGTGTGTGGAGAGCGTCAA |
| R: CCTTCAGAGACAGCCAGGAG |
| Genes | Sequence Name | Sequence (5′–3′) |
|---|---|---|
| miR-101 | Negative control | UUCUCCGAACGUGUCACGUTT (sense) |
| ACGUGACACGUUCGGAGAATT (antisense) | ||
| Mimics | CAGUACUGUGAUAACUGAATT (sense) | |
| UUCAGUUAUCACAGUACUGUA (antisense) | ||
| Inhibitor-negative control | CAGUACUUUUGUGUAGUACAA | |
| Inhibitors | UUCAGUUAUCACAGUACUGUA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Wu, X.; Wang, Q.; Wang, J.; Sun, X.; Ji, D.; Li, Y. Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells. Genes 2022, 13, 1035. https://doi.org/10.3390/genes13061035
Qu J, Wu X, Wang Q, Wang J, Sun X, Ji D, Li Y. Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells. Genes. 2022; 13(6):1035. https://doi.org/10.3390/genes13061035
Chicago/Turabian StyleQu, Jingwen, Xi Wu, Qiang Wang, Jian Wang, Xiaomei Sun, Dejun Ji, and Yongjun Li. 2022. "Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells" Genes 13, no. 6: 1035. https://doi.org/10.3390/genes13061035
APA StyleQu, J., Wu, X., Wang, Q., Wang, J., Sun, X., Ji, D., & Li, Y. (2022). Effect of miR-101 on the Proliferation and Apoptosis of Goat Hair Follicle Stem Cells. Genes, 13(6), 1035. https://doi.org/10.3390/genes13061035






