The Deubiquitinase USP39 Promotes Esophageal Squamous Cell Carcinoma Malignancy as a Splicing Factor
Abstract
:1. Introduction
2. Material and methods
2.1. Cell Culture and Plasmids
2.2. Antibodies and Reagents
2.3. Virus Production and Cell Infection
2.4. Screening of DUB Library
2.5. Human Esophageal Tumor Tissue Collection and Immunohistochemistry Staining
2.6. CCK-8 cell Proliferation Assays
2.7. Flow Cytometry
2.8. Cloning formation assay
2.9. Xenograft Transplantation Experiments
2.10. Immunoprecipitation and Western Blotting
2.11. Sliver Staining and Mass Spectrometry
2.12. RNA Isolation and qPCR
2.13. RNA Immunoprecipitation Sequencing and Data Analysis
2.14. RNA-Seq and Data Analysis
2.15. GO and KEGG Analysis
2.16. Statistical Analysis
3. Result
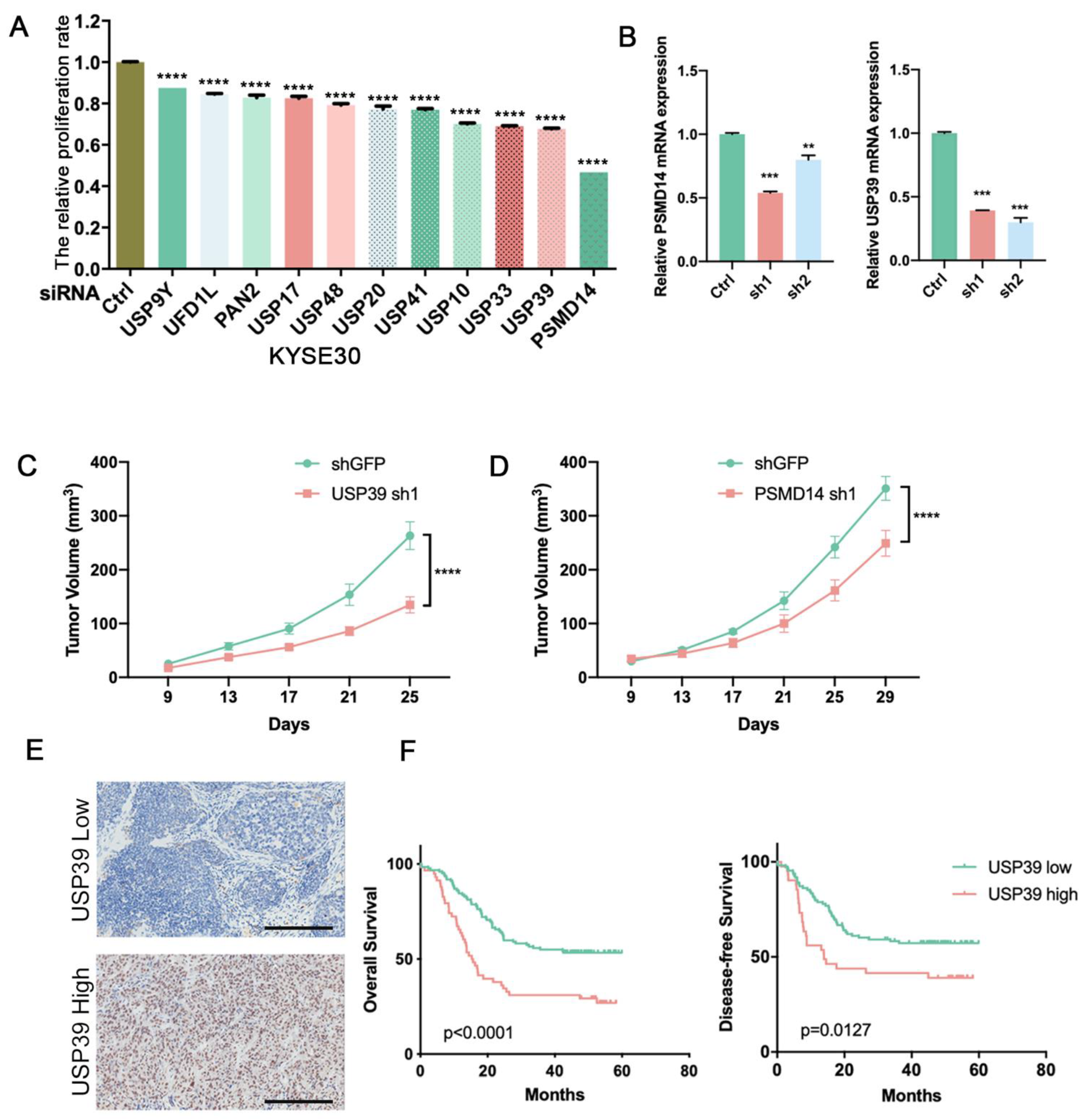
3.1. Identification of DUBs That Affect Tumor Cell Proliferation by siRNA Screening Library
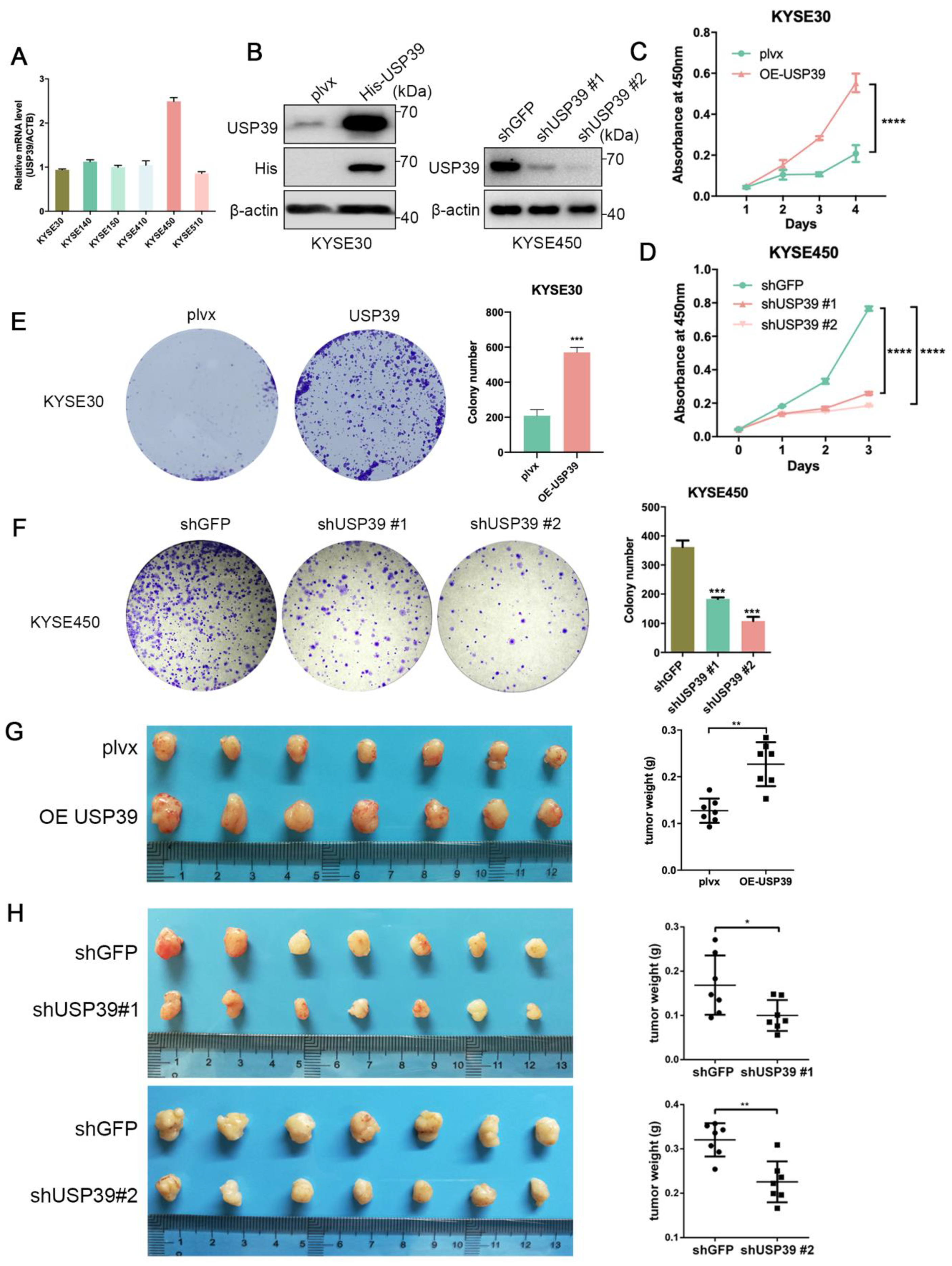
3.2. USP39 Promotes Esophageal Cancer Cell Proliferation, Migration, and Invasion
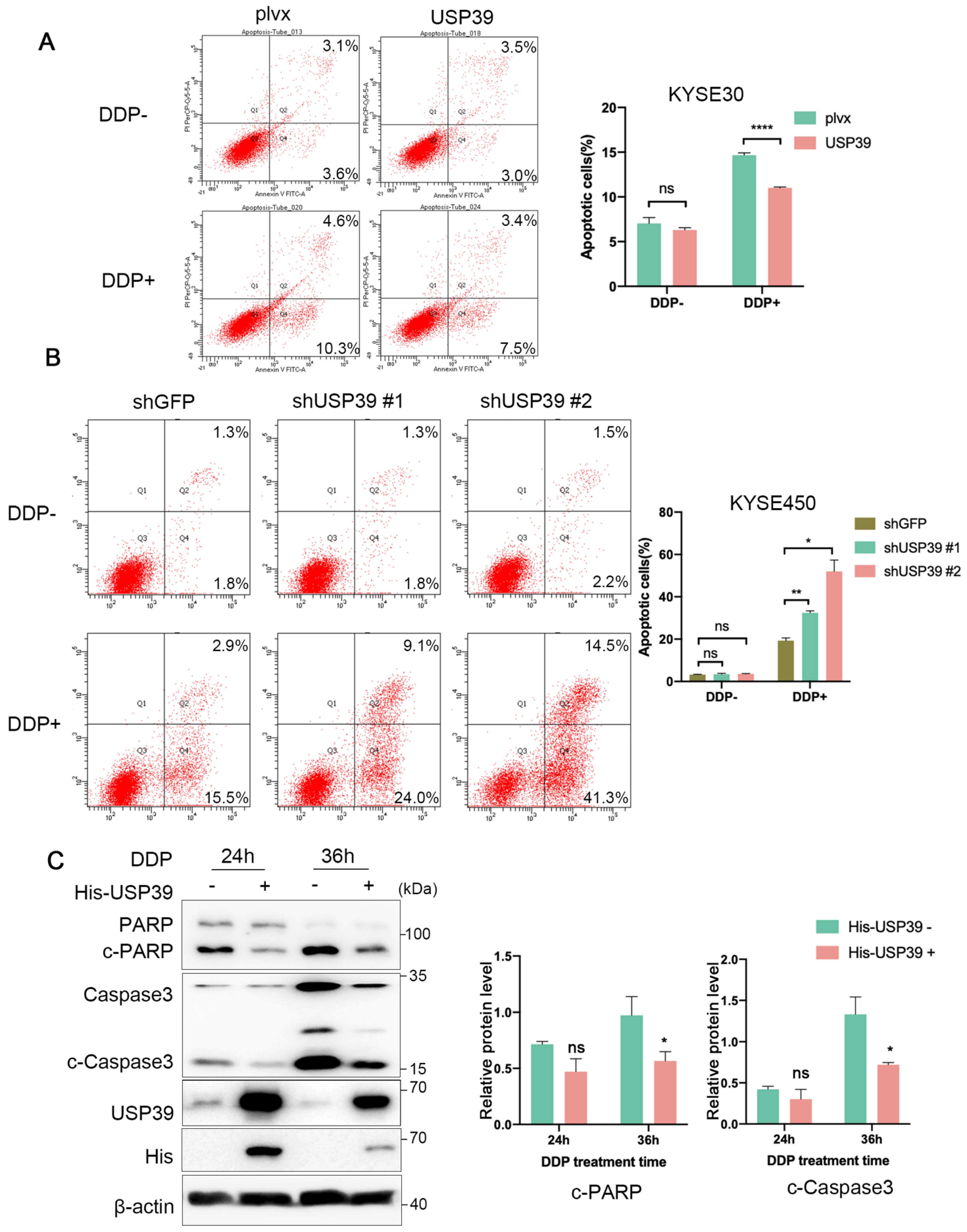
3.3. USP39 Drives Chemoresistance in Esophageal Cancer via Inhibition of Cell Apoptosis
3.4. USP39 Interacts with Several Spliceosome Components
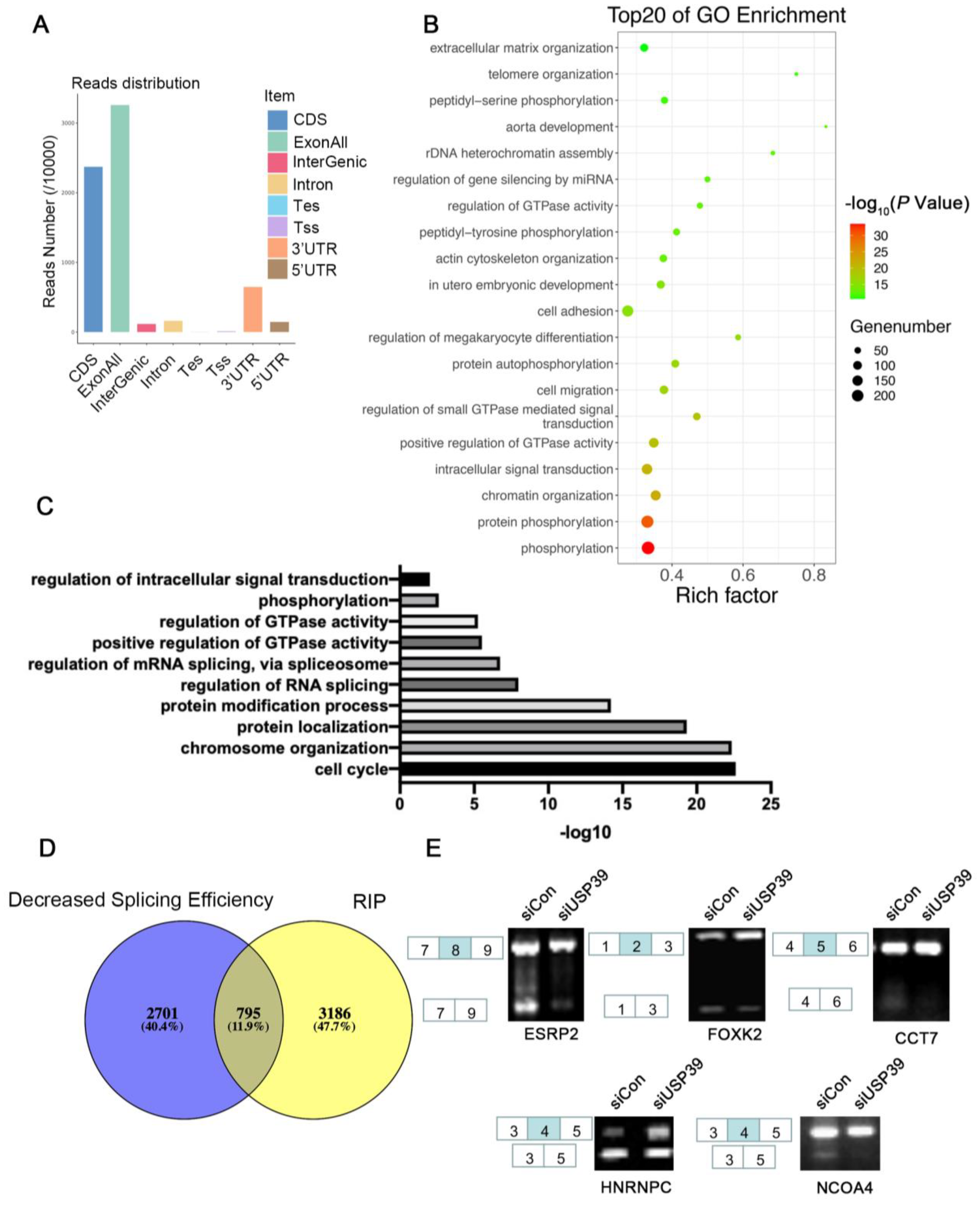
3.5. USP39 Affects Alternative Splicing in ESCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Wu, X.; Chang, W.; Zhao, P.; Zhu, X.; Chen, H.; Nan, Y.; Luo, A.; Zhou, X.; Su, D.; et al. ARID1A Hypermethylation Disrupts Transcriptional Homeostasis to Promote Squamous Cell Carcinoma Progression. Cancer Res. 2020, 80, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wu, X.; Zhao, P.; Nan, Y.; Chang, W.; Zhu, X.; Su, D.; Liu, Z. OTUD1 Activates Caspase-Independent and Caspase-Dependent Apoptosis by Promoting AIF Nuclear Translocation and MCL1 Degradation. Adv. Sci. (Weinh.) 2021, 8, 2002874. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Song, C.; Lei, Y.; Xu, J.; Zhang, G.; Wang, W.; Song, G. Deubiquitinase USP39 and E3 ligase TRIM26 balance the level of ZEB1 ubiquitination and thereby determine the progression of hepatocellular carcinoma. Cell Death Differ 2021, 28, 2315–2332. [Google Scholar] [CrossRef]
- Yuan, J.; Li, X.; Zhang, G.; Cheng, W.; Wang, W.; Lei, Y.; Ma, Q.; Song, G. USP39 mediates p21-dependent proliferation and neoplasia of colon cancer cells by regulating the p53/p21/CDC2/cyclin B1 axis. Mol. Carcinog 2021, 60, 265–278. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Li, J.; Qin, J.; Song, J.; Li, Y.; Zhao, L.; Zhang, X.; Guo, H.; Shao, C.; et al. Splicing factor USP39 promotes ovarian cancer malignancy through maintaining efficient splicing of oncogenic HMGA2. Cell Death Dis. 2021, 12, 294. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Geng, G.; Li, L.; Yin, P.; Nowsheen, S.; Li, Y.; Wu, C.; Liu, J.; Zhao, F.; et al. USP39 regulates DNA damage response and chemo-radiation resistance by deubiquitinating and stabilizing CHK2. Cancer Lett. 2019, 449, 114–124. [Google Scholar] [CrossRef]
- Hadjivassiliou, H.; Rosenberg, O.S.; Guthrie, C. The crystal structure of S. cerevisiae Sad1, a catalytically inactive deubiquitinase that is broadly required for pre-mRNA splicing. RNA 2014, 20, 656–669. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liu, Z.; Zhang, E.; Zhang, P.; Wang, Y.; Hang, J.; Li, Q. USP39 promotes tumorigenesis by stabilizing and deubiquitinating SP1 protein in hepatocellular carcinoma. Cell Signal. 2021, 85, 110068. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.V.; Makarov, E.M.; Luhrmann, R. The 65 and 110 kDa SR-related proteins of the U4/U6.U5 tri-snRNP are essential for the assembly of mature spliceosomes. EMBO J. 2001, 20, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, X.W.; Li, L.; Chen, L.; Liu, X.; Lu, J.L.; Zhu, X.M.; Huang, H.; Yang, Q.W.; Ye, J.Q.; et al. Overexpression of USP39 predicts poor prognosis and promotes tumorigenesis of prostate cancer via promoting EGFR mRNA maturation and transcription elongation. Oncotarget 2016, 7, 22016–22030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, K.; Ji, J.; Zhang, X.; Huang, B.; Chen, A.; Zhang, D.; Li, X.; Wang, X.; Wang, J. RNA splicing factor USP39 promotes glioma progression by inducing TAZ mRNA maturation. Oncogene 2019, 38, 6414–6428. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.W.; Xu, D.; Chen, W.J.; Chen, J.X.; Chen, W.J.; Ye, J.Q.; Gan, S.S.; Zhou, W.; Song, X.; Shi, L.; et al. USP39 promotes malignant proliferation and angiogenesis of renal cell carcinoma by inhibiting VEGF-A165b alternative splicing via regulating SRSF1 and SRPK1. Cancer Cell Int. 2021, 21, 486. [Google Scholar] [CrossRef]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhou, F.; Zuo, Z.; Cheng, H.; Zhou, R. A global view of cancer-specific transcript variants by subtractive transcriptome-wide analysis. PLoS ONE 2009, 4, e4732. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Li, Y.; Lu, Z.; Che, Y.; Sun, S.; Huang, J.; Lei, Y.; Wang, X.; Liu, C.; Zheng, S.; et al. Survival-associated alternative splicing signatures in esophageal carcinoma. Carcinogenesis 2019, 40, 121–130. [Google Scholar] [CrossRef]
- Kidogami, S.; Iguchi, T.; Sato, K.; Yoshikawa, Y.; Hu, Q.; Nambara, S.; Komatsu, H.; Ueda, M.; Kuroda, Y.; Masuda, T.; et al. SF3B4 Plays an Oncogenic Role in Esophageal Squamous Cell Carcinoma. Anticancer Res. 2020, 40, 2941–2946. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; An, H.; Sun, W.; Wang, R.; Liu, M.; Zhang, K. The landscape and biological relevance of aberrant alternative splicing events in esophageal squamous cell carcinoma. Oncogene 2021, 40, 4184–4197. [Google Scholar] [CrossRef] [PubMed]
- Budwit-Novotny, D.A.; McCarty, K.S.; Cox, E.B.; Soper, J.T.; Mutch, D.G.; Creasman, W.T.; Flowers, J.L.; McCarty, K.S., Jr. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986, 46, 5419–5425. [Google Scholar] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, C.; Wang, J.; Xu, D.; Wang, H.; Wang, T.; Li, L.; Li, H.; Nan, P.; Zhang, J.; et al. Chromatin modifier MTA1 regulates mitotic transition and tumorigenesis by orchestrating mitotic mRNA processing. Nat. Commun. 2020, 11, 4455. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Y.; Zhou, H.; Li, L.; Li, Y.; Ding, F.; Cao, X.; Liu, Z. Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 2018, 418, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, H.; Liu, G.; Ji, Q.; Cheng, X.; Li, X.; Liu, W.; Thorne, R.F.; Zhang, R.; Liu, X. The Deubiquitinase USP39 Promotes ESCC Tumorigenesis Through Pre-mRNA Splicing of the mTORC2 Component Rictor. Front. Oncol. 2021, 11, 667495. [Google Scholar] [CrossRef]
- Liu, S.; Rauhut, R.; Vornlocher, H.P.; Luhrmann, R. The network of protein-protein interactions within the human U4/U6.U5 tri-snRNP. RNA 2006, 12, 1418–1430. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, D.E.; Kastner, B.; Dybkov, O.; Hofele, R.V.; Liu, W.T.; Urlaub, H.; Luhrmann, R.; Stark, H. Molecular architecture of the human U4/U6.U5 tri-snRNP. Science 2016, 351, 1416–1420. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Hartmuth, K.; Mohlmann, S.; Urlaub, H.; Ficner, R.; Luhrmann, R. Phosphorylation of human PRP28 by SRPK2 is required for integration of the U4/U6-U5 tri-snRNP into the spliceosome. Nat. Struct. Mol. Biol. 2008, 15, 435–443. [Google Scholar] [CrossRef]
- Bailis, J.M.; Forsburg, S.L. MCM proteins: DNA damage, mutagenesis and repair. Curr. Opin. Genet. Dev. 2004, 14, 17–21. [Google Scholar] [CrossRef]
- Lei, M. The MCM complex: Its role in DNA replication and implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Pan, Y.Y.; You, C.G. CDK1, CCNB1, CDC20, BUB1, MAD2L1, MCM3, BUB1B, MCM2, and RFC4 May Be Potential Therapeutic Targets for Hepatocellular Carcinoma Using Integrated Bioinformatic Analysis. Biomed Res. Int. 2019, 2019, 1245072. [Google Scholar] [CrossRef] [Green Version]
- Soling, A.; Sackewitz, M.; Volkmar, M.; Schaarschmidt, D.; Jacob, R.; Holzhausen, H.J.; Rainov, N.G. Minichromosome maintenance protein 3 elicits a cancer-restricted immune response in patients with brain malignancies and is a strong independent predictor of survival in patients with anaplastic astrocytoma. Clin. Cancer Res. 2005, 11, 249–258. [Google Scholar] [PubMed]
- Lau, K.M.; Chan, Q.K.; Pang, J.C.; Li, K.K.; Yeung, W.W.; Chung, N.Y.; Lui, P.C.; Tam, Y.S.; Li, H.M.; Zhou, L.; et al. Minichromosome maintenance proteins 2, 3 and 7 in medulloblastoma: Overexpression and involvement in regulation of cell migration and invasion. Oncogene 2010, 29, 5475–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Xu, Y.; Xie, Y.; Zhang, L.; Gao, M.; Li, S.; Wang, F. m6A Regulators Is Differently Expressed and Correlated with Immune Response of Esophageal Cancer. Front. Cell. Dev. Biol. 2021, 9, 650023. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Ma, J.; Lu, M.; Liu, Z.; Sun, Y.; Chen, H. The Deubiquitinase USP39 Promotes Esophageal Squamous Cell Carcinoma Malignancy as a Splicing Factor. Genes 2022, 13, 819. https://doi.org/10.3390/genes13050819
Zhu X, Ma J, Lu M, Liu Z, Sun Y, Chen H. The Deubiquitinase USP39 Promotes Esophageal Squamous Cell Carcinoma Malignancy as a Splicing Factor. Genes. 2022; 13(5):819. https://doi.org/10.3390/genes13050819
Chicago/Turabian StyleZhu, Xiaolin, Jianlin Ma, Minyi Lu, Zhihua Liu, Yongkun Sun, and Hongyan Chen. 2022. "The Deubiquitinase USP39 Promotes Esophageal Squamous Cell Carcinoma Malignancy as a Splicing Factor" Genes 13, no. 5: 819. https://doi.org/10.3390/genes13050819
APA StyleZhu, X., Ma, J., Lu, M., Liu, Z., Sun, Y., & Chen, H. (2022). The Deubiquitinase USP39 Promotes Esophageal Squamous Cell Carcinoma Malignancy as a Splicing Factor. Genes, 13(5), 819. https://doi.org/10.3390/genes13050819





