The Length of Leukocyte and Femoral Artery Telomeres in Patients with Peripheral Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Samples
2.2. DNA Preparation and Quantitative Polymerase Chain Reaction (qPCR)
2.3. Statistical Analysis
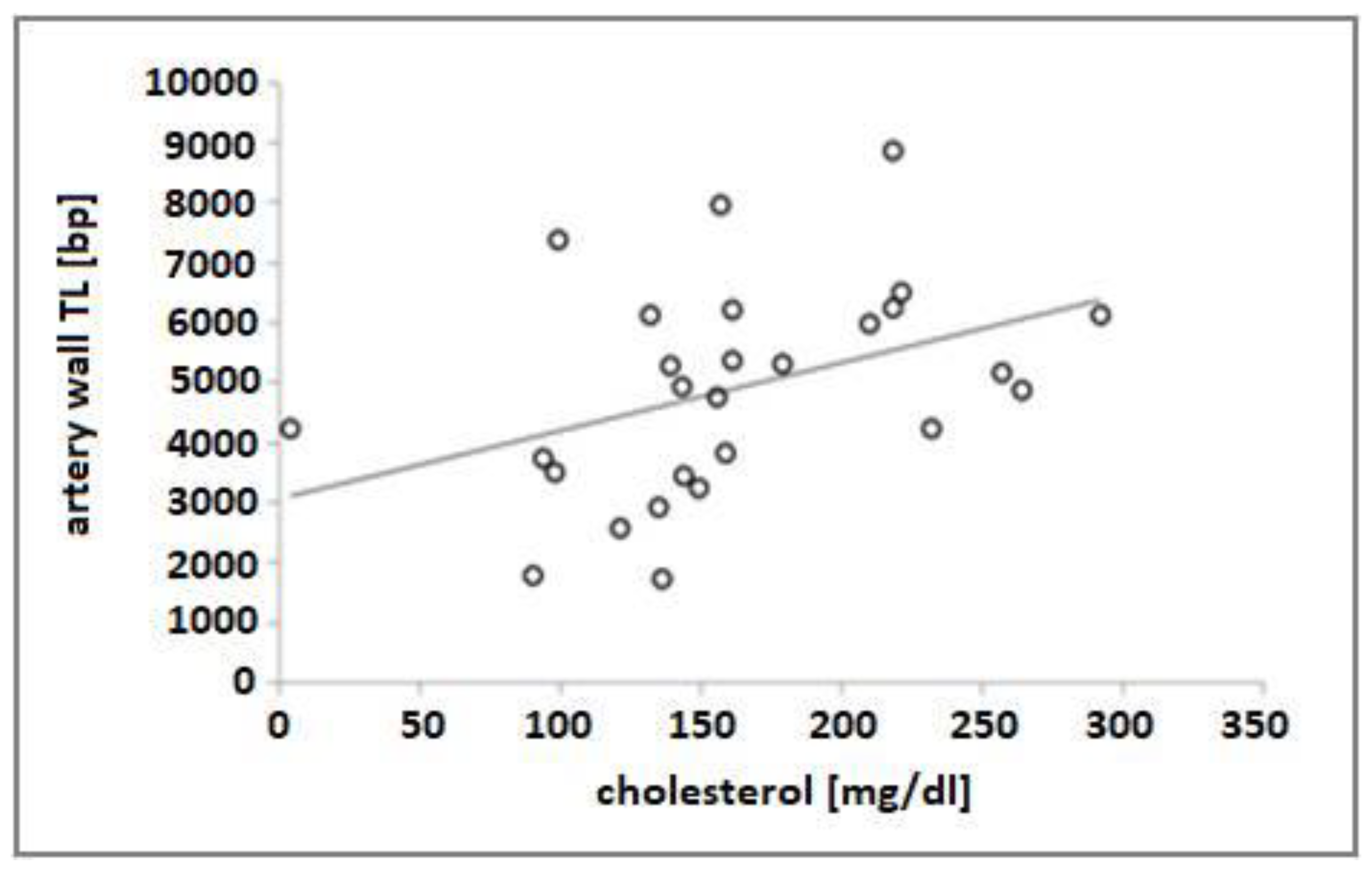
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Blackburn, E.; Epel, E.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Dlouha, D.; Maluskova, J.; Kralova Lesna, I.; Lanska, V.; Hubacek, J.A. Comparison of the relative telomere length measured in leukocytes and eleven different human tissues. Physiol. Res. 2014, 63, 343–350. [Google Scholar] [CrossRef]
- Pańczyszyn, A.; Boniewska-Bernacka, E.; Goc, A. The role of telomeres and telomerase in the senescence of postmitotic cells. DNA Repair 2020, 95, 102956. [Google Scholar] [CrossRef] [PubMed]
- Boniewska-Bernacka, E.; Pańczyszyn, A.; Klinger, M. Telomeres and telomerase in risk assessment of cardiovascular diseases. Exp. Cell Res. 2020, 397, 112361. [Google Scholar] [CrossRef]
- Hoffmann, J.; Richardson, G.; Haendeler, J.; Altschmied, J.; Andrés, V.; Spyridopoulos, I. Telomerase as a Therapeutic Target in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1047–1061. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Lin, Y.; Huang, F.; Ji, H.; Li, Y.; Lin, S.; Chen, X.; Duan, S. Differences in Leukocyte Telomere Length between Coronary Heart Disease and Normal Population: A Multipopulation Meta-Analysis. Biomed. Res. Int. 2019, 2019, 5046867. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Wang, C.Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, C.J.; Demissie, S.; Kimura, M.; Levy, D.; Gardner, J.P.; White, C.; D’Agostino, R.B.; Wolf, P.A.; Polak, J.; Cupples, L.A.; et al. Leukocyte telomere length and carotid artery intimal medial thickness: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1165–1171. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.G.; Ives, S.J.; Walker, A.E.; Cawthon, R.M.; Andtbacka, R.H.; Noyes, D.; Lesniewski, L.A.; Richardson, R.S.; Donato, A.J. Role of arterial telomere dysfunction in hypertension: Relative contributions of telomere shortening and telomere uncapping. J. Hypertens. 2014, 32, 1293–1299. [Google Scholar] [CrossRef] [Green Version]
- Stefler, D.; Malyutina, S.; Maximov, V.; Orlov, P.; Ivanoschuk, D.; Nikitin, Y.; Gafarov, V.; Ryabikov, A.; Voevoda, M.; Bobak, M.; et al. Leukocyte telomere length and risk of coronary heart disease and stroke mortality: Prospective evidence from a Russian cohort. Sci. Rep. 2018, 8, 16627. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Akawi, O.; Fox, S.A.; Li, F.; O’Neil, C.; Balint, B.; Arpino, J.M.; Watson, A.; Wong, J.; Guo, L.; et al. Cardiac-Referenced Leukocyte Telomere Length and Outcomes After Cardiovascular Surgery. JACC Basic Transl. Sci. 2018, 3, 591–600. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Su, X.; Da, M.; Yang, Z.; Duan, W.; Mo, X. Association between leukocyte telomere length and cardiovascular disease in a large general population in the United States. Sci. Rep. 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.R.; De Bacquer, D.; Segers, P.; Van Damme, P.; De Backer, G.G.; Van Oostveldt, P.; Van Criekinge, W.; et al. Asklepios Study Investigators. Systemic telomere length and preclinical atherosclerosis: The Asklepios Study. Eur. Heart J. 2009, 30, 074–081. [Google Scholar] [CrossRef] [Green Version]
- Rietzschel, E.R.; Bekaert, S.; De Meyer, T. Telomeres and Atherosclerosis: The Attrition of an Attractive Hypothesis. J. Am. Coll. Cardiol. 2016, 67, 2477–2479. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Mihara, K.; Bhattacharjee, D.; Mukherjee, M. Telomere length as a potential biomarker of coronary artery disease. Indian J. Med. Res. 2017, 145, 730–737. [Google Scholar] [CrossRef]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proc. Online 2011, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pańczyszyn, A.; Boniewska-Bernacka, E.; Głąb, G. Telomere length in leukocytes and cervical smears of women with high-risk human papillomavirus (HR HPV) infection. Taiwan J. Obstet. Gynecol. 2020, 59, 51–55. [Google Scholar] [CrossRef]
- Cawthon, R. Telomere measurement by the novel monochrome multiplex quantative PCR method. Nucleic Acids Res. 2009, 37, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Gallino, A.; Aboyans, V.; Diehm, C.; Cosentino, F.; Stricker, H.; Falk, E.; Schouten, O.; Lekakis, J.; Amann-Vesti, B.; Siclari, F.; et al. Non-coronary atherosclerosis. Eur. Heart J. 2014, 35, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [Green Version]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Nzietchueng, R.; Elfarra, M.; Nloga, J.; Labat, C.; Carteaux, J.P.; Maureira, P.; Lacolley, P.; Villemot, J.P.; Benetos, A. Telomere length in vascular tissues from patients with atherosclerotic disease. J. Nutr. Health Aging 2011, 15, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Gutmajster, E.; Witecka, J.; Wyskida, M.; Koscinska-Marczewska, J.; Szwed, M.; Owczarz, M.; Mossakowska, M.; Milewicz, A.; Puzianowska-Kuznicka, M.; Zejda, J.; et al. Telomere length in elderly Caucasians weakly correlates with blood cell counts. Sci. World J. 2013, 2013, 153608. [Google Scholar] [CrossRef] [PubMed]
- Compté, N.; Bailly, B.; De Breucker, S.; Goriely, S.; Pepersack, T. Study of the association of total and differential white blood cell counts with geriatric conditions, cardio-vascular diseases, seric IL-6 levels and telomere length. Exp. Gerontol. 2015, 61, 105–112. [Google Scholar] [CrossRef]
- Neuner, B.; Lenfers, A.; Kelsch, R.; Jäger, K.; Brüggmann, N.; van der Harst, P.; Walter, M. Telomere Length Is Not Related to Established Cardiovascular Risk Factors but Does Correlate with Red and White Blood Cell Counts in a German Blood Donor Population. PLoS ONE 2015, 10, e0139308. [Google Scholar] [CrossRef]
- Adams, C.D.; Boutwell, B.B. A Mendelian randomization study of telomere length and blood-cell traits. Sci. Rep. 2020, 10, 12223. [Google Scholar] [CrossRef] [PubMed]
- Mollica, L.; Fleury, I.; Belisle, C.; Provost, S.; Roy, D.C.; Busque, L. No association between telomere length and blood cell counts in elderly individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Den Elzen, W.J.P.; Martin-Ruiz, C.; von Zglinicki, T.; Westendorp, R.G.J.; Kirkwood, T.B.; Gussekloo, J. Telomere length and anaemia in old age: Results from the Newcastle 85-plus Study and the Leiden 85-plus Study. Age Ageing 2011, 40, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ruiz, C.M.; Gussekloo, J.; van Heemst, D.; von Zglinicki, T.; Westendorp, R.G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell 2005, 4, 287–290. [Google Scholar] [CrossRef]
- Mazidi, M.; Penson, P.; Banach, M. Association between telomere length and complete blood count in US adults. Arch. Med. Sci. 2017, 13, 601–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Aviv, A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis 2009, 205, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhou, K.W.; Yang, G.Z.; Chen, C. Association between lipoproteins and telomere length in US adults: Data from the NHANES 1999-2002. Lipids Health Dis. 2019, 18, 80. [Google Scholar] [CrossRef] [Green Version]
- Rehkopf, D.H.; Needham, B.L.; Lin, J.; Blackburn, E.H.; Zota, A.R.; Wojcicki, J.M.; Epel, E.S. Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults. PLoS Med. 2016, 13, e1002188. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Chavakis, T. Immunometabolic control of hematopoiesis. Mol. Aspects Med. 2021, 77, 100923. [Google Scholar] [CrossRef]
- Baragetti, A.; Bonacina, F.; Catapano, A.; Danilo, A.; Norata, G. Effect of Lipids and Lipoproteins on Hematopoietic Cell Metabolism and Commitment in Atherosclerosis. Immunometabolism 2021, 3, e210014. [Google Scholar] [CrossRef]
- Boccardi, V.; Barbieri, M.; Rizzo, M.R.; Marfella, R.; Esposito, A.; Marano, L.; Paolisso, G. A new pleiotropic effect of statins in elderly: Modulation of telomerase activity. FASEB J. 2013, 27, 3879–3885. [Google Scholar] [CrossRef]
- Boccardi, V.; Paolisso, G. The association between statins and telomere shortening. Clin. Lipidol. 2014, 9, 311–315. [Google Scholar] [CrossRef]
- Olivieri, F.; Mazzanti, I.; Abbatecola, A.M.; Recchioni, R.; Marcheselli, F.; Procopio, A.D.; Antonicelli, R. Telomere/Telomerase system: A new target of statins pleiotropic effect? Curr. Vasc. Pharmacol. 2012, 10, 216–224. [Google Scholar] [CrossRef] [PubMed]

| Primer/Oligomer | Sequence | Reference |
|---|---|---|
| Primer TeloF | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT | [18] |
| Primer TeloR | GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT | |
| Primer Albu | CGGCGGCGGGCGGCGCGGGCTGGGCGGaaatgctgcacagaatccttg | [20] |
| Primer Albd | GCCCGGCCCGCCGCGCCCGTCCCGCCGgaaaagcatggtcgcctgtt | |
| Oligomer tel | TTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGG | [18] |
| Oligomer alb | CAGAGTCACCAAATGCTGCACAGAATCCTTGGTGAACAGGCGACCATGCTTTTCAGCTCTGGAA | [19] |
| Variable | n Available | Mean ± SD/Median (Q1; Q3) |
|---|---|---|
| Age, years | 32 | 67.63 ± 8.36 |
| Leukocyte telomeres | 32 | 2381.63 ± 897.96 |
| Artery wall telomeres | 32 | 5131.72 ± 1 884.15 |
| Ratio (artery wall TL/leukocyte TL) | 32 | 2.34 ± 0.88 |
| Glucose, mg/dL | 28 | 108.50 (95.25; 133.75) |
| Cholesterol, mg/dL | 27 | 157.00 (135.50; 218.00) |
| CRP, mg/L | 31 | 3.05 (1.30; 8.27) |
| LDL, mg/dL | 9 | 58.20 (51.70; 95.00) |
| WBCs, 103/uL | 32 | 7.86 ± 2.17 |
| HGB, g/dL | 32 | 13.50 (12.25; 14.70) |
| Neutrophils, 103/µL | 23 | 4.14 (3.55; 5.52) |
| Lymphocytes, 103/µL | 23 | 2.09 ± 0.72 |
| Platelets, 103/µL | 32 | 239.22 ± 73.48 |
| β | 95% CI | Std. β | p | |
|---|---|---|---|---|
| Age, years | −25.44 | −64.36 to 13.49 | −0.24 | 0.192 |
| Glucose, mg/dL | 4.52 | −0.70 to 9.74 | 0.34 | 0.087 |
| Cholesterol, mg/dL | 3.62 | 0.82 to 6.42 | 0.47 | 0.013 |
| CRP, mg/L | 18.02 | −15.98 to 52.03 | 0.20 | 0.287 |
| LDL, mg/dL | −5.10 | −18.81 to 8.60 | −0.31 | 0.408 |
| WBCs, 103/uL | 232.03 | 104.30 to 359.76 | 0.56 | 0.001 |
| HGB, g/dL | −8.80 | −23.49 to 5.89 | −0.22 | 0.231 |
| Neutrophils, 103/uL | 320.42 | 171.45 to 469.39 | 0.75 | <0.001 |
| Lymphocytes, 103/uL | 116.30 | −486.46 to 719.12 | 0.09 | 0.692 |
| Platelets, 103/uL | 2.68 | −1.77 to 7.12 | 0.02 | 0.229 |
| β | 95% CI | Std. β | p | |
|---|---|---|---|---|
| Age, years | −75.22 | −154.46 to 4.02 | −0.33 | 0.062 |
| Glucose, mg/dL | −5.20 | −17.15 to 6.54 | −0.18 | 0.379 |
| Cholesterol, mg/dL | 6.68 | 0.87 to 12.48 | 0.42 | 0.026 |
| CRP, mg/L | −6.24 | −79.38 to 66.89 | −0.03 | 0.863 |
| LDL, mg/dL | 5.93 | −30.48 to 42.34 | 0.17 | 0.712 |
| WBCs, 103/uL | 87.35 | −234.71 to 409.40 | 0.10 | 0.584 |
| HGB, g/dL | −18.15 | −48.99 to 12.69 | −0.21 | 0.239 |
| Neutrophils, 103/uL | 153.50 | −254.65 to 561.75 | 0.17 | 0.443 |
| Lymphocytes, 103/uL | 855.20 | −284.19 to 1994.60 | 0.33 | 0.134 |
| Platelets, 103/uL | 5.17 | −4.20 to 14.53 | 0.20 | 0.269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boniewska-Bernacka, E.; Pańczyszyn, A.; Hobot, J.; Donizy, P.; Ziembik, Z.; Goc, A.; Klinger, M. The Length of Leukocyte and Femoral Artery Telomeres in Patients with Peripheral Atherosclerosis. Genes 2022, 13, 704. https://doi.org/10.3390/genes13040704
Boniewska-Bernacka E, Pańczyszyn A, Hobot J, Donizy P, Ziembik Z, Goc A, Klinger M. The Length of Leukocyte and Femoral Artery Telomeres in Patients with Peripheral Atherosclerosis. Genes. 2022; 13(4):704. https://doi.org/10.3390/genes13040704
Chicago/Turabian StyleBoniewska-Bernacka, Ewa, Anna Pańczyszyn, Jacek Hobot, Piotr Donizy, Zbigniew Ziembik, Anna Goc, and Marian Klinger. 2022. "The Length of Leukocyte and Femoral Artery Telomeres in Patients with Peripheral Atherosclerosis" Genes 13, no. 4: 704. https://doi.org/10.3390/genes13040704
APA StyleBoniewska-Bernacka, E., Pańczyszyn, A., Hobot, J., Donizy, P., Ziembik, Z., Goc, A., & Klinger, M. (2022). The Length of Leukocyte and Femoral Artery Telomeres in Patients with Peripheral Atherosclerosis. Genes, 13(4), 704. https://doi.org/10.3390/genes13040704






