Identification of Genomic Regions and Sources for Wheat Blast Resistance through GWAS in Indian Wheat Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Phenotyping for Wheat Blast
2.2. Genotyping
2.3. Linkage Disequilibrium, Kinship and Population Structure Analysis
2.4. GWAS Analysis
3. Results
3.1. Field Phenotyping for Wheat Blast
3.2. SNP Distribution, Population Structure and Linkage Disequilibrium Analysis
3.3. GWAS for Blast Resistance in the 298 Bread Wheat Genotypes
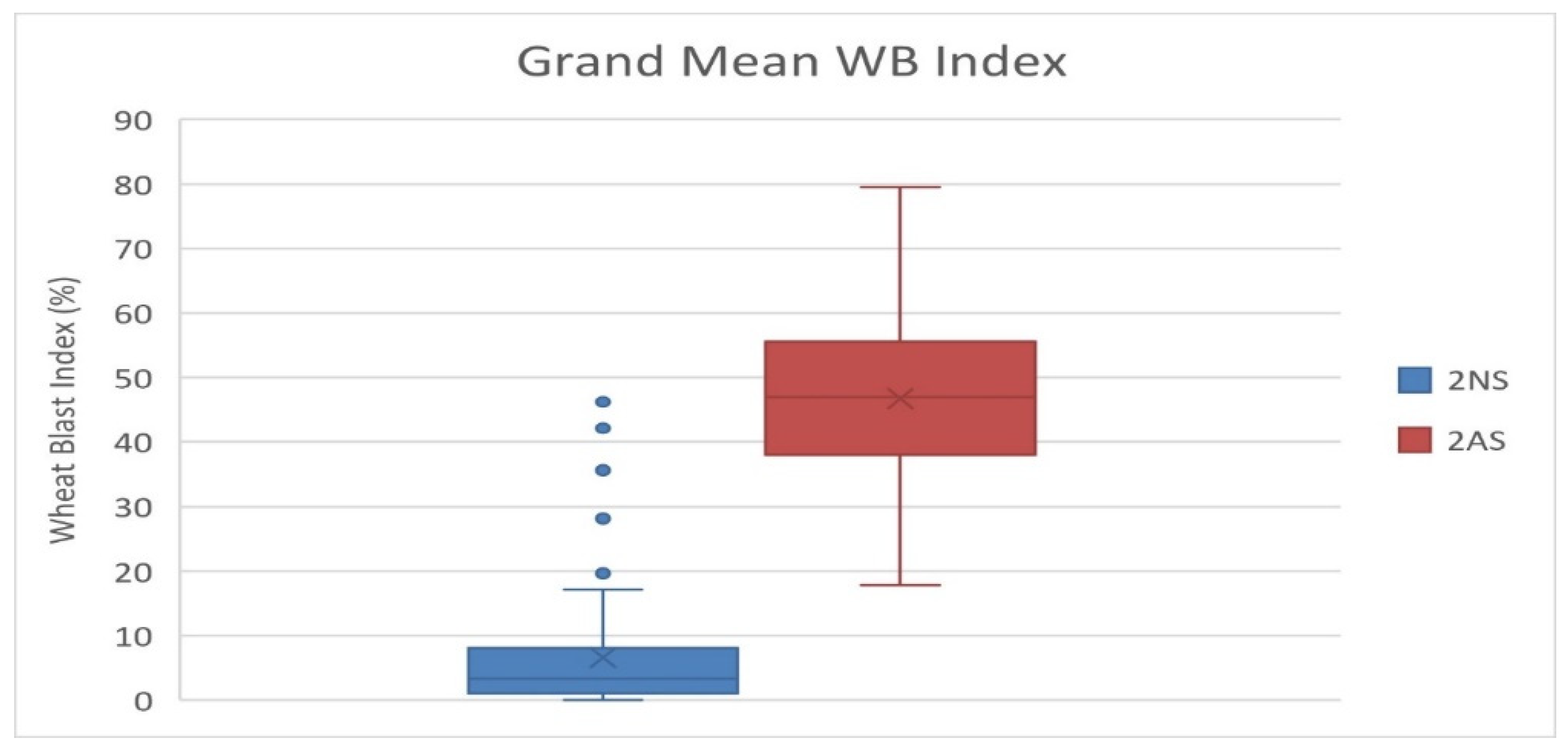
3.4. 2NS Translocation and Wheat Blast Resistance in Wheat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.P.; He, X.; Phuke, R.M.; Joshi, A.K.; Bishnoi, S.K.; Singh, P.K. India’s preparedness to the potential threat from wheat blast. In Current Trends in Wheat and Barley Research Development; ICAR-Indian Agricultural Research Institute, Regional Station: Indore, India, 2019; p. 214. [Google Scholar]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Gupta, A.K.; Singh, M.; Pandey, D.; Kumar, A. Complementary Proteomics, Genomics approaches identifies potential pathogenicity/virulence factors in Tilletia indica induced under the influence of host factor. Sci. Rep. 2019, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Kim, K.-H.; Choi, J. Wheat Blast in Bangladesh: The Current Situation and Future Impacts. Plant Pathol. J. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Gahtyari, N.C.; Roy, C.; Roy, K.K.; He, X.; Tembo, B.; Xu, K.; Juliana, P.; Sonder, K.; Kabir, M.R.; et al. Wheat Blast: A Disease Spreading by Intercontinental Jumps and Its Management Strategies. Front. Plant Sci. 2021, 12, 710707. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.; Rios, J.A.; Eduardo Aucique-Pérez, C.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat blast: Past, present, and future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.; Peterson, G.; Bockus, W.; Kankanala, P.; Dubcovsky, J.; Jordan, K.; Akhunov, E.; Chumley, F.; Baldelomar, F.; Valent, B. The 2NS Translocation from Aegilops ventricosa Confers Resistance to the Triticum Pathotype of Magnaporthe oryzae. Crop Sci. 2016, 56, 990–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat, M.A.; Choi, J. Wheat blast: A new fungal inhabitant to Bangladesh threatening world wheat production. Plant Pathol. J. 2017, 33, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.; Castroagudín, V.L.; Rodrigues, F. Ávila; Rios, J.A.; Aucique-Pérez, C.E.; Moreira, S.I.; Croll, D.; Alves, E.; de Carvalho, G.; Maciel, J.L.N.; et al. Wheat blast: From its origins in South America to its emergence as a global threat. Mol. Plant Pathol. 2019, 20, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Malaker, P.K.; Barma, N.C.D.; Tiwari, T.P.; Collis, W.J.; Duveiller, E.; Singh, P.K.; Joshi, A.K.; Singh, R.P.; Braun, H.-J.; Peterson, G.L.; et al. First Report of Wheat Blast Caused by Magnaporthe oryzae Pathotype Triticum in Bangladesh. Plant Dis. 2016, 100, 2330. [Google Scholar] [CrossRef]
- Callaway, E. Devastating wheat fungus appears in Asia for first time. Nature News 2016, 532, 421–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tembo, B.; Mulenga, R.M.; Sichilima, S.; M’Siska, K.K.; Mwale, M.; Chikoti, P.C.; Singh, P.K.; He, X.; Pedley, K.F.; Peterson, G.L.; et al. Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE 2020, 15, e0238724. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, K.A.; Singh, P.K.; He, X.; Hossain, A.; Kruseman, G.; Erenstein, O. Alternative use of wheat land to implement a potential wheat holiday as wheat blast control: In search of feasible crops in Bangladesh. Land Use Policy 2019, 82, 1–12. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, P.; Hossain, A.; Timsina, J. Rice-wheat system in the northwest Indo-Gangetic plains of South Asia: Issues and technological interventions for increasing productivity and sustainability. Paddy Water Environ. 2021, 19, 345–365. [Google Scholar] [CrossRef]
- Mottaleb, K.A.; Singh, P.; Sonder, K.; Kruseman, G.; Tiwari, T.P.; Barma, N.C.D.; Malaker, P.K.; Braun, H.-J.; Erenstein, O. Threat of wheat blast to South Asia’s food security: An ex-ante analysis. PLoS ONE 2018, 13, e0197555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, V.L.; Anh, N.T.; Tagle, A.G.; Vy, T.T.P.; Inoue, Y.; Takumi, S.; Chuma, I.; Tosa, Y. Rmg8, a New Gene for Resistance to Triticum Isolates of Pyricularia oryzae in Hexaploid Wheat. Phytopathology 2015, 105, 1568–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, S.W.; Mayama, S.; Tosa, Y. Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome 2008, 51, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Tagle, A.G.; Chuma, I.; Tosa, Y. Rmg7, a New Gene for Resistance to Triticum Isolates of Pyricularia oryzae Identified in Tetraploid Wheat. Phytopathology 2015, 105, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hau, V.T.B.; Hirata, K.; Murakami, J.; Nakayashiki, H.; Mayama, S.; Tosa, Y. Rwt4, a wheat gene for resistance to Avena isolates of Magnaporthe oryzae, functions as a gene for resistance to Panicum isolates in Japan. J. Gen. Plant Pathol. 2007, 73, 22–28. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Hau, V.T.B.; Tosa, Y. Identification of genes for resistance to a Digitaria isolate of Magnaporthe grisea in common wheat cultivars. Genome 2009, 52, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Vy, T.T.P.; Hyon, G.-S.; Nga, N.T.T.; Inoue, Y.; Chuma, I.; Tosa, Y. Genetic analysis of host–pathogen incompatibility between Lolium isolates of Pyricularia oryzae and wheat. J. Gen. Plant Pathol. 2013, 80, 59–65. [Google Scholar] [CrossRef]
- Anh, V.L.; Inoue, Y.; Asuke, S.; Vy, T.T.P.; Anh, N.T.; Wang, S.; Chuma, I.; Tosa, Y. Rmg8 and Rmg7, wheat genes for resistance to the wheat blast fungus, recognize the same avirulence gene AVR-Rmg8. Mol. Plant Pathol. 2018, 19, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Horo, J.T.; Asuke, S.; Vy, T.T.P.; Tosa, Y. Effectiveness of the Wheat Blast Resistance Gene Rmg8 in Bangladesh Suggested by Distribution of an AVR-Rmg8 Allele in the Pyricularia oryzae Population. Phytopathology 2020, 110, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Asuke, S.; Vy, T.T.P.; Inoue, Y.; Chuma, I.; Win, J.; Kato, K.; Tosa, Y. A New Resistance Gene in Combination with Rmg8 Confers Strong Resistance Against Triticum Isolates of Pyricularia oryzae in a Common Wheat Landrace. Phytopathology 2018, 108, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goddard, R.; Steed, A.; Chinoy, C.; Ferreira, J.R.; Scheeren, P.L.; Maciel, J.L.N.; Caierão, E.; Torres, G.; Consoli, L.; Santana, F.M.; et al. Dissecting the genetic basis of wheat blast resistance in the Brazilian wheat cultivar BR 18-Terena. BMC Plant Biol. 2020, 20, 398. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kabir, M.R.; Roy, K.K.; Anwar, M.B.; Xu, K.; Marza, F.; Singh, P.K. QTL mapping for field resistance to wheat blast in the Caninde#1/Alondra population. Theor. Appl. Genet. 2020, 133, 2673–2683. [Google Scholar] [PubMed]
- He, X.; Juliana, P.; Kabir, M.R.; Roy, K.K.; Islam, R.; Marza, F.; Peterson, G.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. Screening and Mapping for Head Blast Resistance in a Panel of CIMMYT and South Asian Bread Wheat Germplasm. Front. Genet. 2021, 12, 679162. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Juliana, P.; Kabir, M.R.; Roy, K.K.; Gahtyari, N.C.; Marza, F.; He, X.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. New Genotypes and Genomic Regions for Resistance to Wheat Blast in South Asian Germplasm. Plants 2021, 10, 2693. [Google Scholar] [CrossRef] [PubMed]
- Juliana, P.; He, X.; Kabir, M.R.; Roy, K.K.; Anwar, M.B.; Marza, F.; Poland, J.; Shrestha, S.; Singh, R.P.; Singh, P.K. Genome-wide association mapping for wheat blast resistance in CIMMYT’s international screening nurseries evaluated in Bolivia and Bangla-desh. Sci. Rep. 2020, 10, 15972. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; He, X.; Kabir, M.R.; Roy, K.K.; Anwar, B.; Marza, F.; He, Y.; Jiang, P.; Zhang, X.; Singh, P.K. Genetic sources and loci for wheat head blast resistance identified by genome-wide association analysis. Crop J. 2021. [Google Scholar] [CrossRef]
- Inoue, Y.; Vy, T.T.P.; Tani, D.; Tosa, Y. Suppression of wheat blast resistance by an effector of Pyricularia oryzae is counteracted by a host specificity resistance gene in wheat. New Phytol. 2021, 229, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Steed, A.; Goddard, R.; Gaurav, K.; O’Hara, T.; Schoen, A.; Rawat, N.; Elkot, A.F.; Chonoy, C.; Nicholson, M.H.; et al. A wheat kinase and immune receptor form the host-specificity barrier against the blast fungus. bioRxiv 2022, 1, 477927. [Google Scholar] [CrossRef]
- Inoue, Y.; Vy, T.T.P.; Yoshida, K.; Asano, H.; Mitsuoka, C.; Asuke, S.; Anh, V.L.; Cumagun, C.J.R.; Chuma, I.; Terauchi, R.; et al. Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science 2017, 357, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Q.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Li, H.; Vikram, P.; Singh, R.P.; Kilian, A.; Carling, J.; Song, J.; Burgueno-Ferreira, J.A.; Bhavani, S.; Huerta-Espino, J.; Payne, T.; et al. A high density GBS map of bread wheat and its application for dissecting complex disease resistance traits. BMC Genom. 2016, 16, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helguera, M.; Khan, I.A.; Kolmer, J.; Lijavetzky, D.; Zhong-Qi, L.; Dubcovsky, J. PCR Assays for the Lr37-Yr17-Sr38 Cluster of Rust Resistance Genes and Their Use to Develop Isogenic Hard Red Spring Wheat Lines. Crop Sci. 2003, 43, 1839–1847. [Google Scholar] [CrossRef]
- Seah, S.; Bariana, H.; Jahier, J.; Sivasithamparam, K.; Lagudah, E.S. The introgressed segment carrying rust resistance genes Yr17, Lr37 and Sr38 in wheat can be assayed by a cloned disease resistance gene-like sequence. Theor. Appl. Genet. 2001, 102, 600–605. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, D.A.; vonHoldt, B.M. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Ersoz, E.; Lai, C.-Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Lilin-yin Package ‘CMplot’ Version 3.4.0. 2018. Available online: https://github.com/YinLiLin/CMplot (accessed on 3 January 2022).
- Robinson, H.F. Quantitative genetics in relation to breeding on the centennial of Mendelism. Indian J. Genet. Plant Breed 1966, 26, 171–187. [Google Scholar]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, S.; Gupta, D.R.; Rahman, M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Gupta, D.R.; Hossain, A.; Roy, K.K.; He, X.; Kabir, M.R.; Singh, P.K.; Khan, A.R.; Rahman, M.; Wang, G.-L. Wheat blast: A new threat to food security. Phytopathol. Res. 2020, 2, 28. [Google Scholar] [CrossRef]
- Xu, K.; He, X.; Dreisigacker, S.; He, Z.; Singh, P.K. Anther Extrusion and Its Association with Fusarium Head Blight in CIMMYT Wheat Germplasm. Agronomy 2019, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Dreisigacker, S.; Sansaloni, C.; Duveiller, E.; Singh, R.P.; Singh, P.K. Quantitative Trait Loci Mapping for Spot Blotch Resistance in Two Biparental Mapping Populations of Bread Wheat. Phytopathology 2020, 110, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Mehta, Y.; Guzman, E.; de Viedma, L.; Cubilla, L. Pyricularia blas—A threat to wheat cultivation. Czech J. Genet. Plant Breed. 2011, 47, S130–S134. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://iiwbr.icar.gov.in/wp-content/uploads/2021/08/2020-21-CI-Progress-ReportWebsite-Copy-28072021_compressed.pdf (accessed on 3 January 2022).
- Available online: https://www.aicrpwheatbarleyicar.in/wp-content/uploads/2021/06/2019-20-DirectorsReport.pdf (accessed on 3 January 2022).
- Available online: https://www.aicrpwheatbarleyicar.in/wp-content/uploads/2021/06/Crop-Improvement2018-19-rev.pdf (accessed on 3 January 2022).
- He, X.; Kabir, M.R.; Roy, K.K.; Marza, F.; Chawade, A.; Duveiller, E.; Pierre, C.S.; Singh, P.K. Genetic dissection for head blast resistance in wheat using two mapping populations. Heredity 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cruppe, G. Wheat Blast Management through Identification of Novel Sources of Genetic Resistance and Understanding of Disease Dynamics. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2020. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Harindra, M.W.; Julio, H.E.; Garry, R. Wheat rust in Asia: Meeting the challenges with old and new technologies. New directions for a diverse planet. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; pp. 1–13. [Google Scholar]
- Wang, S.; Wang, J.; Zhang, Z.; Hao, Z.; Zhu, X.; Chai, R. The risk of wheat blast in rice-wheat co-planting regions in China: MoO strains of Pyricularia oryzae cause typical symptom and host reaction on both wheat leaves and spikes. Phytopathol. 2021, 111, 1393–1400. [Google Scholar]
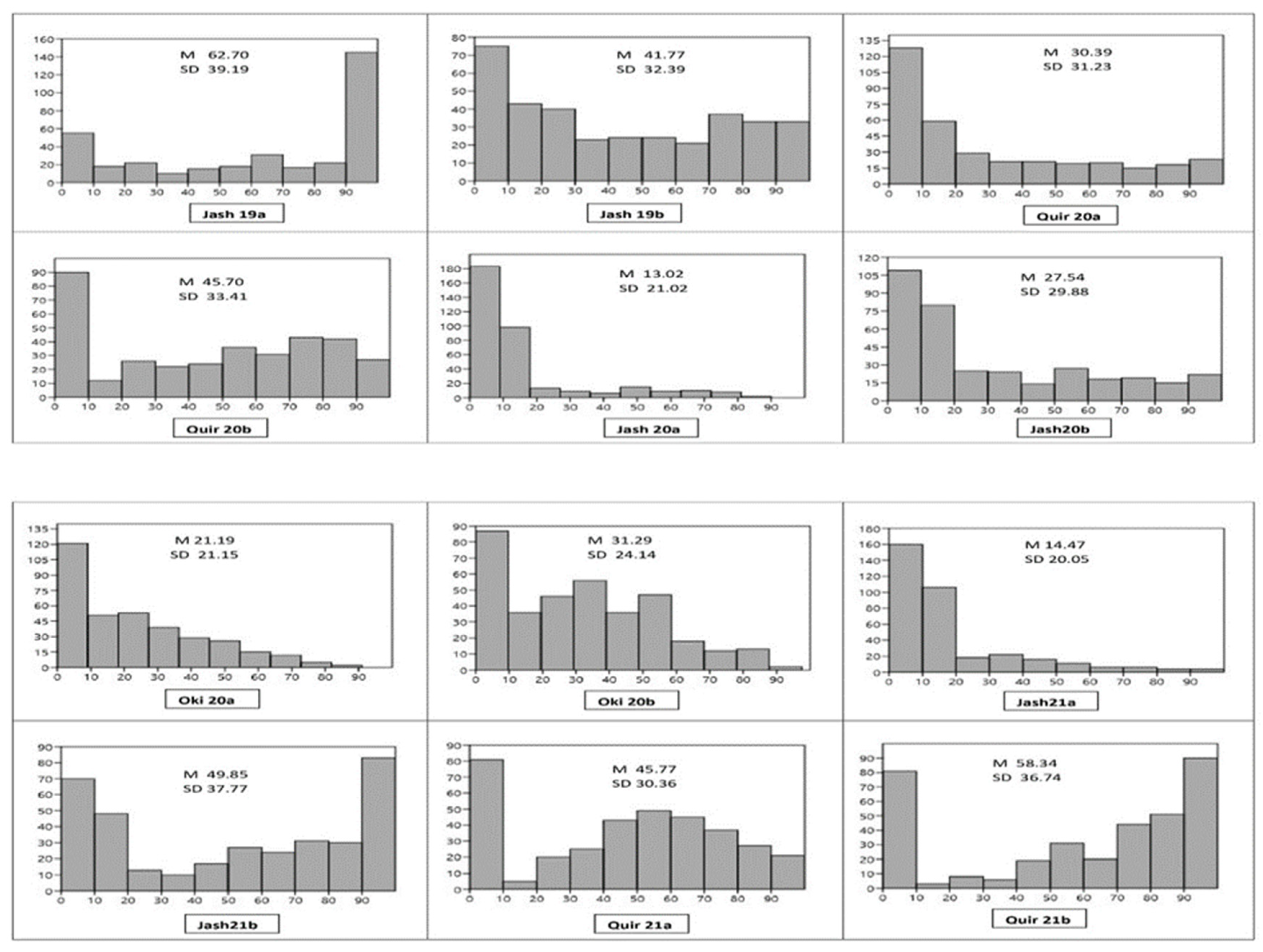
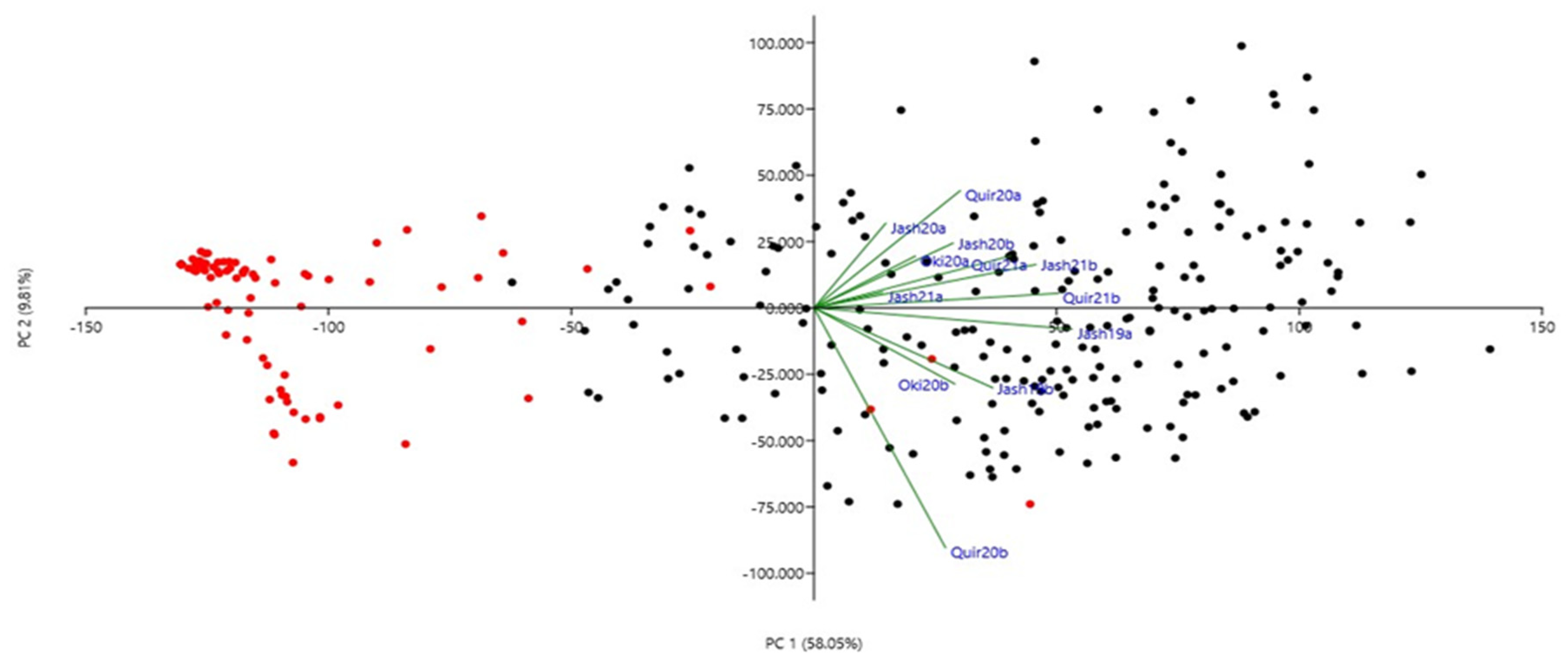
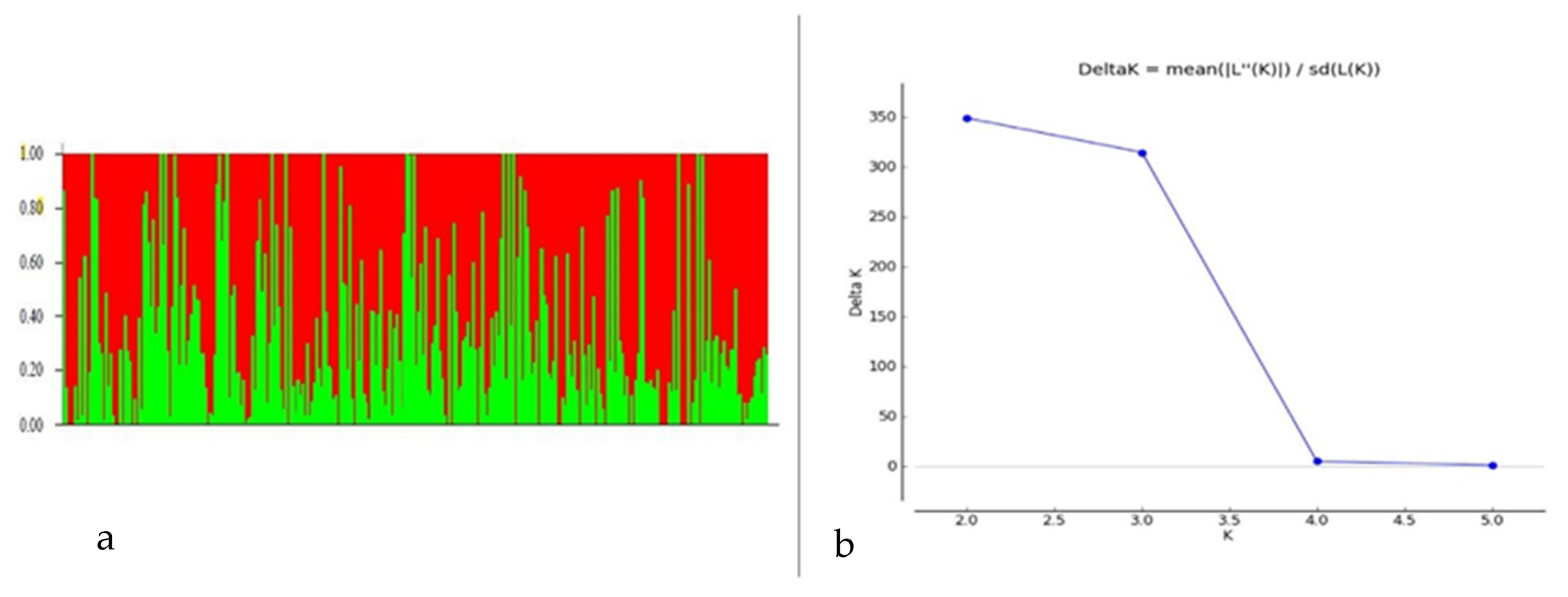
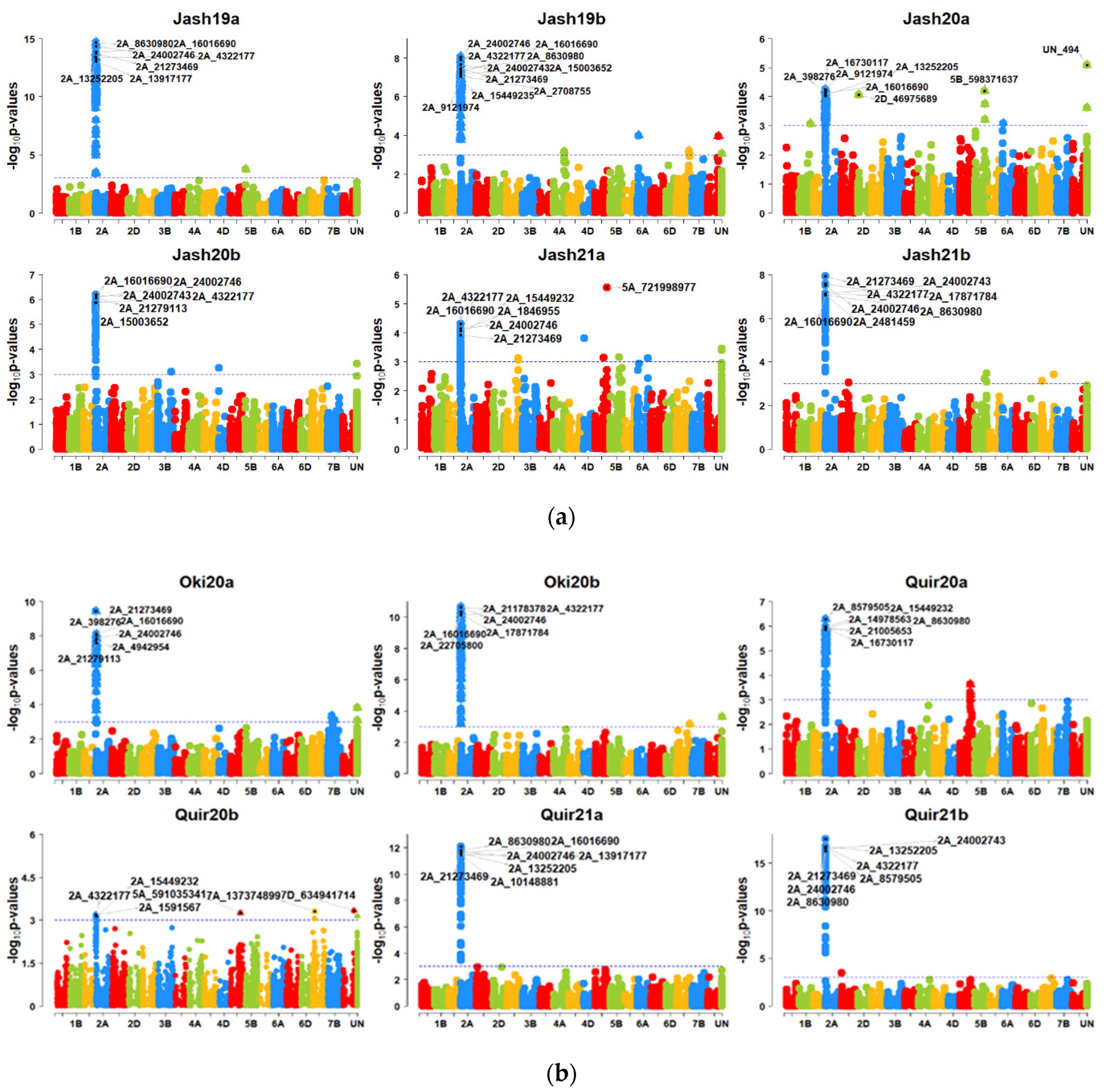
| Location | Source | DF | Mean Squares | F | p | Heritability |
|---|---|---|---|---|---|---|
| Jashore | Genotype | 349 | 3047.65 | 6.4 | <0.0001 | 0.78 |
| Year | 2 | 179,096.65 | 377.83 | <0.0001 | ||
| Genotype × Year | 696 | 658.46 | 1.39 | <0.0001 | ||
| Genotype × Sowing | 349 | 400.88 | 0.85 | 0.962 | ||
| Sowing (Year) | 2 | 140,829.27 | 297.1 | <0.0001 | ||
| Error | 694 | 474.01 | ||||
| Quirusillas | Genotype | 349 | 2504.7084 | 4.86 | <0.0001 | 0.72 |
| Year | 1 | 58,706.8672 | 114.09 | <0.0001 | ||
| Genotype × Year | 348 | 610.2701 | 1.18 | 0.0663 | ||
| Genotype × Sowing | 349 | 613.2954 | 1.18 | 0.0644 | ||
| Sowing (Year) | 1 | 73.5923 | 0.06 | 0.8146 | ||
| Error | 324 | 517.564 | ||||
| Okinawa | Genotype | 349 | 696.319 | 2.11 | <0.0001 | 0.52 |
| Sowing | 1 | 12,235.2133 | 34.51 | <0.0001 | ||
| Error | 347 | 333.9891 |
| Jash19a | Jash19b | Quir20a | Quir20b | Jash20a | Jash20b | Oki20a | Oki20b | Jash21a | Jash21b | Quir21a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jash19b | 0.65 | ||||||||||
| Quir20a | 0.43 | 0.38 | |||||||||
| Quir20b | 0.40 | 0.35 | 0.20 | ||||||||
| Jash20a | 0.33 | 0.28 | 0.44 | 0.08 | |||||||
| Jash20b | 0.47 | 0.41 | 0.37 | 0.21 | 0.44 | ||||||
| Oki20a | 0.47 | 0.36 | 0.60 | 0.26 | 0.45 | 0.42 | |||||
| Oki20b | 0.63 | 0.60 | 0.31 | 0.48 | 0.16 | 0.41 | 0.36 | ||||
| Jash21a | 0.33 | 0.34 | 0.44 | 0.29 | 0.44 | 0.46 | 0.56 | 0.29 | |||
| Jash21b | 0.57 | 0.51 | 0.53 | 0.37 | 0.46 | 0.50 | 0.57 | 0.51 | 0.54 | ||
| Quir21a | 0.66 | 0.52 | 0.60 | 0.34 | 0.39 | 0.49 | 0.63 | 0.56 | 0.40 | 0.63 | |
| Quir21b | 0.68 | 0.50 | 0.56 | 0.41 | 0.32 | 0.45 | 0.57 | 0.61 | 0.37 | 0.61 | 0.78 |
| Algorithm | Marker | Chromosome | Position (Mb) | p Value | R2 | Experiment |
|---|---|---|---|---|---|---|
| All | Multiple SNPs | 2NS | 195997—29397023 | 7.41 × 10−53 to 2.55 × 10−5 | 0.05 to 0.32 | All |
| Farm CPU | 2B_180938790 | 2BS | 180938790 | 1.67 × 10−6 to 5.06 × 10−6 | 0.03 to 0.04 | Jash20b, GM |
| Farm CPU | 3B_794537258 | 3BL | 794537258 | 8.89 × 10−6 to 1.67 × 10−5 | 0.005 to 0.03 | Quir 20a, GM |
| Farm CPU | 4D_25473616 | 4DS | 25473616 | 1.10 × 10−5 to 2.22 × 10−5 | 0.04 to 0.05 | Jash 21a, Oki 20a |
| Farm CPU | 5A_618682953 | 5AL | 618682953 | 5.84 × 10−7 to 1.06 × 10−5 | 0.05 to 0.06 | Oki 20b, Quir 21b |
| Farm CPU | 6A_75053670 | 6AS | 75053670 | 3.11 × 10−8 to 5.07 × 10−7 | 0.04 to 0.05 | Jash19a, Quir21a |
| Farm CPU | 7A_752501634 | 7AL | 752501634 | 5.41 × 10−6 to 2.17 × 10−5 | 0.004 to 0.02 | Jash19b, Jash21b |
| Entry | Origin | Pedigree | WB Index (%) |
|---|---|---|---|
| MACS6736 | Indian | NI 5439/HD2934 | 17.79 |
| DBW297 | CIMMYT | SOKOLL/3/PASTOR//HXL 7573/2*BAU/4/MASSIV/PPR47.89C | 21.10 |
| DBW286 | Indian | DBW 43/DPW 621-50 | 22.71 |
| DBW273 | CIMMYT | FRANCOLIN #1*2//ND 643/2* WBLLI | 22.78 |
| KRL423 | CIMMYT | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/GLADIUS | 23.71 |
| PBW805 | CIMMYT | OASIS/SKAUZ//4*BCN/3/2*PASTOR/4/PBW631 | 24.29 |
| UP3028 | CIMMYT | BECARD#1/CIRNO C 2008//BECARD | 24.80 |
| PBW804 | CIMMYT | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/HUW234 + LR34/PRINIA//PBW34 3*2/KUKUNA/3/ROLF07 | 25.45 |
| PBW773 | CIMMYT | FRANCOLIN#1*2/KIRITATI | 27.98 |
| HD3339 | CIMMYT | FRANCOLIN#1//WBLL1*2/BRAMBLING | 28.06 |
| WH1259 | CIMMYT | SNB//CMH79A.955/3*CNO79/3/ATTILA/4/CHEN/AE.SQUARROSA (TAUS)//BCN/3/2*KAUZ/5/KINGBIRD#1 | 28.73 |
| MP1361 | CIMMYT | CHEN/AEGILOPS SQUARROSA (TAUS)//BCN/3/BAV92/4/BERKUT/5/BAVIS/JWS140 | 29.68 |
| JAUW672 | CIMMYT | SERI.18*2/3/KAUZ*2/BOW//KAUZ/4/CROC | 29.69 |
| MP1360 | CIMMYT | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/GLADIUS/MP 1285 | 29.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuke, R.M.; He, X.; Juliana, P.; Kabir, M.R.; Roy, K.K.; Marza, F.; Roy, C.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. Identification of Genomic Regions and Sources for Wheat Blast Resistance through GWAS in Indian Wheat Genotypes. Genes 2022, 13, 596. https://doi.org/10.3390/genes13040596
Phuke RM, He X, Juliana P, Kabir MR, Roy KK, Marza F, Roy C, Singh GP, Chawade A, Joshi AK, et al. Identification of Genomic Regions and Sources for Wheat Blast Resistance through GWAS in Indian Wheat Genotypes. Genes. 2022; 13(4):596. https://doi.org/10.3390/genes13040596
Chicago/Turabian StylePhuke, Rahul M., Xinyao He, Philomin Juliana, Muhammad R. Kabir, Krishna K. Roy, Felix Marza, Chandan Roy, Gyanendra P. Singh, Aakash Chawade, Arun K. Joshi, and et al. 2022. "Identification of Genomic Regions and Sources for Wheat Blast Resistance through GWAS in Indian Wheat Genotypes" Genes 13, no. 4: 596. https://doi.org/10.3390/genes13040596
APA StylePhuke, R. M., He, X., Juliana, P., Kabir, M. R., Roy, K. K., Marza, F., Roy, C., Singh, G. P., Chawade, A., Joshi, A. K., & Singh, P. K. (2022). Identification of Genomic Regions and Sources for Wheat Blast Resistance through GWAS in Indian Wheat Genotypes. Genes, 13(4), 596. https://doi.org/10.3390/genes13040596








